Abstract
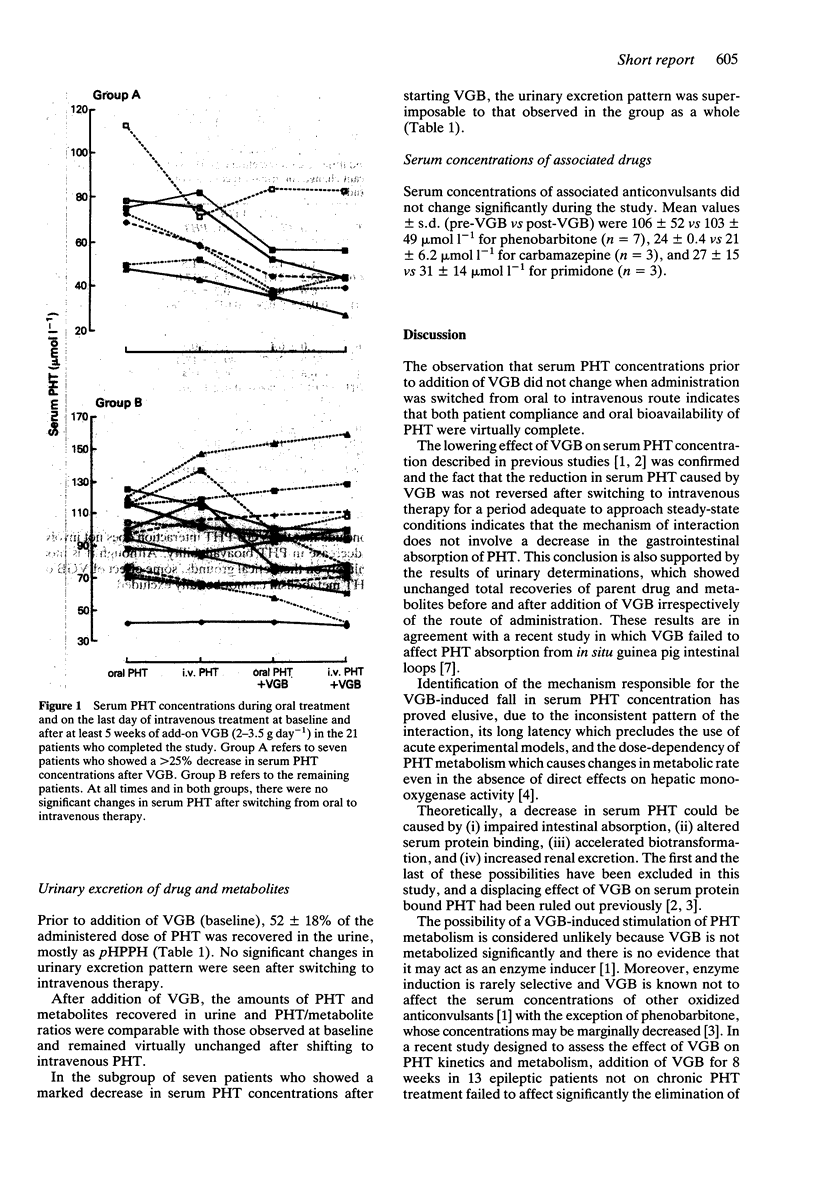
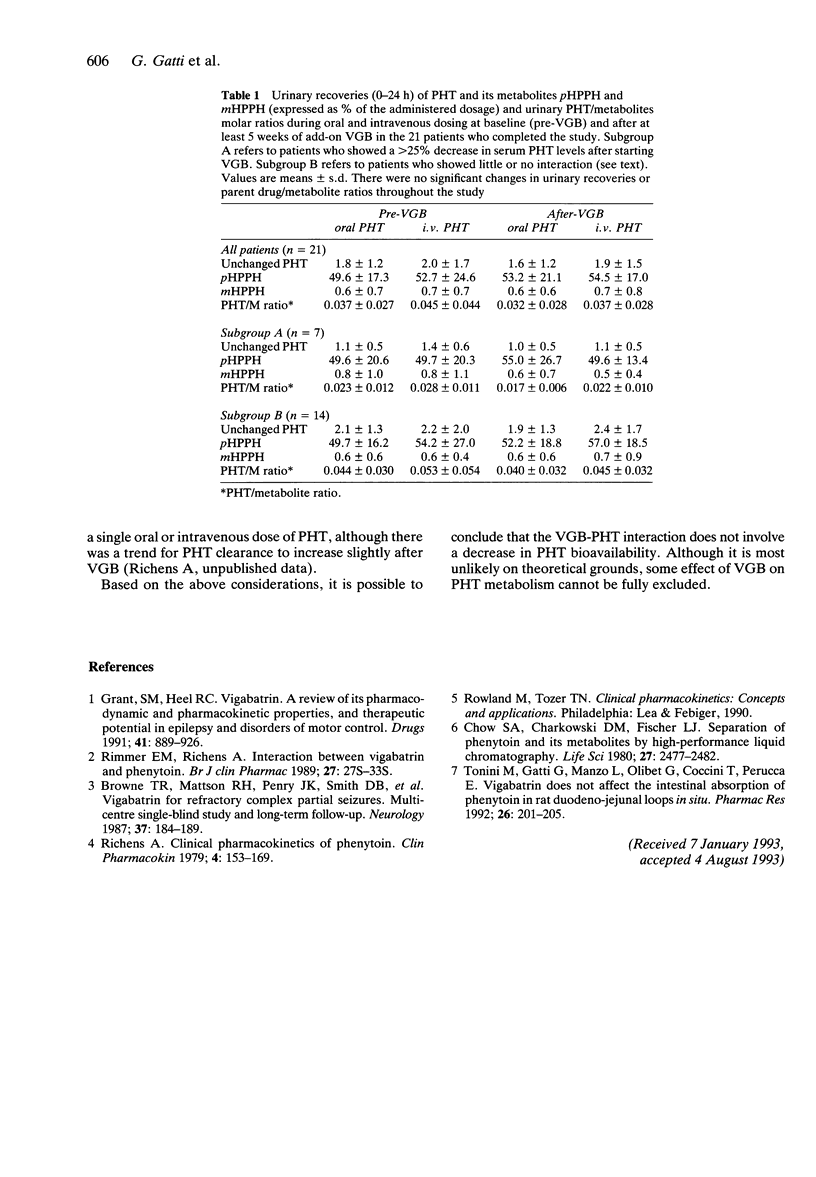
The possibility that vigabatrin (VGB) decreases serum phenytoin (PHT) concentration by lowering the oral bioavailability of PHT was investigated in 21 patients with epilepsy. Each patient was switched from oral to intravenous PHT for 5 days before and after combined treatment with VGB. After VGB (2-3.5 g day(-1) for at least 5 weeks), serum PHT concentrations decreased slightly from 87 +/- 25 to 76 +/- 31 micromol l(-1) (means +/- s.d., P < 0.05), but in a subgroup of seven patients the decrease was more prominent (from 72 +/- 22 to 49 +/- 17 micromol l(-1), P < 0.005). At baseline (before VGB), serum PHT remained unaffected (85 +/- 30 micromol l(-1)) after switching PHT dosage to the intravenous route, indicating that the oral availability of the drug was virtually complete. During VGB treatment, serum PHT was also unchanged (74 +/- 34 micromol l(-1)) after switching from oral to intravenous therapy, and this was also true for the subgroup of patients showing a prominent interaction (48 +/- 18 micromol l(-1)). The urinary recoveries of PHT and its metabolites pHPPH and mHPPH remained constant throughout the study. It is concluded that the oral availability of PHT is unaffected by VGB and that the VBG-induced decrease in serum PHT is mediated by alternative mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browne T. R., Mattson