Abstract
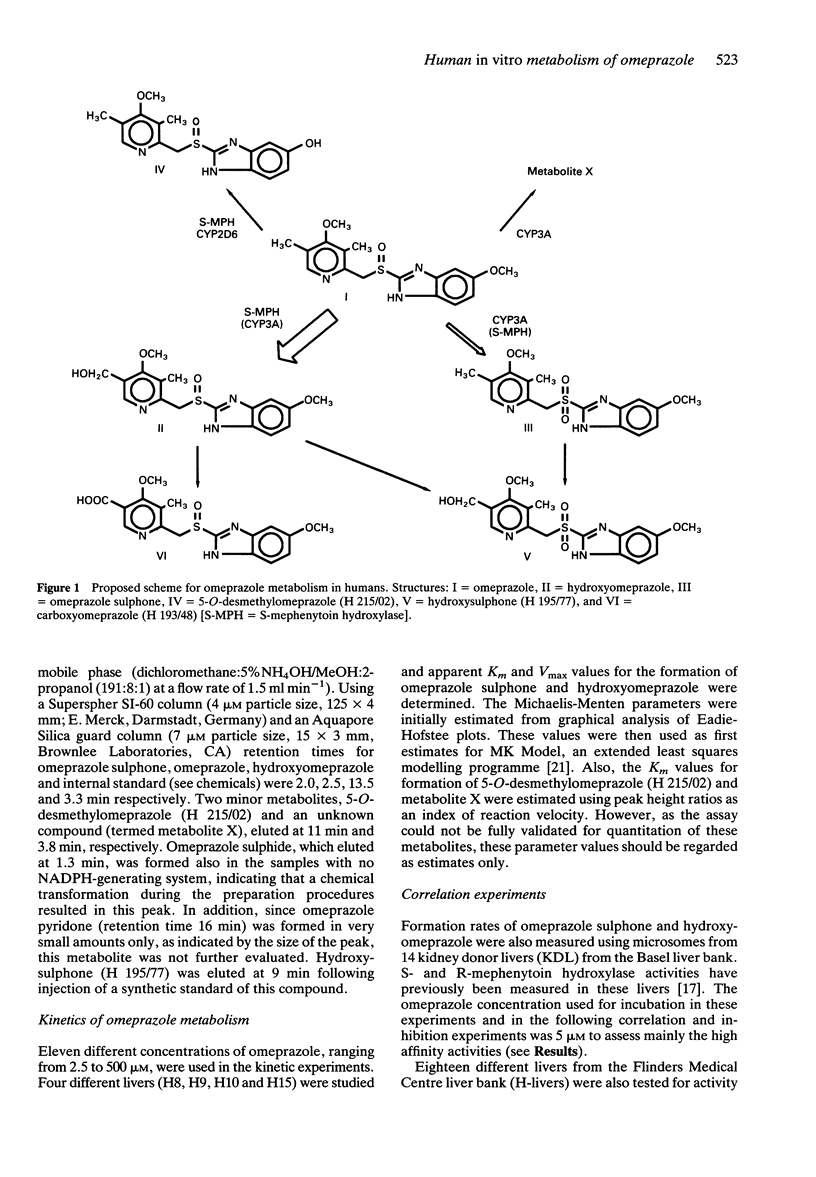
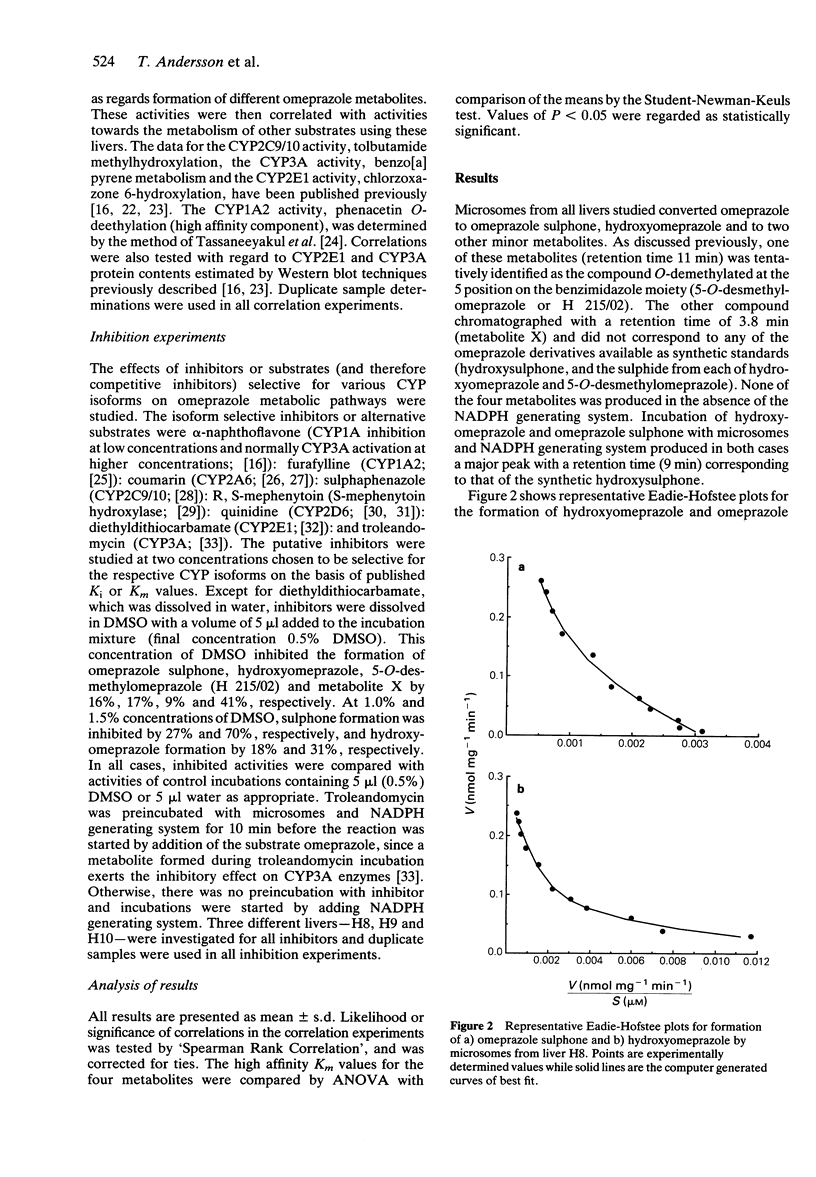
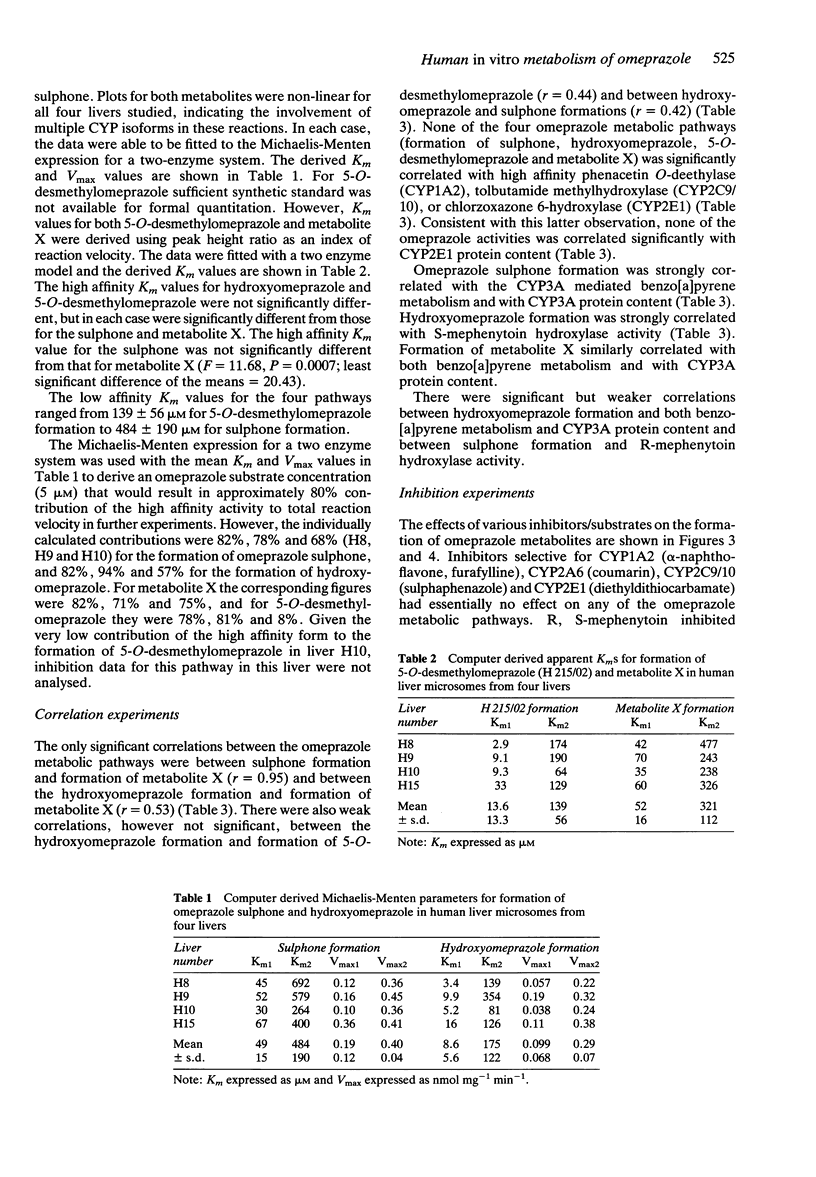
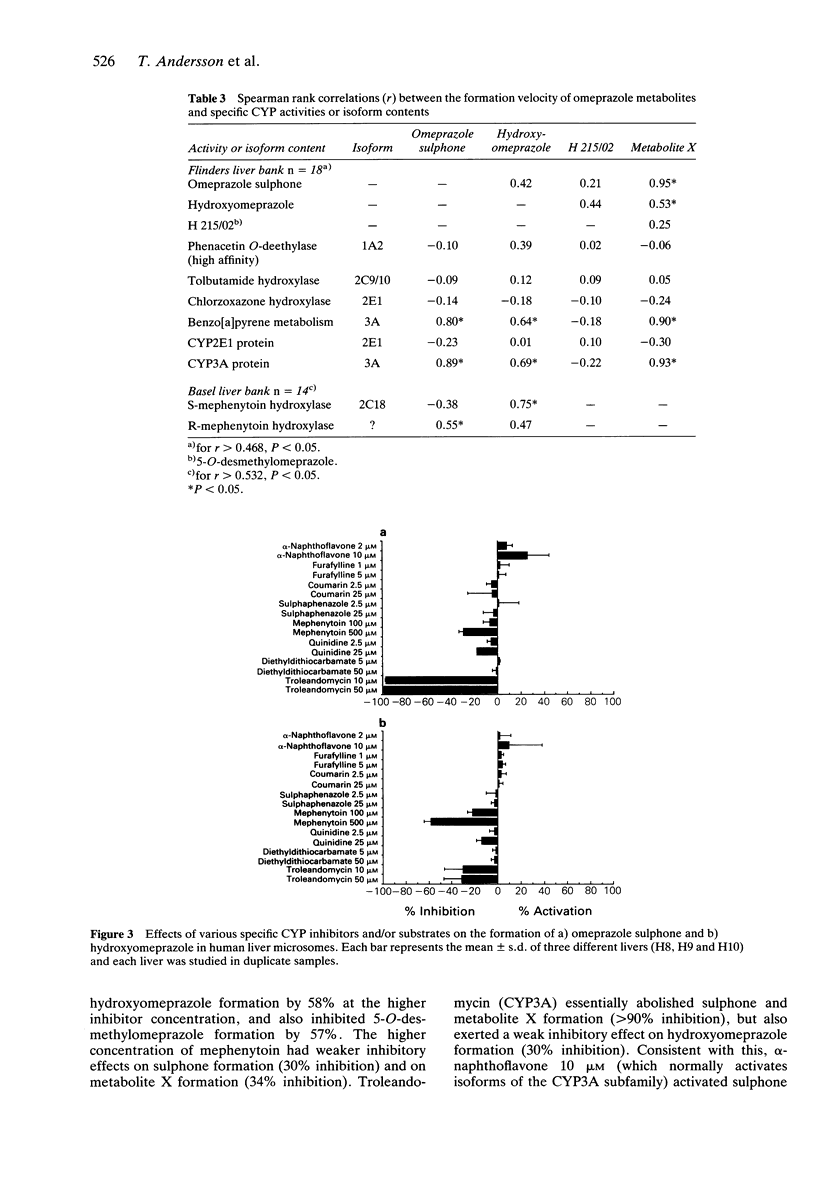
1 The in vitro metabolism of omeprazole was studied in human liver microsomes in order to define the metabolic pathways and identify the cytochrome P450 (CYP) isoforms responsible for the formation of the major omeprazole metabolites. 2 The four major metabolites identified in vitro, in tentative order of importance, were hydroxyomeprazole, omeprazole sulphone, 5-O-desmethylomeprazole, and an unidentified compound termed metabolite X. Omeprazole pyridone was also detected but could not be quantitated. Incubation of hydroxyomeprazole and omeprazole sulphone with human microsomes resulted in both cases in formation of the hydroxysulphone. The kinetics of formation of the four primary metabolites studied were biphasic suggesting the involvement of multiple CYP isoforms in each case. Further studies used substrate concentrations at which the high affinity activities predominated. 3 Formation of the major metabolite, hydroxyomeprazole, was significantly correlated with S-mephenytoin hydroxylase and with benzo[a]pyrene metabolism and CYP3A content. Inhibition studies with isoform selective inhibitors also indicated a dominant role of S-mephenytoin hydroxylase with some CYP3A contribution in the formation of hydroxyomeprazole. Correlation and inhibition data for the sulphone and metabolite X were consistent with a predominant role of the CYP3A subfamily