Abstract
In our studies on the biological function of the mengovirus leader protein, we identified a casein kinase II (CK-2) phosphorylation site in the protein. Here we report that the mengovirus leader protein can be phosphorylated by CK-2 in vitro. Expression of a recombinant leader protein in which the consensus CK-2 sequence around threonine 47 was disturbed resulted in a mutant protein that could no longer be phosphorylated. The CK-2 consensus sequence was modified by site-directed mutagenesis and subsequently introduced into a mengovirus cDNA clone to investigate the effect of the phosphorylation of the leader protein on virus replication and on the host cell response. Modifications by which the CK-2 consensus sequence was disturbed resulted in mutant viruses with reduced growth kinetics. We demonstrated that the integrity of the CK-2 phosphorylation site of the mengovirus leader protein was specifically related to the suppression of NF-κB activation and subsequent suppression of alpha/beta interferon production in infected cells. We also found that the integrity of the CK-2 phosphorylation site of the leader protein coincided with an increase of ferritin expression in the infected cell. These data indicate that the leader protein suppresses the iron-mediated activation of NF-κB and thereby inhibits alpha/beta interferon expression in the infected cell.
The genus Cardiovirus belongs to the picornavirus family and includes the members mengovirus, encephalomyocarditis virus (EMCV), and Theiler's murine encephalomyelitis virus (TMEV). Its genome consists of a 7.8-kb single-stranded RNA molecule of positive polarity and contains a large 5′ untranslated region. The 5′ untranslated region contains an internal ribosome entry site from which viral translation is initiated in a cap-independent manner. The genome is functionally equivalent to cellular mRNA and encodes a single polyprotein that is processed by virus-encoded proteases (20, 24). The polyprotein is subdivided into three regions, P1, P2, and P3. Proteins encoded by the P2 and P3 regions are involved mainly in the replication of the viral RNA genome, whereas the P1 region encodes the capsid proteins. In contrast with most other picornaviruses, the cardiovirus polyprotein also contains an N-terminal leader peptide in which a zinc-binding domain and a C-terminal acidic region are found (6, 11). The function of the cardiovirus leader protein is still obscure.
Previous research indicates that the cardiovirus leader protein may interfere with the antiviral host cell response. We showed that a mengovirus mutant in which the leader protein-encoding region was largely deleted was no longer able to replicate in mouse fibroblasts. This leader deletion mutant could be rescued by 2-aminopurine, an inhibitor of the alpha/beta interferon-related pathways (33). Recently, Van Pesch et al. (27) found that the leader protein of TMEV is specifically involved in the repression of the antiviral immediate-early host cell response. Especially the beta interferon production in TMEV-infected cells was abolished due to leader peptide activity (27). The alpha/beta interferons are important factors that contribute to the immediate-early responses against virus infections. For these innate immune responses, the transcriptional activator nuclear factor κB (NF-κB) plays a crucial role. In most cell types, NF-κB is held in the cytoplasm by the association with its inhibitor protein, IκB. Upon exposure of cells to a wide variety of stimuli, including viruses, the IκB protein is phosphorylated. This phosphorylation event triggers IκB for degradation by the ubiquitin-related proteasome pathway. Degradation of IκB leads to the exposure of a nuclear localization signal on NF-κB that results in the translocation of this transcription factor to the nucleus. In combination with other transcription factors such as interferon regulatory factors (IRFs) and CBP/p300, NF-κB is involved in the transcription of immunoregulatory factors such as alpha/beta interferon and interleukin-6 (28).
Reports on the activation of NF-κB by cytokines and other extracellular stimuli show that disturbance of the redox state of the cell due to production of reactive oxygen species (ROS) is involved in this activation process (26). One of the mediators in the regulation of the cellular redox state is intracellular iron and the iron-binding protein ferritin (14, 15). It was shown that the cytokine tumor necrosis factor alpha induces the synthesis of ferritin mediated by the transcription factor NF-κB (14). It was reported by Mulvey et al. (18) that cardiovirus infections result in an increase of ferritin expression. Enhanced ferritin synthesis in the infected cell may correlate with the suppression of the antiviral host cell response.
Recently, Dvorak et al. showed that a tyrosine residue of the EMCV leader protein is phosphorylated during infection by an unknown kinase (11). The function of this phosphorylation event is still unknown. In this study, we investigated the possible phosphorylation of the mengovirus leader protein in connection with the immediate-early antiviral host cell response. Here, we identified a functional casein kinase II (CK-2) phosphorylation site in the mengovirus leader protein. Further experiments were performed to gain insight into the biological significance of mengovirus leader protein phosphorylation in the virus-mediated suppression of the immediate-early host cell response. We found that the integrity of the CK-2 phosphorylation site in the mengovirus leader is specifically correlated with the increase of ferritin expression and the suppression of NF-κB activation and subsequent inhibition of alpha/beta interferon synthesis.
MATERIALS AND METHODS
Cells and viruses.
Virus propagation and viral RNA transfections were performed with mouse fibroblasts (L929 cells) and HeLa cells. The cells were grown in minimal essential medium (MEM) supplemented with 10% fetal bovine serum. After infection, cells were fed with MEM containing 3% serum, and after transfection, MEM containing 10% serum was added. Serial 10-fold dilutions were tested with eight replicates in 96-well plates containing baby hamster kidney cell (BHK-21) monolayers (25). Fifty percent tissue culture infective doses (TCID50) were calculated as described by to Reed and Muench (23). Wild-type mengovirus vM16.1 was obtained upon transfection of in vitro-transcribed RNA from the mengovirus cDNA pM16.1 (10). pM16.1 was a generous gift from A. Palmenberg. The recombinant mengovirus vM16.1ΔL(12-52), in which the major part of leader protein-encoding region is deleted, and the recombinant mengovirus vM16.1Δ2A, in which the 2A-encoding region is deleted, were described previously (33, 34).
