Abstract
1. The saphenous vein (SV) and internal thoracic artery (ITA) are the most commonly used conduits for coronary artery bypass surgery (CABS). The ITA shows better long term patency than the SV, at least in part due to their different responses to agonists, as well as physical differences between the ITA and SV at the time of grafting. 2. Angiotensin II (A II), a potent endogenous vasoconstrictor circulates at augmented levels during and after CABS, but little is known about the effects of A II on the SV and ITA. 3. We studied the contractile effects of A II on SV and ITA as intact rings from a heterogeneous group of patients undergoing CABS. Two groups of SV samples were studied; freshly excised SV (FSV) with no further manipulation and SV that had been surgically prepared for use as a bypass conduit (PSV). We also assessed the function of the endothelium in FSV, PSV and ITA, by measuring the relaxation of preconstricted rings to bradykinin. In some tissues endothelial presence was examined histologically. 4. Surgical preparation of SV affected the contractile ability of the smooth muscle, as PSV contracted less than FSV to potassium chloride (KCl, 90 mM) (P < 0.0001). Loss of endothelial function was seen in 25% of FSV, 50% of PSV and 33% of ITA. 5. A II caused concentration dependent contractions in all rings, over the same concentration range (1 nM-100 nM). In rings of FSV the presence of functional endothelium attenuated the response, median values with endothelium being less than half that without endothelium (P < 0.0007, at 100 nM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


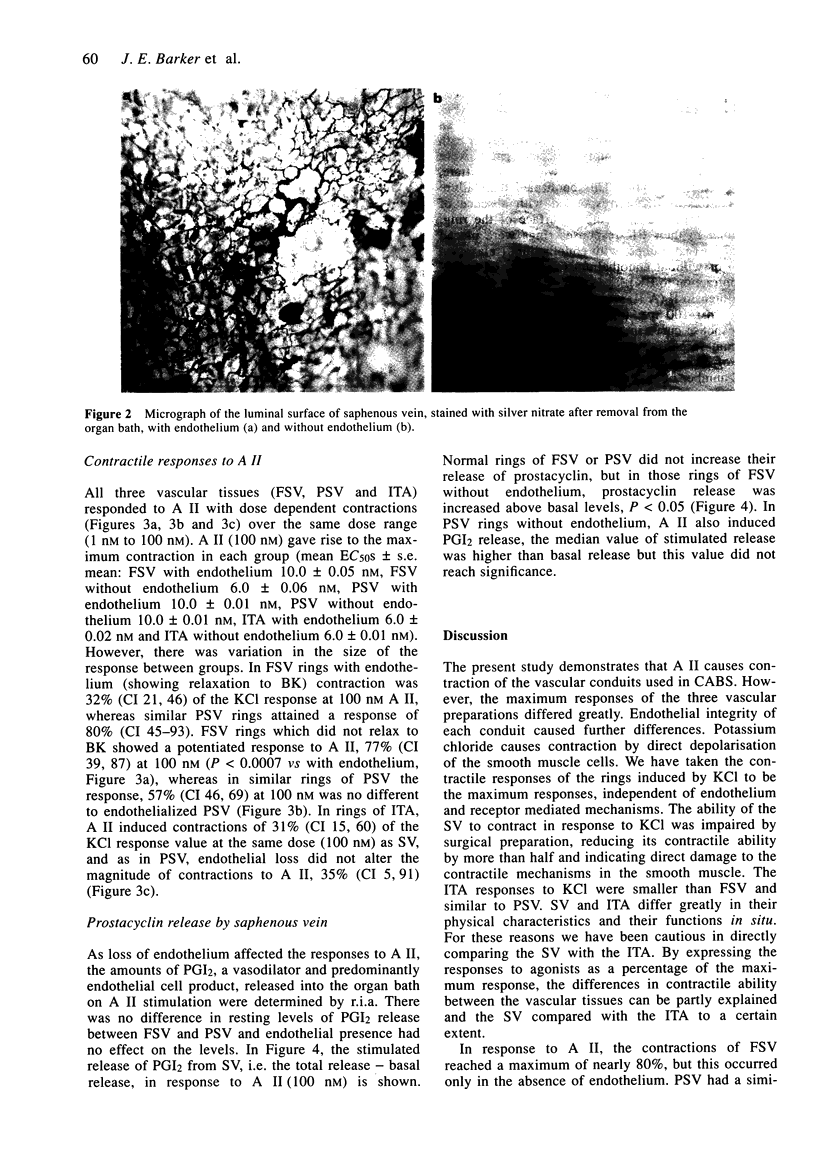
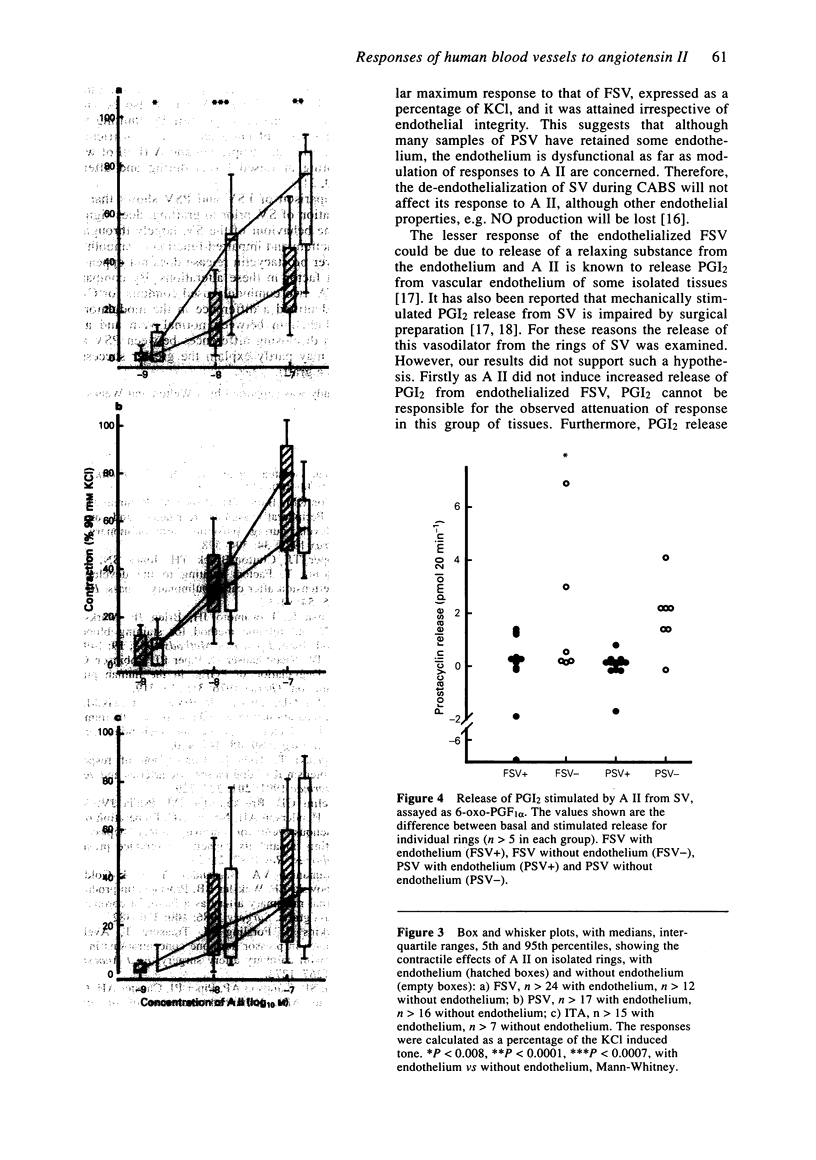

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. P., Chester A. H., Piper P. J., Sampson A. P., Akl E. S., Yacoub M. H. Effects of leukotrienes C4 and D4 on human isolated saphenous veins. Br J Clin Pharmacol. 1992 Nov;34(5):409–414. [PMC free article] [PubMed] [Google Scholar]
- Angelini G. D., Breckenridge I. M., Butchart E. G., Armistead S. H., Middleton K. M., Henderson A. H., Newby A. C. Metabolic damage to human saphenous vein during preparation for coronary artery bypass grafting. Cardiovasc Res. 1985 Jun;19(6):326–334. doi: 10.1093/cvr/19.6.326. [DOI] [PubMed] [Google Scholar]
- Angelini G. D., Breckenridge I. M., Psaila J. V., Williams H. M., Henderson A. H., Newby A. C. Preparation of human saphenous vein for coronary artery bypass grafting impairs its capacity to produce prostacyclin. Cardiovasc Res. 1987 Jan;21(1):28–33. doi: 10.1093/cvr/21.1.28. [DOI] [PubMed] [Google Scholar]