in formation of these metabolites. Formation of 5-O-desmethylomeprazole was inhibited by both R, S-mephenytoin and quinidine, indicating that both S-mephenytoin hydroxylase and CYP2D6 may mediate this reaction in human liver microsomes and in vivo. 4 The Vmax/Km (indicator of intrinsic clearance in vivo) for hydroxyomeprazole was four times greater than that for omeprazole sulphone. Consistent with findings in vivo, the results predict that omeprazole clearance in vivo would be reduced in poor metabolisers of mephenytoin due to reduction in the dominant partial metabolic clearance to hydroxyomeprazole.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Cederberg C., Edvardsson G., Heggelund A., Lundborg P. Effect of omeprazole treatment on diazepam plasma levels in slow versus normal rapid metabolizers of omeprazole. Clin Pharmacol Ther. 1990 Jan;47(1):79–85. doi: 10.1038/clpt.1990.12. [DOI] [PubMed] [Google Scholar]
- Andersson T., Regårdh C. G., Dahl-Puustinen M. L., Bertilsson L. Slow omeprazole metabolizers are also poor S-mephenytoin hydroxylators. Ther Drug Monit. 1990 Jul;12(4):415–416. doi: 10.1097/00007691-199007000-00020. [DOI] [PubMed] [Google Scholar]
- Andersson T., Regårdh C. G., Lou Y. C., Zhang Y., Dahl M. L., Bertilsson L. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics. 1992 Feb;2(1):25–31. doi: 10.1097/00008571-199202000-00005. [DOI] [PubMed] [Google Scholar]