Construction of mengovirus mutants.
Site-directed mutagenesis was performed as described previously (33). Mutants in which the potential phosphorylation sites were disturbed were constructed by the replacement of single amino acid codons within the leader-encoding region. The following oligonucleotides were used: for replacement of threonine at position 3 by alanine, 5′-CTC TTG TTC CAT GGT GGC AGC CAT ATT ATT GTC-3′; for replacement of threonine at position 15 by alanine, 5′-GCA TTT TGG GCA TTC TTC GAA TGC CAT GGA ATG AGC ACA-3′; for replacement of threonine at position 47 by alanine, 5′-ATC ATC TTC ACC ATC TGC CAA CGA CTC CTC AGG-3′; for replacement of glutamic acid at position 50 by alanine, 5′-ATC GAA CAC ATC ATC GGC GCC ATC AGT CAA CGA CTC-3′; and for insertion of a glycine at position 49, 5′-GGA CAC ATC ATC TTC ACC ACC ATC AGT TAA CGA CTC CTC AGG GTA-3′. The mutated fragments were cloned into pM16.1, and the nucleotide sequences of the mutant cDNAs were verified as described previously (33).
Expression of recombinant proteins.
The prokaryotic expression plasmids pGEX4T-ML (mengovirus leader protein), pGEX4T-ML(T3A), pGEX4T-ML(T15A), and pGEX4T-ML(T47A) were constructed by PCR amplification and cloning of the mengovirus leader protein-encoding region into the prokaryotic expression vector pGEX-4T1 (Amersham Pharmacia Biotech). The mengovirus cDNA pM16.1 and the mengovirus mutant cDNAs were used as templates for PCR. The oligonucleotide 5′-TGG TTT TCC GGA TCC ATG GCT ACA ACC ATG-3′ was used as the forward primer and contained a BamHI restriction site (underlined). The oligonucleotide 5′-TGG TTT TCC GGA TCC ATG GCT GCC ACC ATG-3′ was used as the forward primer for the amplification of the T3A leader mutant. The oligonucleotide 5′-GGA TGA GGT GAA TTC TTA TTG TGT CTC GAA CAC-3′ was used as the reverse primer and contained an EcoRI restriction site. Amplification was performed with ULTma DNA polymerase according the instructions of the manufacturer (Perkin-Elmer Cetus). The PCR product was digested with BamHI and EcoRI and ligated into pGEX-4T1. The inserted fragment was confirmed by sequence analysis. Recombinant proteins were expressed from the pGEX-4T-ML constructs as N-terminal fusion proteins with glutathione S-transferase. The fusion proteins were expressed in Escherichia coli BL21 (Novagen) and purified with the affinity matrix glutathione-Sepharose (Amersham Pharmacia Biotech). The mengovirus leader proteins were released from the bound glutathione S-transferase by digestion with biotinylated thrombin (Novagen). The resulting proteins were purified from the biotinylated thrombin with streptavidin-agarose (Novagen). Purified recombinant wild-type leader protein was used to immunize a rabbit to raise leader protein-specific antiserum.
In vitro phosphorylation assay.
In vitro phosphorylation of recombinant mengovirus leader proteins was tested with either CK-2 (Promega) or rabbit reticulocyte lysate (Promega). The CK-2 assay was performed in a reaction mixture of 50 μl containing 0.2 μg of leader protein, 5 U of CK-2 in 25 mM Tris-HCl (pH 7.4), 200 mM NaCl, 10 mM MgCl2, 0.1 mM ATP, and 5 μCi of [γ-32P]ATP (Amersham Pharmacia Biotech). Phosphorylation in a reticulocyte lysate was performed in a reaction mixture of 50 μl containing 0.2 μg of leader protein, 35 μl of rabbit reticulocyte lysate, 0.1 mM ATP, and 5 μCi of [γ-32P]ATP. Reactions were performed at 37°C for 1 to 5 min, after which 10-μl samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
In vivo phosphorylation assay.
In vivo phosphorylation of the mengovirus leader protein during infection was tested in L929 cells labeled with inorganic [32P]phosphate. L929 cells were grown for 3 h in phosphate-free MEM (Life Technologies) supplemented with 10% phosphate-free fetal bovine serum and 1 mCi of [32P]orthophosphate (Amersham Biosciences) per ml. Monolayers of L929 cells were inoculated with virus at a multiplicity of infection (MOI) of 50 TCID50 per cell. Virus suspensions were replaced by fresh medium after 30 min of adsorption at 36°C. Cellular extracts were prepared by lysis with a buffer containing 0.5 M Tris-HCl (pH 8.0), 0.15 M sodium chloride, 1% Nonidet P-40, 0.05% SDS, and 0.1 mM phenylmethylsulfonyl fluoride. Immunoprecipitation of the leader protein was performed with rabbit antiserum raised against the recombinant wild-type mengovirus leader protein and an amount of in vivo 32P-labeled cellular lysate corresponding to 106 cells. The assay was performed by standard procedures as described previously (2).
Transfection of cells with RNA transcripts.
pM16.1 (recombinant) plasmids were linearized by digestion with SalI and transcribed in vitro with T7 RNA polymerase as described previously (33). Cells were transfected in duplicate with 4 μg of RNA by the DEAE-dextran method (33).
Single-cycle growth analysis.
Confluent L929 cell monolayers were infected with virus at an MOI of 1 TCID50 per cell and grown at 36°C for 2, 4, 6, and 8 h (33). Viruses were released by three successive cycles of freezing and thawing and titrated at 36°C.