R. H., Penry J. K., Smith D. B., Treiman D. M., Wilder B. J., Ben-Menachem E., Napoliello M. J., Sherry K. M., Szabo G. K. Vigabatrin for refractory complex partial seizures: multicenter single-blind study with long-term follow-up. Neurology. 1987 Feb;37(2):184–189. doi: 10.1212/wnl.37.2.184. [DOI] [PubMed] [Google Scholar]
- Chow S. A., Charkowski D. M., Fischer L. J. Separation of phenytoin and its metabolites by high-performance liquid chromatography. Life Sci. 1980 Dec 22;27(25-26):2477–2482. doi: 10.1016/0024-3205(80)90525-1. [DOI] [PubMed] [Google Scholar]
- Grant S. M., Heel R. C. Vigabatrin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in epilepsy and disorders of motor control. Drugs. 1991 Jun;41(6):889–926. doi: 10.2165/00003495-199141060-00007. [DOI] [PubMed] [Google Scholar]
- Richens A. Clinical pharmacokinetics of phenytoin. Clin Pharmacokinet. 1979 May-Jun;4(3):153–169. doi: 10.2165/00003088-197904030-00001. [DOI] [PubMed] [Google Scholar]
- Rimmer E. M., Richens A. Interaction between vigabatrin and phenytoin. Br J Clin Pharmacol. 1989;27 (Suppl 1):27S–33S. doi: 10.1111/j.1365-2125.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini M., Gatti G., Manzo L., Olibet G., Coccini T., Perucca E. Vigabatrin does not affect the intestinal absorption of phenytoin in rat duodeno-jejunal loops in situ. Pharmacol Res. 1992 Sep;26(2):201–205. doi: 10.1016/s1043-6618(05)80133-1. [DOI] [PubMed] [Google Scholar]