- Bardhan K. D., Bianchi Porro G., Bose K., Daly M., Hinchliffe R. F., Jonsson E., Lazzaroni M., Naesdal J., Rikner L., Walan A. A comparison of two different doses of omeprazole versus ranitidine in treatment of duodenal ulcers. J Clin Gastroenterol. 1986 Aug;8(4):408–413. doi: 10.1097/00004836-198608000-00005. [DOI] [PubMed] [Google Scholar]
- Doecke C. J., Veronese M. E., Pond S. M., Miners J. O., Birkett D. J., Sansom L. N., McManus M. E. Relationship between phenytoin and tolbutamide hydroxylations in human liver microsomes. Br J Clin Pharmacol. 1991 Feb;31(2):125–130. doi: 10.1111/j.1365-2125.1991.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellenius E., Berglindh T., Sachs G., Olbe L., Elander B., Sjöstrand S. E., Wallmark B. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+)ATPase. Nature. 1981 Mar 12;290(5802):159–161. doi: 10.1038/290159a0. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Müller-Enoch D., Blair I. A. Oxidation of quinidine by human liver cytochrome P-450. Mol Pharmacol. 1986 Sep;30(3):287–295. [PubMed] [Google Scholar]
- Inaba T., Jurima M., Mahon W. A., Kalow W. In vitro inhibition studies of two isozymes of human liver cytochrome P-450. Mephenytoin p-hydroxylase and sparteine monooxygenase. Drug Metab Dispos. 1985 Jul-Aug;13(4):443–448. [PubMed] [Google Scholar]
- Klinkenberg-Knol E. C., Jansen J. M., Festen H. P., Meuwissen S. G., Lamers C. B. Double-blind multicentre comparison of omeprazole and ranitidine in the treatment of reflux oesophagitis. Lancet. 1987 Feb 14;1(8529):349–351. doi: 10.1016/s0140-6736(87)91726-0. [DOI] [PubMed] [Google Scholar]