In vivo labeling of virus-infected cells.
Monolayers of L929 cells were inoculated with virus at an MOI of 50 TCID50 per cell. Virus suspensions were replaced by fresh medium after 30 min of adsorption at 36°C. Monolayers were washed three times with phosphate-buffered saline (pH 7.2) at different times after infection, as indicated, and incubated for 30 min with methionine-free medium containing 10 μCi of l-[35S]methionine (Amersham Pharmacia Biotech) per ml. Cellular extracts were prepared by lysis with a buffer containing 0.5 M Tris-HCl (pH 8.0), 0.15 M sodium chloride, 1% Nonidet P-40, 0.05% SDS, and 0.1 mM phenylmethylsulfonyl fluoride. Protein synthesis was further analyzed by immunoprecipitation, Western blotting, or SDS-PAGE and autoradiography.
Alpha/beta interferon assay.
The production of alpha/beta interferon by infected L929 cells was determined according to the method of Chinsangaram et al. (7) with some modifications. L929 cells were infected with wild-type and mutant mengoviruses for 48 h at an MOI of 0.2 TCID50 per cell. In order to inactivate virus, cell culture supernatants of infected L929 cells were brought to pH 2 by the addition of 2 M HCl and stored at 4°C for 24 h, after which the pH was restored to 7 by the addition of 2 M NaOH. Virus inactivation was checked on BHK-21 cells. Interferon production was checked by treatment of L929 cells with a 1:10 dilution of virus-inactivated cell culture supernatant 24 h prior to infection with serial dilutions of wild-type mengovirus. As a control, L929 cells were treated with various amounts of mouse alpha/beta interferon (Sigma). Beta interferon levels in cell culture supernatants of infected HeLa cells were quantified by using a beta interferon-specific enzyme-linked immunosorbent assay according to the instructions of the manufacturer (Biosource).
iNOS assay.
Inducible nitric oxide synthase (iNOS) activity was determined by measurement of NO production in infected cells by using Griess reagent (31). Briefly, 100 μl of cell culture supernatant was mixed with 100 μl of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine in 5% phosphoric acid). The optical density at 550 nm was measured in a Bio-Rad microplate reader. The production of iNOS in cells was determined by Western blotting with polyclonal rabbit antibodies against iNOS (Santa Cruz Biotechnology).
Ferritin assay.
L929 cells were labeled in vivo with l-[35S]methionine as described above. Immunoprecipitation of ferritin was performed with rabbit anti-human ferritin antibodies (Dako) and an amount of in vivo-labeled cellular lysate corresponding to 106 cells. The assay was performed by standard procedures as described previously (2).
NF-κB activation assay.
Activation of NF-κB was tested by the transfection of L929 cells with the plasmid pNFkB-Luc (Clontech) by using the transfecting reagent Fugene-6 (Roche) according to the manufacturer's instructions. At 48 h after transfection, cells were infected with wild-type and mutant mengoviruses for 6 h at an MOI of 10 TCID50 per cell. Luciferase activity was measured in cellular lysates by using the Luciferase Assay System (Promega) according to the instructions of the manufacturer.
RESULTS
Threonine 47 of the recombinant mengovirus leader protein is phosphorylated by CK-2.
Regulation of various cellular processes is often modulated by phosphorylation of involved protein factors. To study the role of protein phosphorylation in leader protein activity, we screened this protein for potential phosphorylation sites. Only three potential CK-2 sites were found in the leader protein. CK-2 is a ubiquitous, highly conserved serine/threonine protein kinase found in eukaryotic cells. The enzyme recognizes a broad range of substrates with the consensus amino acid sequence (S, T) X X (D, E), in which the serine or threonine residue is the phosphate acceptor (22). The presence of an aspartic acid or glutamic acid residue at position +3, relative to the phosphate acceptor is essential for phosphorylation. The presence of additional acidic amino acid residues at positions −1, +1, +2, +4, and +5 will improve the CK-2-mediated phosphorylation. The three potential CK-2 phosphorylation sites in the mengovirus leader protein that were identified were at a threonine residue at position 3 (T3) (AT*TMEQE), a threonine residue at position 15 (T15) (MT*FEECP), and a threonine residue at position 47 (T47) (LT*DGEDD) (the phosphate acceptor is indicated by an asterisk).
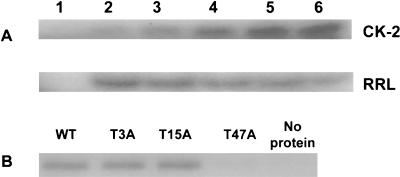
The wild-type leader protein was expressed to investigate whether the protein could be phosphorylated by CK-2. Figure 1A shows that the recombinant mengovirus leader protein was efficiently phosphorylated by CK-2 in vitro. To investigate whether the leader protein could be phosphorylated in a rabbit reticulocyte lysate, the recombinant leader protein was added to a reticulocyte lysate in the presence of 32P-labeled ATP. As shown in Fig. 1A, the wild-type leader protein was rapidly phosphorylated, with a maximum incorporation of radioactive phosphate reached within 1 min.
FIG. 1.
In vitro phosphorylation of the mengovirus leader protein by CK-2. (A) Time course of the phosphorylation of recombinant wild-type leader protein. Reactions were performed as described in Materials and Methods. Reactions were done in the absence of leader protein for 5 min (lane 1) or in the presence of 200 ng of leader protein for 1, 2, 3, 4, and 5 min (lanes 2, 3, 4, 5, and 6, respectively). Reactions were performed with CK-2 (upper panel) or in rabbit reticulocyte lysate (RRL) (lower panel). Reactions were analyzed by SDS-PAGE. (B) In vitro phosphorylation of recombinant leader proteins for 5 min by CK-2. Reactions were performed with 200 ng of recombinant wild-type (WT) leader protein or mutant (T3A, T15A, or T47A) leader protein or in the absence of leader protein. Reactions were analyzed by SDS-PAGE.
