Abstract
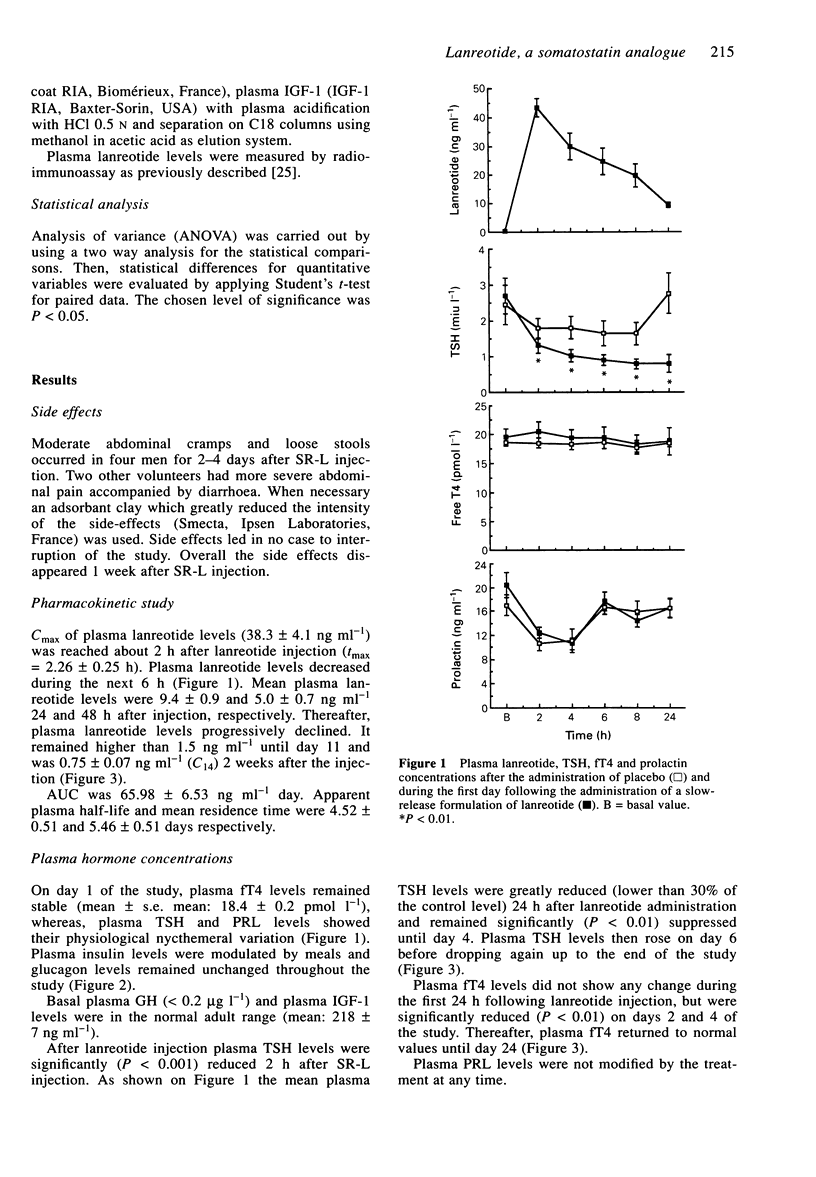
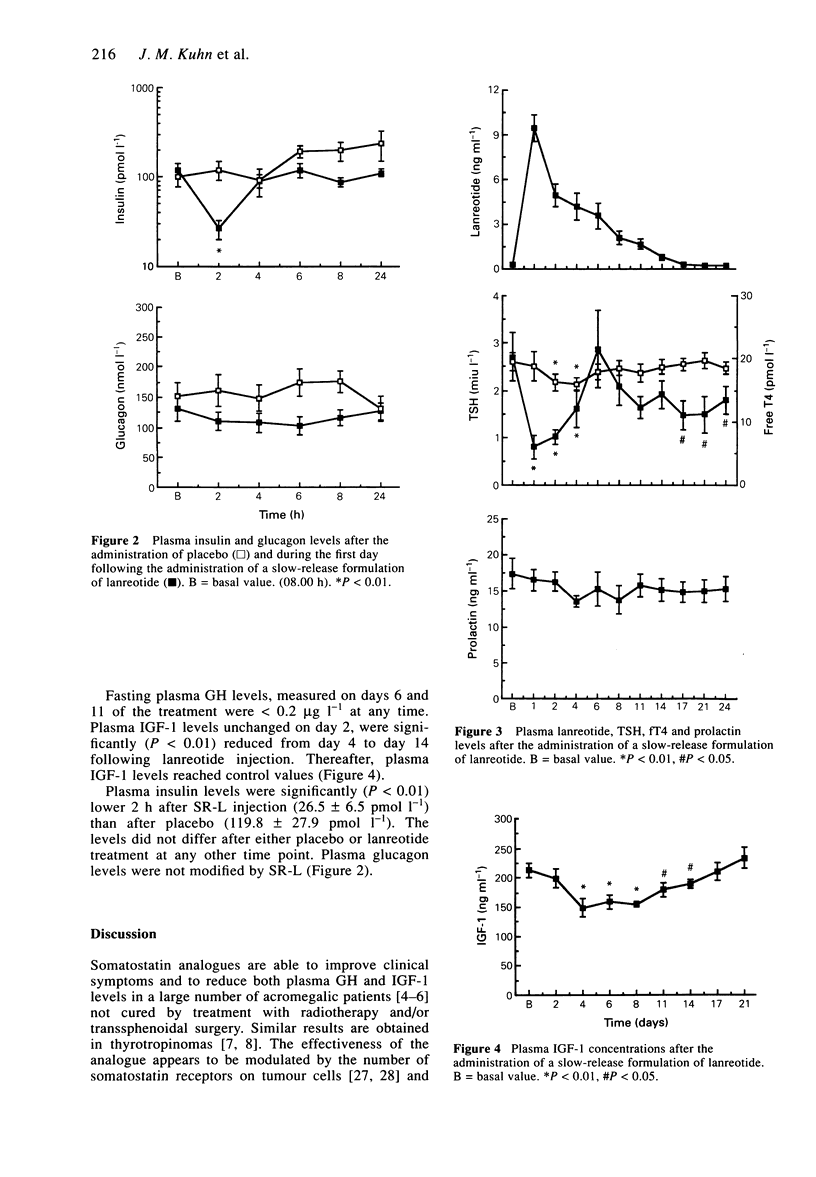
1. The aims of the study were to assess the pharmacokinetic parameters and the hormonal effects of the slow-release formulation of the somatostatin analogue (SR-L) in normal male volunteers. 2. Eight healthy males were studied. For the determination of basal values blood was sampled before the injection of vehicle and then every other hour for 8 h in order to measure plasma GH, prolactin (PRL), TSH, free thyroxin (fT4), insulin and glucagon levels. Plasma insulin-like growth factor 1 (IGF-1) levels were measured on a single sample. On day 1 of the study, 30 mg SR-L was administered intramuscularly. Blood was drawn just before injection and then every other hour for a period of 8 h. Thereafter, blood was sampled three times a week for 3 weeks in order to measure lanreotide, IGF-1, TSH, fT4 and PRL concentrations. Plasma GH was determined on days 6 and 11 of the study. 3. Plasma lanreotide concentrations rose to 38.3 +/- 4.1 ng ml-1 2 h following injection. The levels then progressively decreased, remaining above 1.5 ng ml-1 until day 11 and reaching 0.92 +/- 0.28 ng ml-1 2 weeks after injection. The apparent plasma half-life and mean residence time were 4.52 +/- 0.50 and 5.48 +/- 0.51 days respectively. 4. By comparison with the control day, plasma insulin concentrations only decreased 2 h following injection, whereas plasma glucagon did not change at any time. 5. Plasma TSH concentrations were significantly (P < 0.01) reduced from 2 h to day 4 following SR-L injection.2+ '
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck-Peccoz P., Mariotti S., Guillausseau P. J., Medri G., Piscitelli G., Bertoli A., Barbarino A., Rondena M., Chanson P., Pinchera A. Treatment of hyperthyroidism due to inappropriate secretion of thyrotropin with the somatostatin analog SMS 201-995. J Clin Endocrinol Metab. 1989 Jan;68(1):208–214. doi: 10.1210/jcem-68-1-208. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Reisine T. Molecular biology of somatostatin receptors. Trends Neurosci. 1993 Jan;16(1):34–38. doi: 10.1016/0166-2236(93)90050-v. [DOI] [PubMed] [Google Scholar]
- Bertherat J., Brue T., Enjalbert A., Gunz G., Rasolonjanahary R., Warnet A., Jaquet P., Epelbaum J. Somatostatin receptors on thyrotropin-secreting pituitary adenomas: comparison with the inhibitory effects of octreotide upon in vivo and in vitro hormonal secretions. J Clin Endocrinol Metab. 1992 Aug;75(2):540–546. doi: 10.1210/jcem.75.2.1353505. [DOI] [PubMed] [Google Scholar]