- Küpfer A., Preisig R. Pharmacogenetics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur J Clin Pharmacol. 1984;26(6):753–759. doi: 10.1007/BF00541938. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W. Y., Dombrosky P., Atkins W. M., Villafranca J. J. Terbium(III) luminescence study of tyrosine emission from Escherichia coli glutamine synthetase. Biochemistry. 1991 Apr 9;30(14):3427–3431. doi: 10.1021/bi00228a011. [DOI] [PubMed] [Google Scholar]
- Lind T., Andersson T., Skånberg I., Olbe L. Biliary excretion of intravenous [14C] omeprazole in humans. Clin Pharmacol Ther. 1987 Nov;42(5):504–508. doi: 10.1038/clpt.1987.188. [DOI] [PubMed] [Google Scholar]
- Lind T., Cederberg C., Ekenved G., Haglund U., Olbe L. Effect of omeprazole--a gastric proton pump inhibitor--on pentagastrin stimulated acid secretion in man. Gut. 1983 Apr;24(4):270–276. doi: 10.1136/gut.24.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus M. E., Burgess W. M., Veronese M. E., Huggett A., Quattrochi L. C., Tukey R. H. Metabolism of 2-acetylaminofluorene and benzo(a)pyrene and activation of food-derived heterocyclic amine mutagens by human cytochromes P-450. Cancer Res. 1990 Jun 1;50(11):3367–3376. [PubMed] [Google Scholar]
- Meier U. T., Dayer P., Malè P. J., Kronbach T., Meyer U. A. Mephenytoin hydroxylation polymorphism: characterization of the enzymatic deficiency in liver microsomes of poor metabolizers phenotyped in vivo. Clin Pharmacol Ther. 1985 Nov;38(5):488–494. doi: 10.1038/clpt.1985.213. [DOI] [PubMed] [Google Scholar]