- Angelini G. D., Christie M. I., Bryan A. J., Lewis M. J. Surgical preparation impairs release of endothelium-derived relaxing factor from human saphenous vein. Ann Thorac Surg. 1989 Sep;48(3):417–420. doi: 10.1016/s0003-4975(10)62869-x. [DOI] [PubMed] [Google Scholar]
- Berti F., Rossoni G., Biasi G., Buschi A., Mandelli V., Tondo C. Defibrotide, by enhancing prostacyclin generation, prevents endothelin-1 induced contraction in human saphenous veins. Prostaglandins. 1990 Oct;40(4):337–350. doi: 10.1016/0090-6980(90)90099-h. [DOI] [PubMed] [Google Scholar]
- Cooper T. J., Clutton-Brock T. H., Jones S. N., Tinker J., Treasure T. Factors relating to the development of hypertension after cardiopulmonary bypass. Br Heart J. 1985 Jul;54(1):91–95. doi: 10.1136/hrt.54.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello K. B., Stewart D. J., Baffour R. Endothelin is a potent constrictor of human vessels used in coronary revascularization surgery. Eur J Pharmacol. 1990 Sep 21;186(2-3):311–314. doi: 10.1016/0014-2999(90)90450-k. [DOI] [PubMed] [Google Scholar]
- Dignan R. J., Yeh T., Jr, Dyke C. M., Lee K. F., Lutz H. A., 3rd, Ding M., Wechsler A. S. Reactivity of gastroepiploic and internal mammary arteries. Relevance to coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1992 Jan;103(1):116–123. [PubMed] [Google Scholar]
- Hawkins S., Forsling M., Treasure T., Aveling W. Changes in pressor hormone concentrations in association with coronary artery surgery. Renin and vasopressin responses. Br J Anaesth. 1986 Nov;58(11):1267–1272. doi: 10.1093/bja/58.11.1267. [DOI] [PubMed] [Google Scholar]
- Jose P., Niederhauser U., Piper P. J., Robinson C., Smith A. P. Degradation of porstaglandin F2alpha in the human pulmonary circulation. Thorax. 1976 Dec;31(6):713–719. doi: 10.1136/thx.31.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K. H., von Segesser L., Müller-Glauser W., Siebenmann R., Schneider K., Lüscher T. F., Turina M. Internal-mammary coronary artery grafts: is their superiority also due to a basically intact endothelium? Thorac Cardiovasc Surg. 1989 Jun;37(3):187–189. doi: 10.1055/s-2007-1020315. [DOI] [PubMed] [Google Scholar]
