Abstract
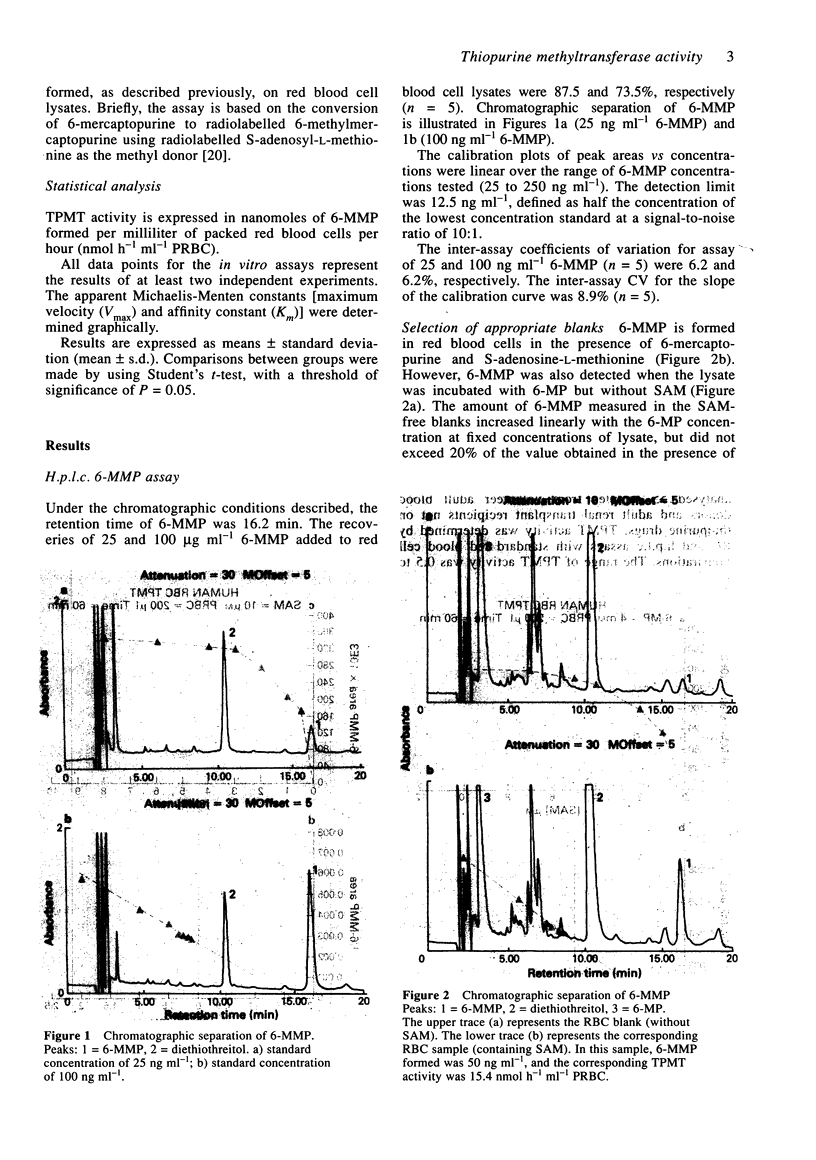
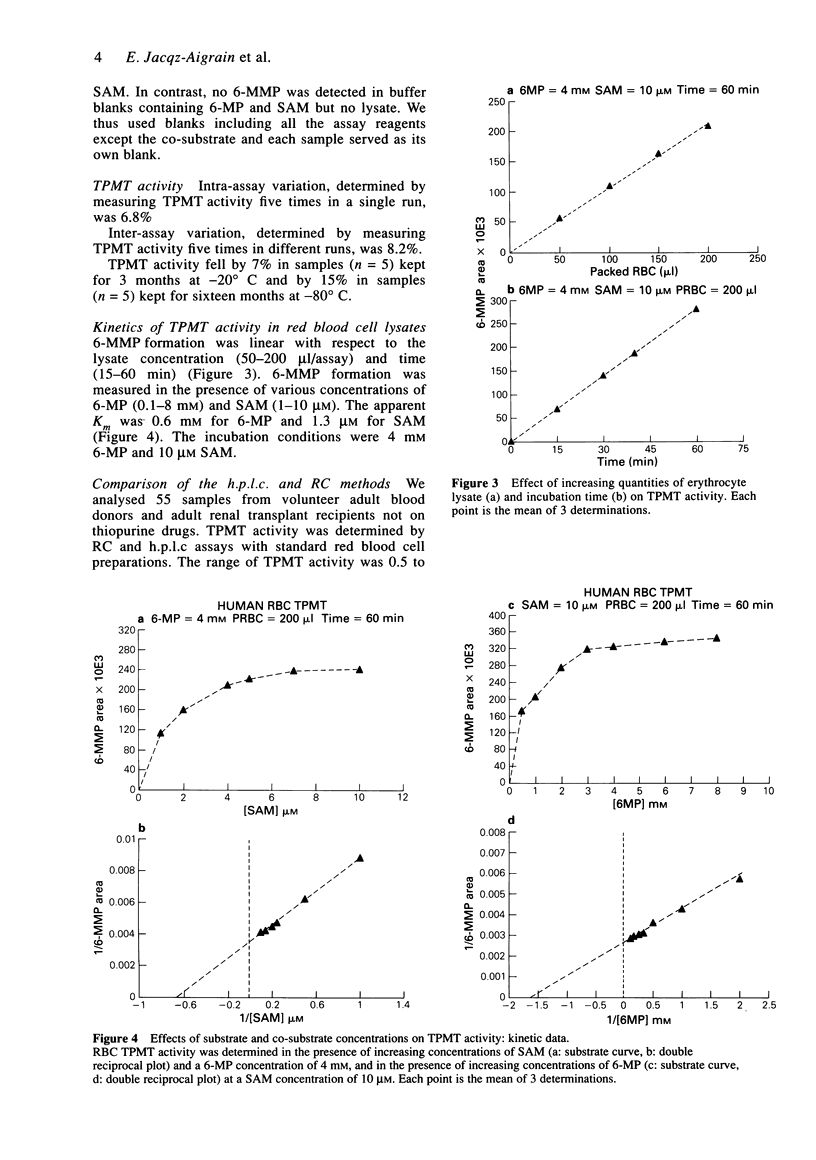
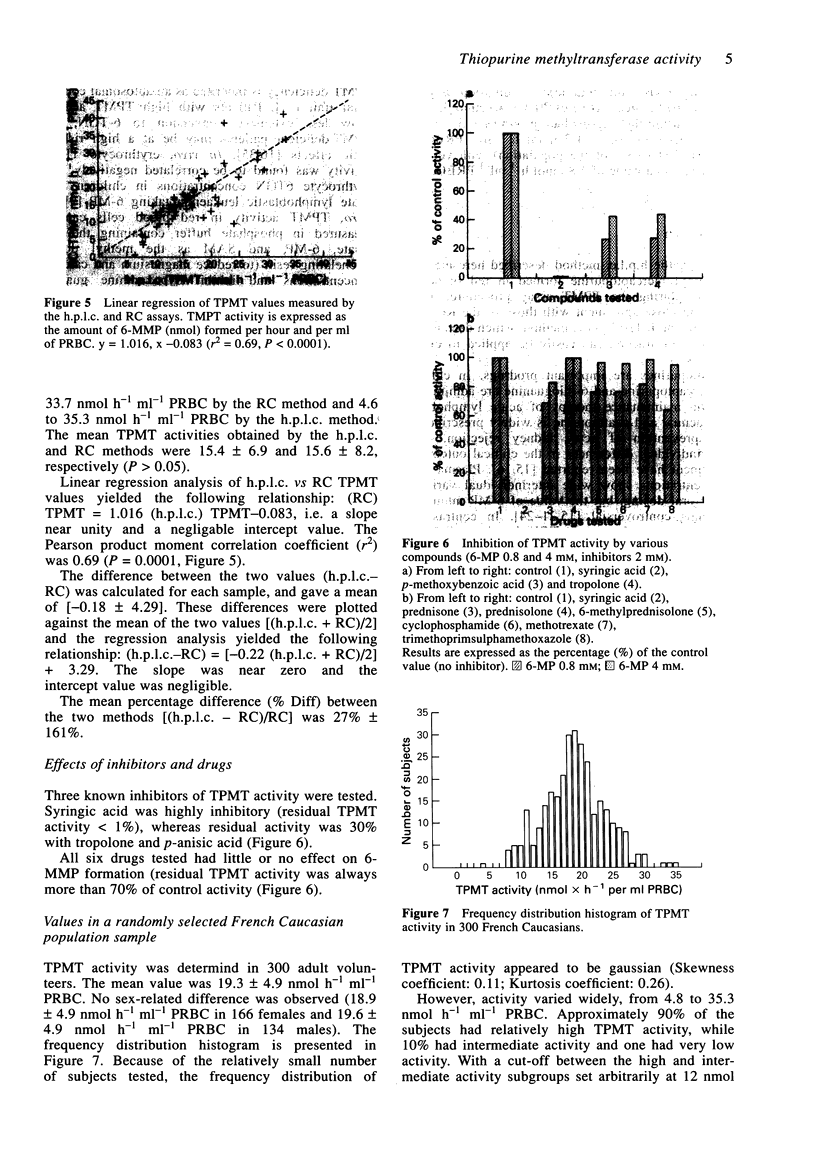
1. Thiopurine methyltransferase (TPMT) is a cytosolic enzyme involved in the catabolism of thiopurine drugs, which are used to treat cancer patients and organ transplant recipients. Because TPMT activity is polymorphic and under genetic control, large interindividual variations in the immunosuppressive activity and toxicity of these drugs may, at least in part, be inherited. 2. We have developed a specific h.p.l.c. method for measuring 6-methyl mercaptopurine formed from 6-mercaptopurine (6-MP) in red blood cell lysates during the TPMT assay procedure. In blinded assays of 55 samples from adult blood donors, the results of the h.p.l.c. method correlated with those of the radiochemical reference method (r = 0.83, P < 0.001). 3. Using this h.p.l.c. assay, we tested the effect of known inhibitors of TPMT activity (syringic acid, p-anisic acid and tropolone) in vitro and showed that they were highly inhibitory. We also found that drugs often administered concomitantly with 6-MP (prednisone, prednisolone, 6-methylprednisolone, cyclophosphamide, methotrexate, and trimethoprim-sulphamethoxazole) had little or no effect on TPMT activity in vitro. 4. In a group of 300 French individuals, TMPT activity was highly variable, ranging from 4.7 to 35.3 nmol h-1 ml-1 of packed red blood cells (nmol h-1 ml-1 PRBC) with a mean value of 19.3 +/- 4.9. TMPT activity was not influenced by sex. 5. This sensitive and reproducible h.p.l.c. assay for TPMT activity in red blood cells may prove useful for prospective clinical studies designed to optimise dosage regimens of thiopurine drugs (detection limit for 6-methyl mercaptopurine is 5 ng ml-1, intra- and inter-assay variations are 6.8 and 8.2%, respectively).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames M. M., Selassie C. D., Woodson L. C., Van Loon J. A., Hansch C., Weinshilboum R. M. Thiopurine methyltransferase: structure-activity relationships for benzoic acid inhibitors and thiophenol substrates. J Med Chem. 1986 Mar;29(3):354–358. doi: 10.1021/jm00153a009. [DOI] [PubMed] [Google Scholar]
- Bland J. M., Altman D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986 Feb 8;1(8476):307–310. [PubMed] [Google Scholar]