- Bonfils S., Ruszniewski P., Costil V., Laucournet H., Vatier J., Rene E., Mignon M. Prolonged treatment of Zollinger-Ellison syndrome by long-acting somatostatin. Lancet. 1986 Mar 8;1(8480):554–555. doi: 10.1016/s0140-6736(86)90905-0. [DOI] [PubMed] [Google Scholar]
- Bruno J. F., Xu Y., Song J., Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11151–11155. doi: 10.1073/pnas.89.23.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson P., Weintraub B. D., Harris A. G. Octreotide therapy for thyroid-stimulating hormone-secreting pituitary adenomas. A follow-up of 52 patients. Ann Intern Med. 1993 Aug 1;119(3):236–240. doi: 10.7326/0003-4819-119-3-199308010-00010. [DOI] [PubMed] [Google Scholar]
- Christensen S. E., Weeke J., Orskov H., Møller N., Flyvbjerg A., Harris A. G., Lund E., Jørgensen J. Continuous subcutaneous pump infusion of somatostatin analogue SMS 201-995 versus subcutaneous injection schedule in acromegalic patients. Clin Endocrinol (Oxf) 1987 Sep;27(3):297–306. doi: 10.1111/j.1365-2265.1987.tb01156.x. [DOI] [PubMed] [Google Scholar]
- De Leon D. D., Bakker B., Wilson D. M., Hintz R. L., Rosenfeld R. G. Demonstration of insulin-like growth factor (IGF-I and -II) receptors and binding protein in human breast cancer cell lines. Biochem Biophys Res Commun. 1988 Apr 15;152(1):398–405. doi: 10.1016/s0006-291x(88)80727-7. [DOI] [PubMed] [Google Scholar]
- Drieu K., Devissaguet J. P., Duboistesselin R., Dray F., Ezan E. Pharmacokinetics of D-Trp6 LHRH in man: sustained release polymer microsphere study (I.M. route). Prog Clin Biol Res. 1987;243A:435–437. [PubMed] [Google Scholar]
- Ezzat S., Snyder P. J., Young W. F., Boyajy L. D., Newman C., Klibanski A., Molitch M. E., Boyd A. E., Sheeler L., Cook D. M. Octreotide treatment of acromegaly. A randomized, multicenter study. Ann Intern Med. 1992 Nov 1;117(9):711–718. doi: 10.7326/0003-4819-117-9-711. [DOI] [PubMed] [Google Scholar]
- Feldman M., Kiser R. S., Unger R. H., Li C. H. Beta-endorphin and the endocrine pancreas. Studies in healthy and diabetic human beings. N Engl J Med. 1983 Feb 17;308(7):349–353. doi: 10.1056/NEJM198302173080701. [DOI] [PubMed] [Google Scholar]
- Gulya K., Pelton J. T., Hruby V. J., Yamamura H. I. Cyclic somatostatin octapeptide analogues with high affinity and selectivity toward mu opioid receptors. Life Sci. 1986 Jun 16;38(24):2221–2229. doi: 10.1016/0024-3205(86)90574-6. [DOI] [PubMed] [Google Scholar]