- Pessayre D., Tinel M., Larrey D., Cobert B., Funck-Brentano C., Babany G. Inactivation of cytochrome P-450 by a troleandomycin metabolite. Protective role of glutathione. J Pharmacol Exp Ther. 1983 Mar;224(3):685–691. [PubMed] [Google Scholar]
- Regårdh C. G., Andersson T., Lagerström P. O., Lundborg P., Skånberg I. The pharmacokinetics of omeprazole in humans--a study of single intravenous and oral doses. Ther Drug Monit. 1990 Mar;12(2):163–172. doi: 10.1097/00007691-199003000-00010. [DOI] [PubMed] [Google Scholar]
- Renberg L., Simonsson R., Hoffmann K. J. Identification of two main urinary metabolites of [14C]omeprazole in humans. Drug Metab Dispos. 1989 Jan-Feb;17(1):69–76. [PubMed] [Google Scholar]
- Robson R. A., Matthews A. P., Miners J. O., McManus M. E., Meyer U. A., Hall P. M., Birkett D. J. Characterisation of theophylline metabolism in human liver microsomes. Br J Clin Pharmacol. 1987 Sep;24(3):293–300. doi: 10.1111/j.1365-2125.1987.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesardic D., Boobis A. R., Murray B. P., Murray S., Segura J., de la Torre R., Davies D. S. Furafylline is a potent and selective inhibitor of cytochrome P450IA2 in man. Br J Clin Pharmacol. 1990 Jun;29(6):651–663. doi: 10.1111/j.1365-2125.1990.tb03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn D. R., Kobayashi K., Chiba K., Lee K. H., Shin S. G., Ishizaki T. Disposition kinetics and metabolism of omeprazole in extensive and poor metabolizers of S-mephenytoin 4'-hydroxylation recruited from an Oriental population. J Pharmacol Exp Ther. 1992 Sep;262(3):1195–1202. [PubMed] [Google Scholar]