- Bostrom B., Erdmann G. Cellular pharmacology of 6-mercaptopurine in acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1993 Feb;15(1):80–86. [PubMed] [Google Scholar]
- Chocair P. R., Duley J. A., Simmonds H. A., Cameron J. S. The importance of thiopurine methyltransferase activity for the use of azathioprine in transplant recipients. Transplantation. 1992 May;53(5):1051–1056. doi: 10.1097/00007890-199205000-00016. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Horner M., Chu Y. Q., Kalwinsky D., Roberts W. M. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr. 1991 Dec;119(6):985–989. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Tang B. K., Campbell M. E., Kalow W. Effect of allopurinol on caffeine disposition in man. Br J Clin Pharmacol. 1986 Apr;21(4):454–458. doi: 10.1111/j.1365-2125.1986.tb05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. M., Tang B. K., Kalow W. Variability in caffeine metabolism. Clin Pharmacol Ther. 1983 May;33(5):591–602. doi: 10.1038/clpt.1983.80. [DOI] [PubMed] [Google Scholar]
- Guerciolini R., Szumlanski C., Weinshilboum R. M. Human liver xanthine oxidase: nature and extent of individual variation. Clin Pharmacol Ther. 1991 Dec;50(6):663–672. doi: 10.1038/clpt.1991.205. [DOI] [PubMed] [Google Scholar]
- Hale J. P., Lilleyman J. S. Importance of 6-mercaptopurine dose in lymphoblastic leukaemia. Arch Dis Child. 1991 Apr;66(4):462–466. doi: 10.1136/adc.66.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayder S., Lafolie P., Björk O., Peterson C. 6-mercaptopurine plasma levels in children with acute lymphoblastic leukemia: relation to relapse risk and myelotoxicity. Ther Drug Monit. 1989 Nov;11(6):617–622. doi: 10.1097/00007691-198911000-00001. [DOI] [PubMed] [Google Scholar]
- Jacqz E., Hall S. D., Branch R. A. Genetically determined polymorphisms in drug oxidation. Hepatology. 1986 Sep-Oct;6(5):1020–1032. doi: 10.1002/hep.1840060534. [DOI] [PubMed] [Google Scholar]
- Jones C. D., Smart C., Titus A., Blyden G., Dorvil M., Nwadike N. Thiopurine methyltransferase activity in a sample population of black subjects in Florida. Clin Pharmacol Ther. 1993 Mar;53(3):348–353. doi: 10.1038/clpt.1993.31. [DOI] [PubMed] [Google Scholar]
- Klemetsdal B., Straume B., Wist E., Aarbakke J. Identification of factors regulating thiopurine methyltransferase activity in a Norwegian population. Eur J Clin Pharmacol. 1993;44(2):147–152. doi: 10.1007/BF00315472. [DOI] [PubMed] [Google Scholar]