- Heiman M. L., Murphy W. A., Coy D. H. Differential binding of somatostatin agonists to somatostatin receptors in brain and adenohypophysis. Neuroendocrinology. 1987 Jun;45(6):429–436. doi: 10.1159/000124788. [DOI] [PubMed] [Google Scholar]
- Heron I., Thomas F., Dero M., Gancel A., Ruiz J. M., Schatz B., Kuhn J. M. Pharmacokinetics and efficacy of a long-acting formulation of the new somatostatin analog BIM 23014 in patients with acromegaly. J Clin Endocrinol Metab. 1993 Mar;76(3):721–727. doi: 10.1210/jcem.76.3.8095269. [DOI] [PubMed] [Google Scholar]
- Heron I., Thomas F., Dero M., Poutrain J. R., Henane S., Catus F., Kuhn J. M. Traitement de l'acromégalie par une forme à libération prolongée du lanréotide. Un nouvel analogue de la somatostatine. Presse Med. 1993 Mar 27;22(11):526–531. [PubMed] [Google Scholar]
- Hindmarsh P. C., Pringle P. J., Di Silvio L., Brook C. G. A preliminary report on the role of somatostatin analogue (SMS 201-995) in the management of children with tall stature. Clin Endocrinol (Oxf) 1990 Jan;32(1):83–91. doi: 10.1111/j.1365-2265.1990.tb03753.x. [DOI] [PubMed] [Google Scholar]
- Itoh S., Tanaka K., Kumagae M., Takeda F., Morio K., Kogure M., Hasegawa M., Horiuchi T., Watabe T., Miyabe S. Effect of subcutaneous injection of a long-acting analogue of somatostatin (SMS 201-995) on plasma thyroid-stimulating hormone in normal human subjects. Life Sci. 1988;42(26):2691–2699. doi: 10.1016/0024-3205(88)90245-7. [DOI] [PubMed] [Google Scholar]
- Jalil R., Nixon J. R. Biodegradable poly(lactic acid) and poly(lactide-co-glycolide) microcapsules: problems associated with preparative techniques and release properties. J Microencapsul. 1990 Jul-Sep;7(3):297–325. doi: 10.3109/02652049009021842. [DOI] [PubMed] [Google Scholar]
- Koper J. W., Hofland L. J., van Koetsveld P. M., den Holder F., Lamberts S. W. Desensitization and resensitization of rat pituitary tumor cells in long-term culture to the effects of the somatostatin analogue SMS 201-995 on cell growth and prolactin secretion. Cancer Res. 1990 Oct 1;50(19):6238–6242. [PubMed] [Google Scholar]
- Kuhn J. M., Basin C., Beaudoin C., Emy P., Mollard M., de Rougé B., Schatz B. Influence de la dose et des modalités d'administration du BIM 23014 sur la sécrétion d'hormone de croissance de l'acromégale. Ann Endocrinol (Paris) 1992;53(5-6):208–214. [PubMed] [Google Scholar]