In order to identify CK-2 sites in the leader protein, recombinant leader mutant proteins in which the threonine residue at position 3, 15, or 47 was replaced by alanine were expressed. The mutant recombinant leader proteins T3A, T15A, and T47A were subsequently analyzed in the CK-2 phosphorylation assay. Both the T3A and the T15A leader proteins were efficiently phosphorylated by CK-2 (Fig. 1B). However, the T47A leader protein could no longer be phosphorylated by CK-2 (Fig. 1B). These results show that the threonine at position 47 of the mengovirus leader is the substrate for CK-2. The same results were found when the recombinant proteins were analyzed for phosphorylation in a reticulocyte lysate (data not shown). These results strongly argue for the presence of a functional CK-2 phosphorylation site at threonine 47.
Phenotypic characterization of the T47A mengovirus mutant.
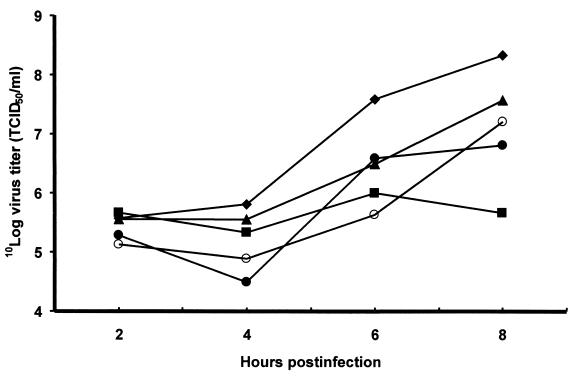
To examine the functional importance of the CK-2 phosphorylation site for virus replication, the T47A mutation was introduced into the mengovirus cDNA clone pM16.1. A cytopathic effect was observed upon transfection of the RNA into L929 cells. The mutated virus vM16(T47A) was further characterized by single-cycle growth analysis (Fig. 2). The mutation T47A in the mengovirus leader protein resulted in a disabled virus with a virus yield of about 10% of that of the wild type (Fig. 2). These results suggest that phosphorylation of the leader protein is important for efficient virus growth.
FIG. 2.
Single-cycle growth curves of wild-type virus vM16 (♦), vM16ΔL(12-64) (▪), vM16T47A (▴), vM16E50A (○), and vM16insG49 (•). L929 cells were infected at an MOI of 1 and harvested at 2, 4, 6, and 8 h postinfection. Virus titers were determined by titration (23).
In order to demonstrate the in vivo phosphorylation of the leader protein, L929 cells were labeled with [32P]orthophosphate for 3 h preinfection. In this 3-h period the major portion of intracellular ATP will be radioactively labeled. Phosphorylation of the leader protein was tested by immunoprecipitation of lysates obtained from [32P]ATP-labeled cells either mock infected or infected with wild-type mengovirus, vM16.1ΔL(12-52), vM16.1Δ2A, or vM16T47A. We were not able to detect the phosphorylated leader protein in samples taken at 3 and 5 h postinfection. This is probably due to the unstable character of the leader protein, as described previously for the EMCV leader protein (11).
To investigate the possibility that threonine 47 is involved in leader protein activity other than as a phosphate acceptor, two additional mutants in which the CK-2 consensus sequence flanking the threonine 47 residue was disturbed were constructed. A mutant was made in which the glutamic acid residue at position 50 was replaced by alanine, creating the sequence LT*DGADD. Another mutant was made by insertion of a glycine at position 49, thereby creating the sequence LT*DGGEDD. Upon transfection, two mutant viruses, vM16E50A and vM16insG49, were obtained. Both of these mutant viruses exhibited growth characteristics similar to those of vM16T47A (Fig. 2). These results support the idea that the disabled phenotype of the vM16T47A mutant is caused by a disturbed CK-2 consensus sequence, rather than by another function of the threonine residue, and that the integrity of the phosphorylation site of the leader protein is important for its function.
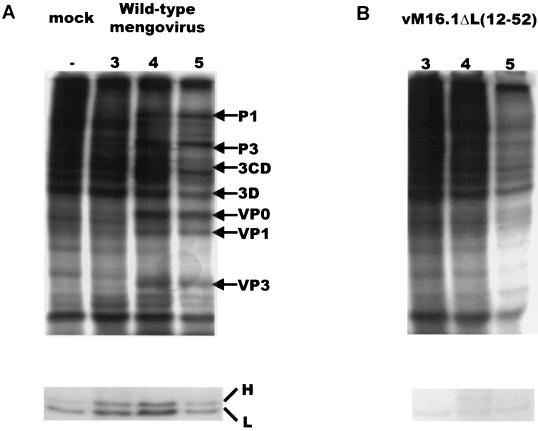
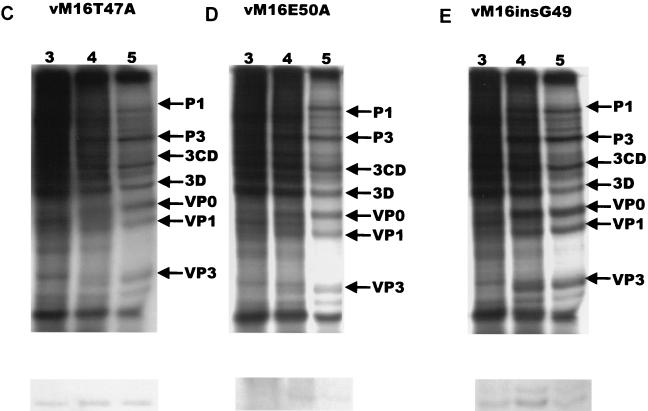
Protein synthesis in infected cells was studied by in vivo labeling experiments. L929 cells were infected with wild-type mengovirus, a mutant [vM16.1ΔL(12-52)] in which the leader protein was deleted (33), and mutant viruses vM16T47A, vM16E50A, and vM16insG49 and were pulse-labeled at 3, 4, and 5 h after infection. At the latest time point, wild-type mengovirus-infected cells produce exclusively viral proteins and host cell protein synthesis is inhibited (Fig. 3A) (33). The recombinant mengovirus vM16.1ΔL(12-52) showed a decreased inhibition of host cell protein synthesis and a decreased production of viral proteins compared to wild-type virus, as was previously shown (33) (Fig. 2 and 3B). However, host cell protein synthesis shutoff and viral translation were not seriously affected by mutations which disturbed the leader protein phosphorylation site (Fig. 3C, D, and E).