- Klemetsdal B., Tollefsen E., Loennechen T., Johnsen K., Utsi E., Gisholt K., Wist E., Aarbakke J. Interethnic difference in thiopurine methyltransferase activity. Clin Pharmacol Ther. 1992 Jan;51(1):24–31. doi: 10.1038/clpt.1992.4. [DOI] [PubMed] [Google Scholar]
- Koren G., Ferrazini G., Sulh H., Langevin A. M., Kapelushnik J., Klein J., Giesbrecht E., Soldin S., Greenberg M. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990 Jul 5;323(1):17–21. doi: 10.1056/NEJM199007053230104. [DOI] [PubMed] [Google Scholar]
- Lee E. J., Kalow W. Thiopurine S-methyltransferase activity in a Chinese population. Clin Pharmacol Ther. 1993 Jul;54(1):28–33. doi: 10.1038/clpt.1993.105. [DOI] [PubMed] [Google Scholar]
- Lennard L. Assay of 6-thioinosinic acid and 6-thioguanine nucleotides, active metabolites of 6-mercaptopurine, in human red blood cells. J Chromatogr. 1987 Dec 25;423:169–178. doi: 10.1016/0378-4347(87)80340-7. [DOI] [PubMed] [Google Scholar]
- Lennard L., Hale J. P., Lilleyman J. S. Red blood cell hypoxanthine phosphoribosyltransferase activity measured using 6-mercaptopurine as a substrate: a population study in children with acute lymphoblastic leukaemia. Br J Clin Pharmacol. 1993 Oct;36(4):277–284. doi: 10.1111/j.1365-2125.1993.tb00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S. Are children with lymphoblastic leukaemia given enough 6-mercaptopurine? Lancet. 1987 Oct 3;2(8562):785–787. doi: 10.1016/s0140-6736(87)92511-6. [DOI] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S., Van Loon J., Weinshilboum R. M. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990 Jul 28;336(8709):225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989 Dec;7(12):1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [DOI] [PubMed] [Google Scholar]
- Lennard L., Maddocks J. L. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercaptopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol. 1983 Jan;35(1):15–18. doi: 10.1111/j.2042-7158.1983.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43(4):329–339. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Lilleyman J. S., Weinshilboum R. M. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther. 1987 Jan;41(1):18–25. doi: 10.1038/clpt.1987.4. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Weinshilboum R. M. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989 Aug;46(2):149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- Lilleyman J. S., Lennard L., Rees C. A., Morgan G., Maddocks J. L. Childhood lymphoblastic leukaemia: sex difference in 6-mercaptopurine utilization. Br J Cancer. 1984 Jun;49(6):703–707. doi: 10.1038/bjc.1984.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod H. L., Miller D. R., Evans W. E. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993 May 1;341(8853):1151–1151. doi: 10.1016/0140-6736(93)93168-z. [DOI] [PubMed] [Google Scholar]
- Relling M. V., Lin J. S., Ayers G. D., Evans W. E. Racial and gender differences in N-acetyltransferase, xanthine oxidase, and CYP1A2 activities. Clin Pharmacol Ther. 1992 Dec;52(6):643–658. doi: 10.1038/clpt.1992.203. [DOI] [PubMed] [Google Scholar]
- Remy C. N. Ribonucleotides and ribonucleosides as methyl acceptors for S-adenosylmethionine: (amino- and thio-)purine methyl-transferases. Incorporation of 6-amino-2-methylaminopurine into ribonucleic acids. Biochim Biophys Acta. 1967 Apr 18;138(2):258–275. [PubMed] [Google Scholar]
- Rundles R. W., Elion G. B. Mercaptopurine "bioavailability". N Engl J Med. 1984 Apr 5;310(14):929–929. doi: 10.1056/NEJM198404053101421. [DOI] [PubMed] [Google Scholar]
- Sather H., Miller D., Nesbit M., Heyn R., Hammond D. Differences in prognosis for boys and girls with acute lymphoblastic leukaemia. Lancet. 1981 Apr 4;1(8223):739–743. doi: 10.1016/s0140-6736(81)92623-4. [DOI] [PubMed] [Google Scholar]
- Schütz E., Gummert J., Mohr F., Oellerich M. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993 Feb 13;341(8842):436–436. doi: 10.1016/0140-6736(93)93028-y. [DOI] [PubMed] [Google Scholar]
- Soria-Royer C., Legendre C., Mircheva J., Premel S., Beaune P., Kreis H. Thiopurine-methyl-transferase activity to assess azathioprine myelotoxicity in renal transplant recipients. Lancet. 1993 Jun 19;341(8860):1593–1594. doi: 10.1016/0140-6736(93)90729-z. [DOI] [PubMed] [Google Scholar]
- Szumlanski C. L., Honchel R., Scott M. C., Weinshilboum R. M. Human liver thiopurine methyltransferase pharmacogenetics: biochemical properties, liver-erythrocyte correlation and presence of isozymes. Pharmacogenetics. 1992 Aug;2(4):148–159. [PubMed] [Google Scholar]
- Tidd D. M., Paterson A. R. A biochemical mechanism for the delayed cytotoxic reaction of 6-mercaptopurine. Cancer Res. 1974 Apr;34(4):738–746. [PubMed] [Google Scholar]