- Loop F. D., Lytle B. W., Cosgrove D. M., Stewart R. W., Goormastic M., Williams G. W., Golding L. A., Gill C. C., Taylor P. C., Sheldon W. C. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986 Jan 2;314(1):1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Diederich D., Siebenmann R., Lehmann K., Stulz P., von Segesser L., Yang Z. H., Turina M., Grädel E., Weber E. Difference between endothelium-dependent relaxation in arterial and in venous coronary bypass grafts. N Engl J Med. 1988 Aug 25;319(8):462–467. doi: 10.1056/NEJM198808253190802. [DOI] [PubMed] [Google Scholar]
- Nakatsu K., Kawamoto J. H., Brien J. F., Marks G. S. A facile, reliable method for staining blood vessel endothelium. J Pharmacol Methods. 1988 Apr;19(2):149–154. doi: 10.1016/0160-5402(88)90035-6. [DOI] [PubMed] [Google Scholar]
- Subramanian V. A., Hernandez Y., Tack-Goldman K., Grabowski E. F., Weksler B. B. Prostacyclin production by internal mammary artery as a factor in coronary artery bypass grafts. Surgery. 1986 Aug;100(2):376–383. [PubMed] [Google Scholar]
- Tadjkarimi S., O'Neil G. S., Luu T. N., Allen S. P., Schyns C. J., Chester A. H., Yacoub M. H. Comparison of cyclic GMP in human internal mammary artery and saphenous vein: implications for coronary artery bypass graft patency. Cardiovasc Res. 1992 Mar;26(3):297–300. doi: 10.1093/cvr/26.3.297. [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Bain W. H., Russell M., Brannan J. J., Morton I. J. Peripheral vascular resistance and angiotensin II levels during pulsatile and non-pulsatile cardiopulmonary bypass. Thorax. 1979 Oct;34(5):594–598. doi: 10.1136/thx.34.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M., Toda N. Comparison of responses to angiotensin II of dog mesenteric arteries and veins. Eur J Pharmacol. 1991 Aug 29;201(2-3):223–229. doi: 10.1016/0014-2999(91)90349-u. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., Diederich D., Schneider K., Siebenmann R., Stulz P., von Segesser L., Turina M., Bühler F. R., Lüscher T. F. Endothelium-derived relaxing factor and protection against contractions induced by histamine and serotonin in the human internal mammary artery and in the saphenous vein. Circulation. 1989 Oct;80(4):1041–1048. doi: 10.1161/01.cir.80.4.1041. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., von Segesser L., Bauer E., Stulz P., Turina M., Lüscher T. F. Different activation of the endothelial L-arginine and cyclooxygenase pathway in the human internal mammary artery and saphenous vein. Circ Res. 1991 Jan;68(1):52–60. doi: 10.1161/01.res.68.1.52. [DOI] [PubMed] [Google Scholar]