FIG. 3.
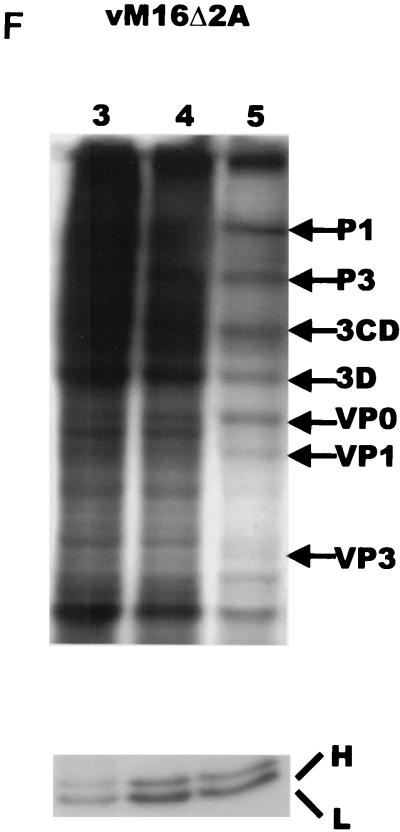
Protein synthesis in infected L929 cells. Cells were mock infected (A) or infected with wild-type mengovirus (A), vM16ΔL(12-52) (B), vM16T47A (C), vM16E50A (D), vM16insG49 (E), or vM16Δ2A (F) as indicated at an MOI of 50 TCID50/cell. At the time points indicated above the lanes (in hours postinfection), cells were pulse-labeled with [35S]methionine for 30 min. Cells were lysed and directly analyzed by SDS-PAGE on 12.5% polyacrylamide gels (upper panels) or used for immunoprecipitation of ferritin (lower panels). Viral proteins and heavy (H) and light (L) ferritin chains are indicated.
The presence of the CK-2 phosphorylation site of the mengovirus leader protein is essential for suppression of the antiviral host cell response.
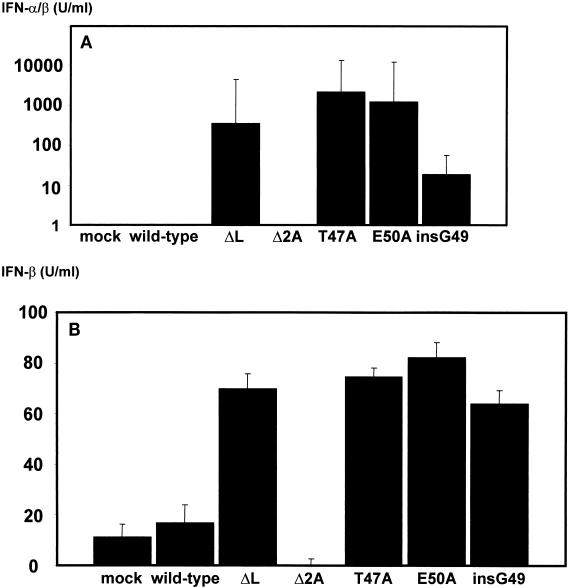
Recently, it was reported that the TMEV leader protein inhibits alpha/beta interferon production (27). The effect of mengovirus leader protein phosphorylation on the immediate-early production of alpha/beta interferon was tested in L929 cells that were either mock infected or infected with wild-type mengovirus, vM16.1ΔL(12-52), or the phosphorylation mutants vM16T47A, vM16E50A, and vM16insG49. As a control, interferon production was also examined in cells infected with the mengovirus mutant vM16.1Δ2A, in which the 2A-encoding region was deleted (34). This mutant virus also exhibits reduced growth in L929 cells, but the leader protein is still fully intact. Supernatants were collected and used to prime uninfected L929 cells 24 h before infection with serial dilutions of wild-type mengovirus. For comparison, a standard curve of serial dilutions of mouse alpha/beta interferon was made. Protection against mengovirus infection was already observed at an interferon concentration of 0.01 U/ml. At higher interferon concentrations, increasing amounts of virus were necessary to cause a cytopathic effect. By using the standard curve, alpha/beta interferon concentrations in cell culture supernatants of infected cells could be estimated. Figure 4A summarizes the results of three independent experiments. Detectable amounts of alpha/beta interferon were not found in mock-infected L929 cells or cells infected with wild-type mengovirus or vM16.1Δ2A. In cells infected with vM16.1ΔL(12-52), vM16T47A, vM16E50A, or vM16insG49, alpha/beta interferon was produced (Fig. 4A). Beta interferon production was measured in mock-infected HeLa cells or cells infected with wild-type mengovirus or mutant viruses. The beta interferon levels produced in HeLa cells were similar to the levels of alpha/beta interferon found in L929 cells (Fig. 4B). From these results we concluded that the mengovirus leader protein is directly involved in the suppression of alpha/beta interferon production and that this activity depends on the integrity of a CK-2 phosphorylation site at threonine 47.