- Tinel M., Berson A., Pessayre D., Letteron P., Cattoni M. P., Horsmans Y., Larrey D. Pharmacogenetics of human erythrocyte thiopurine methyltransferase activity in a French population. Br J Clin Pharmacol. 1991 Dec;32(6):729–734. [PMC free article] [PubMed] [Google Scholar]
- Van Loon J. A., Weinshilboum R. M. Human lymphocyte thiopurine methyltransferase pharmacogenetics: effect of phenotype on 6-mercaptopurine-induced inhibition of mitogen stimulation. J Pharmacol Exp Ther. 1987 Jul;242(1):21–26. [PubMed] [Google Scholar]
- Van Loon J. A., Weinshilboum R. M. Thiopurine methyltransferase biochemical genetics: human lymphocyte activity. Biochem Genet. 1982 Aug;20(7-8):637–658. doi: 10.1007/BF00483962. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Raymond F. A., Pazmiño P. A. Human erythrocyte thiopurine methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1978 May 2;85(3):323–333. doi: 10.1016/0009-8981(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S. L. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980 Sep;32(5):651–662. [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum R. Pharmacogenetics of methyl conjugation and thiopurine drug toxicity. Bioessays. 1987 Aug;7(2):78–82. doi: 10.1002/bies.950070207. [DOI] [PubMed] [Google Scholar]
- Woodson L. C., Ames M. M., Selassie C. D., Hansch C., Weinshilboum R. M. Thiopurine methyltransferase. Aromatic thiol substrates and inhibition by benzoic acid derivatives. Mol Pharmacol. 1983 Nov;24(3):471–478. [PubMed] [Google Scholar]
- Woodson L. C., Dunnette J. H., Weinshilboum R. M. Pharmacogenetics of human thiopurine methyltransferase: kidney-erythrocyte correlation and immunotitration studies. J Pharmacol Exp Ther. 1982 Jul;222(1):174–181. [PubMed] [Google Scholar]
- Woodson L. C., Weinshilboum R. M. Human kidney thiopurine methyltransferase. Purification and biochemical properties. Biochem Pharmacol. 1983 Mar 1;32(5):819–826. doi: 10.1016/0006-2952(83)90582-8. [DOI] [PubMed] [Google Scholar]
- Zimm S., Collins J. M., Riccardi R., O'Neill D., Narang P. K., Chabner B., Poplack D. G. Variable bioavailability of oral mercaptopurine. Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med. 1983 Apr 28;308(17):1005–1009. doi: 10.1056/NEJM198304283081705. [DOI] [PubMed] [Google Scholar]