- Kuhn J. M., Basin C., Mollard M., De Rouge B., Schatz B., Wolf L. M. Effects of the new somatostatin analogue (BIM 23014) on blood glucose homeostasis in normal men. Eur J Clin Invest. 1992 Dec;22(12):793–799. doi: 10.1111/j.1365-2362.1992.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Kuhn J. M., Basin C., Mollard M., de Rougé B., Baudoin C., Obach R., Tolis G. Pharmacokinetic study and effects on growth hormone secretion in healthy volunteers of the new somatostatin analogue BIM 23014. Eur J Clin Pharmacol. 1993;45(1):73–77. doi: 10.1007/BF00315353. [DOI] [PubMed] [Google Scholar]
- Kvols L. K., Moertel C. G., O'Connell M. J., Schutt A. J., Rubin J., Hahn R. G. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986 Sep 11;315(11):663–666. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Uitterlinden P., Verschoor L., van Dongen K. J., del Pozo E. Long-term treatment of acromegaly with the somatostatin analogue SMS 201-995. N Engl J Med. 1985 Dec 19;313(25):1576–1580. doi: 10.1056/NEJM198512193132504. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Zweens M., Klijn J. G., van Vroonhoven C. C., Stefanko S. Z., Del Pozo E. The sensitivity of growth hormone and prolactin secretion to the somatostatin analogue SMS 201-995 in patients with prolactinomas and acromegaly. Clin Endocrinol (Oxf) 1986 Aug;25(2):201–212. doi: 10.1111/j.1365-2265.1986.tb01683.x. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Dickson R. B., Gelmann E. P., Rosen N., Knabbe C., Bates S., Bronzert D., Huff K., Kasid A. Growth regulation of human breast carcinoma occurs through regulated growth factor secretion. J Cell Biochem. 1987 Sep;35(1):1–16. doi: 10.1002/jcb.240350102. [DOI] [PubMed] [Google Scholar]
- O'Carroll A. M., Lolait S. J., König M., Mahan L. C. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Pelton J. T., Kazmierski W., Gulya K., Yamamura H. I., Hruby V. J. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J Med Chem. 1986 Nov;29(11):2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- Prévost G., Foehrlé E., Thomas F., Pihan I., Veber N., Starzec A., Israël L. Growth of human breast cancer cell lines is inhibited by the somatostatin analog BIM23014. Endocrinology. 1991 Jul;129(1):323–329. doi: 10.1210/endo-129-1-323. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Landolt A. M. The growth hormone responses to octreotide in acromegaly correlate with adenoma somatostatin receptor status. J Clin Endocrinol Metab. 1989 Apr;68(4):844–850. doi: 10.1210/jcem-68-4-844. [DOI] [PubMed] [Google Scholar]
- Sassolas G., Khalfallah Y., Chayvialle J. A., Cohen R., Merabet S., Casez J. P., Calvet P., Cabrera P. Effects of the somatostatin analog BIM 23014 on the secretion of growth hormone, thyrotropin, and digestive peptides in normal men. J Clin Endocrinol Metab. 1989 Feb;68(2):239–246. doi: 10.1210/jcem-68-2-239. [DOI] [PubMed] [Google Scholar]