FIG. 4.
(A) Production of alpha/beta interferon in infected L929 cells. Cells were infected with the virus indicated at an MOI of 0.2 TCID50/ml for 48 h. Cell culture supernatants were treated for 24 h at pH 2, neutralized, and used for priming of fresh L929 cells. Interferon concentrations were calculated by using a standard curve of serial dilutions of alpha/beta interferon. (B) Production of beta interferon in infected HeLa cells. Cells were infected with the virus indicated at an MOI of 0.2 TCID50/ml for 48 h. Beta interferon production in cell culture supernatants was measured by enzyme-linked immunosorbent assay. Means and standard deviations from three independent experiments are indicated.
The integrity of the CK-2 phosphorylation site of the mengovirus leader protein is essential for apoferritin synthesis in mengovirus-infected cells.
It has been reported that within the first 5 h after mengovirus infection, the synthesis of both light- and heavy-chain apoferritin is increased approximately threefold, while the overall cellular protein synthesis is severely decreased (4, 17, 18). This induction of apoferritin appears to be a cardiovirus-specific event, since enhanced apoferritin expression was observed in human HeLa cells after infection with mengovirus and TMEV but not after infection with poliovirus (18).
Immediate-early host cell responses, such as the production of nitric oxide and cytokine-mediated processes, are related to intracellular iron homeostasis (8, 9, 26). For this reason, the possible relationship between the leader protein function and ferritin production in mengovirus-infected cells was examined. Mock-infected L929 cells or cells infected with wild-type virus, vM16ΔL(12-52), or vM16T47A were pulse-labeled with [35S]methionine at various time points after infection, after which the ferritin production was assayed by immunoprecipitation. Figure 3 shows a typical example of the ferritin assay. Figure 3A shows that the ferritin expression was significantly increased already at 3 h postinfection in wild-type mengovirus-infected L929 cells. However, no increase of ferritin was observed in vM16ΔL(12-52)-infected cells (Fig. 3B). Notably, in L929 cells infected with vM16ΔL(12-52) the overall protein synthesis was decreased without enhancement of ferritin synthesis (Fig. 3B). These data suggest that induction of ferritin synthesis is indeed a virus-related phenomenon and is caused not by the inhibition of protein synthesis in general but directly by the activity of the leader protein. Disturbance of the CK-2 phosphorylation site in mutant viruses vM16T47A, vM16E50A, and vM16insG49 also did not result in an increase of ferritin. The lower ferritin levels in mutant virus-infected cells compared to the ferritin production in mock-infected cells may be caused by the inhibition of host cell translation in mutant virus-infected cells, while ferritin production is not upregulated as it is in wild-type-infected cells. These results illustrate that the presence of the CK-2 phosphorylation site in the mengovirus leader protein is required for the biological effect of the leader protein on ferritin expression (Fig. 3C, D, and E).
Ferritin production was examined in cells infected with the mengovirus mutant vM16.1Δ2A, in which the 2A-encoding region is deleted but the leader protein is still intact (34). Infection of L929 cells with vM16.1Δ2A resulted in an increase of ferritin synthesis similar to that found in wild-type mengovirus-infected cells (Fig. 3F). These results point to the specific role of the mengovirus leader protein in the induction of ferritin expression.
Apoferritin expression is not related to iNOS induction.
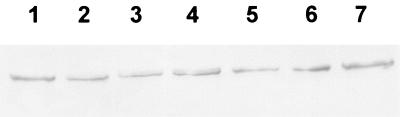
Increased concentrations of free iron in the cytoplasm give rise to the formation of ROS such as nitric oxide, hydroxyl radicals, and hydrogen peroxide. The synthesis of nitric oxide in infected cells is directly associated with intracellular iron homeostasis (9). Therefore, the effect of mengovirus leader protein mutations on the production of nitric oxide and the synthesis of iNOS was examined. L929 cells were either mock infected or infected with wild-type mengovirus, vM16.1ΔL(12-52), vM16T47A, vM16E50A, or vM16insG49. At between 1 and 9 h postinfection, cell culture supernatants were tested at 1-h intervals for the presence of nitric oxide by using Griess reagent (31). At the same time points, lysates of infected L929 cells were made and tested by Western blotting for the presence of iNOS. Neither production of nitric oxide nor a change in intracellular levels of iNOS was found in mock- and virus-infected cells (Fig. 5).
FIG. 5.
Synthesis of iNOS in infected L929 cells. Cells were mock infected (lane 1) or infected with wild-type mengovirus (lane 2), vM16ΔL(12-52) (lane 3), vM16T47A (lane 4), vM16E50A (lane 5), vM16insG49 (lane 6), or vM16Δ2A (lane 7) at an MOI of 50 TCID50/cell. At 5 h postinfection, cells were lysed and analyzed by Western blotting with antibodies against iNOS.
Integrity of the CK-2 phosphorylation site of the mengovirus leader protein is essential for the suppression of NF-κB activation in mengovirus-infected cells.