- Tassaneeyakul W., Birkett D. J., Veronese M. E., McManus M. E., Tukey R. H., Quattrochi L. C., Gelboin H. V., Miners J. O. Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J Pharmacol Exp Ther. 1993 Apr;265(1):401–407. [PubMed] [Google Scholar]
- Veronese M. E., Doecke C. J., Mackenzie P. I., McManus M. E., Miners J. O., Rees D. L., Gasser R., Meyer U. A., Birkett D. J. Site-directed mutation studies of human liver cytochrome P-450 isoenzymes in the CYP2C subfamily. Biochem J. 1993 Jan 15;289(Pt 2):533–538. doi: 10.1042/bj2890533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walan A., Bader J. P., Classen M., Lamers C. B., Piper D. W., Rutgersson K., Eriksson S. Effect of omeprazole and ranitidine on ulcer healing and relapse rates in patients with benign gastric ulcer. N Engl J Med. 1989 Jan 12;320(2):69–75. doi: 10.1056/NEJM198901123200201. [DOI] [PubMed] [Google Scholar]
- Wallmark B., Lorentzon P., Larsson H. The mechanism of action of omeprazole--a survey of its inhibitory actions in vitro. Scand J Gastroenterol Suppl. 1985;108:37–51. [PubMed] [Google Scholar]
- Yamano S., Tatsuno J., Gonzalez F. J. The CYP2A3 gene product catalyzes coumarin 7-hydroxylation in human liver microsomes. Biochemistry. 1990 Feb 6;29(5):1322–1329. doi: 10.1021/bi00457a031. [DOI] [PubMed] [Google Scholar]
- Yun C. H., Shimada T., Guengerich F. P. Purification and characterization of human liver microsomal cytochrome P-450 2A6. Mol Pharmacol. 1991 Nov;40(5):679–685. [PubMed] [Google Scholar]