- Setyono-Han B., Henkelman M. S., Foekens J. A., Klijn G. M. Direct inhibitory effects of somatostatin (analogues) on the growth of human breast cancer cells. Cancer Res. 1987 Mar 15;47(6):1566–1570. [PubMed] [Google Scholar]
- Tauber M. T., Tauber J. P., Vigoni F., Harris A. G., Rochicchioli P. Effect of the long-acting somatostatin analogue SMS 201-995 on growth rate and reduction of predicted adult height in ten tall adolescents. Acta Paediatr Scand. 1990 Feb;79(2):176–181. doi: 10.1111/j.1651-2227.1990.tb11435.x. [DOI] [PubMed] [Google Scholar]
- Thermos K., Reisine T. Somatostatin receptor subtypes in the clonal anterior pituitary cell lines AtT-20 and GH3. Mol Pharmacol. 1988 Apr;33(4):370–377. [PubMed] [Google Scholar]
- Timsit J., Chanson P., Larger E., Duet M., Mosse A., Guillausseau P. J., Harris A. G., Moulonguet M., Warnet A., Lubetzki J. The effect of subcutaneous infusion versus subcutaneous injections of a somatostatin analogue (SMS 201-995) on the diurnal GH profile in acromegaly. Acta Endocrinol (Copenh) 1987 Sep;116(1):108–112. doi: 10.1530/acta.0.1160108. [DOI] [PubMed] [Google Scholar]
- Tran V. T., Beal M. F., Martin J. B. Two types of somatostatin receptors differentiated by cyclic somatostatin analogs. Science. 1985 Apr 26;228(4698):492–495. doi: 10.1126/science.2858917. [DOI] [PubMed] [Google Scholar]
- Vance M. L., Harris A. G. Long-term treatment of 189 acromegalic patients with the somatostatin analog octreotide. Results of the International Multicenter Acromegaly Study Group. Arch Intern Med. 1991 Aug;151(8):1573–1578. [PubMed] [Google Scholar]
- Walker J. M., Bowen W. D., Atkins S. T., Hemstreet M. K., Coy D. H. Mu-opiate binding and morphine antagonism by octapeptide analogs of somatostatin. Peptides. 1987 Sep-Oct;8(5):869–875. doi: 10.1016/0196-9781(87)90074-x. [DOI] [PubMed] [Google Scholar]
- Weeke J., Hansen A. P., Lundaek K. Inhibition by somatostatin of basal levels of serum thyrotropin (TSH) in normal men. J Clin Endocrinol Metab. 1975 Jul;41(1):168–171. doi: 10.1210/jcem-41-1-168. [DOI] [PubMed] [Google Scholar]
- Williams T. C., Kelijman M., Crelin W. C., Downs T. R., Frohman L. A. Differential effects of somatostatin (SRIH) and a SRIH analog, SMS 201-995, on the secretion of growth hormone and thyroid-stimulating hormone in man. J Clin Endocrinol Metab. 1988 Jan;66(1):39–45. doi: 10.1210/jcem-66-1-39. [DOI] [PubMed] [Google Scholar]