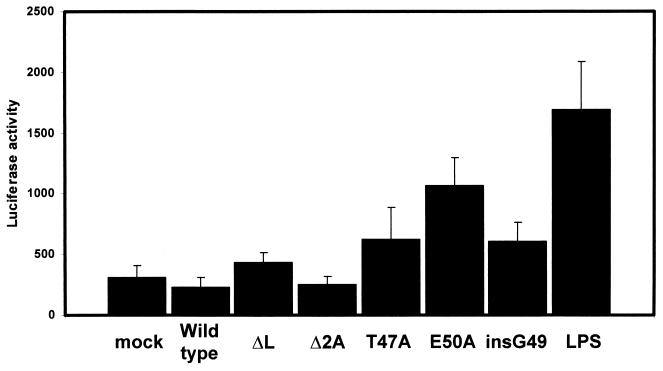
Induction of alpha/beta interferon expression depends on the activation of the transcription factor NF-κB. Increased concentrations of free iron in the cytoplasm give rise to the formation of ROS other than nitric oxide, which are thought to be involved in the activation of NF-κB (26, 30). For this reason, the activation of NF-κB in mengovirus-infected cells was examined. L929 cells were transfected with the reporter plasmid pNFκB-Luc, in which luciferase gene expression is placed under control of four tandem copies of the NF-κB consensus sequence. Transfected cells were mock infected or infected with wild-type virus, vM16ΔL(12-52), vM16T47A, vM16E50A, vM16insG49, or vM16Δ2A. As a control, transfected cells were treated with lipopolysaccharide.
Figure 6 shows the NF-κB activation under the various conditions. Wild-type mengovirus infection resulted in a small reduction in luciferase activity compared to the situation found in mock-infected cells. In contrast, with cells infected with wild-type virus, luciferase activity in cells infected with leader peptide mengovirus mutants is increased compared to that in mock-infected cells. Although levels of reporter gene induction in cells infected with leader peptide mutants are relatively modest, an increase of luciferase activity was found in mutant virus-infected cells compared to mock-infected cells and cells infected with wild-type mengovirus or vM16Δ2A. The luciferase activity in cells infected with vM16T47A, vM16E50A, and vM16insG49 was especially increased. The limited increase of luciferase activity in vM16ΔL(12-52)-infected cells might be caused by the overall inhibition of translation as shown in Fig. 3B. Luciferase activity in cells infected with vM16Δ2A was similar to that in wild-type virus-infected cells, indicating that reduced virus growth is not a determinant for NF-κB activation. The experiment was performed three times with the same results. These results demonstrate that the mengovirus leader activity is directly involved in the suppression of NF-κB activation. Moreover, reduction of NF-κB-dependent transcription depends on the integrity of the phosphorylation site at threonine 47.
FIG. 6.
Activation of NF-κB in infected L929 cells. Cellular monolayers were transfected with an expression plasmid for luciferase under control of four repeats of the NF-κB binding site. At 48 h posttransfection, cells were mock infected or infected with wild-type mengovirus, vM16ΔL(12-52), vM16Δ2A, vM16T47A, vM16E50A, or vM16insG49 at an MOI of 10 TCID50/ml. As a control, cells were treated with 100 μg of lipopolysaccharide per ml. At 6 h postinfection, cellular lysates were tested for luciferase activity. Error bars indicate standard deviations.
DISCUSSION
In this report we showed that the mengovirus leader protein is a suppressor of the antiviral host cell response, possibly through the production of ferritin in infected cells. We also showed that the integrity of a CK-2 phosphorylation site at threonine 47 of the leader protein is required for the suppression of alpha/beta interferon production as well as for the production of ferritin.
Recently, Dvorak et al. described the presence of a tyrosine phosphorylation site in the leader protein of EMCV (11). They described the presence of a phosphorylated form of the leader protein in HeLa cells infected with the wild-type virus. However, the signals that they obtained were very weak due to the labile character of the leader protein after it is released from the polyprotein and became visible only after immunoprecipitation followed by Western blotting with phosphotyrosine-specific antibodies (11). In our experiments, we found no signal in the in vivo phosphorylation assay at the position of the leader protein. Apart from the differences in labeling procedures (incubation of cells with [γ-32P]ATP previously versus [32P]orthophosphate in our experiments), the contrasting results might be explained by an increased instability of the leader protein in L929 cells compared to HeLa cells. Nevertheless, phenotypic characterization showed that the amino acid composition in the threonine 47 region is essential for proper leader protein function. Any modification of the potential CK-2 site is detrimental for virus-induced ferritin induction and inhibition of the antiviral host cell response. However, since phosphorylation could not be directly demonstrated in vivo, because of the short half-life of the protein, structural effects other than phosphorylation in the region of potential CK-2 sites may in principle cause the effects of the leader protein on the suppression of alpha/beta interferon by inhibition of the iron/ferritin-mediated activation of NF-κB as well.
Our results are in agreement with the finding of Van Pesch et al. (27), who recently showed that the homologous leader protein of TMEV inhibits immediate-early alpha/beta interferon production (27). We previously reported that a mengovirus mutant with a deletion of the leader protein showed disabled virus growth (33). Notably, the mutant virus could be rescued by 2-aminopurine, an inhibitor of alpha/beta interferon-related signaling pathways (32, 33). These results support the idea that the mengovirus leader protein interferes with the antiviral host cell response. Van Pesch et al. showed that the TMEV leader peptide specifically inhibits the transcription of the immediate-early alpha/beta interferon genes but not transcription of other, late alpha interferon genes. For this reason, they postulated that the TMEV leader protein would interact with IRF-3. Expression of immediate-early and late interferon genes depends on factors such as IRF-3, IRF-7, and NF-κB. In addition to a possible inhibition of IRF-3, our results indicate that in wild-type mengovirus-infected cells NF-κB activation is suppressed and correlates with the suppression of beta interferon.
Interestingly, despite the effect of virus replication on host cell translation shutoff, it was found previously that mengovirus infection causes an enhancement of the translation of both heavy- and light-chain ferritin mRNAs in L929 cells (4, 17, 18). In those studies, it was suggested that the ferritin particles produced in infected cells might play a role in the inhibition of host cell translation. In this report, we show that the mengovirus leader protein is responsible for the induction of ferritin in infected cells. However, since the leader protein phosphorylation mutants were capable to inhibit host cell translation without the enhancement of ferritin expression, it is obvious that the induction of ferritin cannot be associated alone with the inhibition of host cell translation as previously suggested (4, 18, 18).
As a consequence of electron transfer reactions in mitochondria and other organelles, small amounts of ROS are produced in normal respiring cells. ROS include nitric oxide, hydroxyl radicals, superoxide, and peroxides. ROS are capable of causing oxidative damage to macromolecules. Therefore, ROS are scavenged by cellular antioxidant proteins such as glutathione. High doses of ROS generated by environmental stress are cytotoxic and are referred to as oxidative stress. Recent reports suggest that ROS may act as a mediator in signal transduction pathways in which cells react to the surplus of intracellular ROS with the induction of gene expression of proteins involved in the regulation of the cellular redox state. The transcription factor NF-κB plays an important role in stress responses. Based on the finding that antioxidant proteins inhibit NF-κB activation, it was suggested that NF-κB activity is regulated by intracellular ROS levels (30). An important component that participates in ROS formation via the Fenton reaction is free intracellular iron (13). Since intracellular iron homeostasis is regulated by ferritin (1), it was suggested that ferritin might serve as a cytoprotective protein, minimizing oxygen free radical formation by sequestering intracellular iron. This idea is supported by the finding that exposure of cells to inducers of ROS such as hydrogen peroxide and tumor necrosis factor alpha results in the induction of ferritin synthesis (1, 5, 12, 14, 19, 21, 29).
The observation that the mengovirus leader protein interferes with both the cellular iron homeostasis and the activation of NF-κB provides an explanation for the mechanism by which the leader protein downregulates the antiviral host cell response (Fig. 7). Induction of ferritin expression in mengovirus-infected cells will limit the availability of iron for the production of free hydroxyl radicals (16). As a consequence, NF-κB activation and thereby alpha/beta interferon expression are suppressed in mengovirus-infected cells. Experiments to determine the relationship between ferritin and iron concentrations and the suppression of host cell defense mechanisms are in progress.
FIG. 7.
Role of ferritin and iron in the oxidative stress response. Various environmental factors, such as virus infections, cytokines, and UV radiation, enzymatically generate peroxides. Under the influence of iron ions, the peroxides are further reduced to hydroxyl radicals and other ROS that are capable of activating NF-κB. Ferritin forms an antioxidant system that traps free iron and thereby reduces the host cell response.
In general, picornavirus infections provoke a cellular antiviral response. An essential step in this antiviral response is the activation of the double-stranded-RNA-dependent protein kinase PKR and subsequent activation of NF-κB-mediated expression of genes such as that for beta interferon (28). Picornaviruses have developed mechanisms to prevent or diminish the effects of the host cell antiviral response, such as the proteolytic degradation of PKR by enterovirus 2A proteinase (3). Until now, such a mechanism has not been found for cardioviruses. Our data described in this paper show that the mengovirus leader protein interrupts the antiviral host cell response by suppression of NF-κB activation, possibly via interaction with cellular iron metabolism.
REFERENCES
- 1.Aisen, P., C. Enns, and M. Wessling-Resnick. 2001. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell. Biol. 33:940-959. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. A. Smith, J. G. Seidman, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Black, T. L., G. N. Barber, and M. G. Katze. 1993. Degradation of the interferon-induced 68,000-Mr protein kinase by poliovirus requires RNA. J. Virol. 67:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boege, U., R. Hancharyk, and D. G. Scraba. 1987. The synthesis of a particle-forming cellular protein is enhanced by Mengo virus infection. Virology 159:358-367. [DOI] [PubMed] [Google Scholar]
- 5.Cairo, G., L. Tacchini, G. Pogliaghi, E. Anzon, A. Tomasi, and A. Bernelli-Zazzera. 1995. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the “free” iron pool. J. Biol. Chem. 270:700-703. [DOI] [PubMed] [Google Scholar]
- 6.Chen, H. H., W. P. Kong, and R. P. Roos. 1995. The leader peptide of Theiler's murine encephalomyelitis virus is a zinc-binding protein. J. Virol. 69:8076-8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinsangaram, J., M. E. Piccone, and M. J. Grubman. 1999. Ability of foot-and-mouth disease virus to form plaques in cell culture is associated with suppression of alpha/beta interferon. J. Virol. 73:9891-9898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connelly, L., M. Palacios-Callender, C. Ameixa, S. Moncada, and A. J. Hobbs. 2001. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J. Immunol. 166:3873-3881. [DOI] [PubMed] [Google Scholar]
- 9.Dlaska, M., and G. Weiss. 1999. Central role of transcription factor NF-IL6 for cytokine and iron-mediated regulation of murine inducible nitric oxide synthase expression. J. Immunol. 162:6171-6177. [PubMed] [Google Scholar]
- 10.Duke, G. M., and A. C. Palmenberg. 1989. Cloning and synthesis of infectious cardiovirus RNAs containing short, discrete poly(C) tracts. J. Virol. 63:1822-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dvorak, C. M. T., D. J. Hall, M. Hill, M. Riddle, A. Pranter, J. Dillman, M. Deibel, and A. C. Palmenberg. 2001. Leader protein of encephalomyocarditis virus binds zinc, is phosphorylated during viral infection, and affects the efficiency of genome translation. Virology 290:261-271. [DOI] [PubMed] [Google Scholar]
- 12.Gray, C. P., A. V. Franco, P. Arosio, and P. Hersey. 2001. Immunosuppressive effects of melanoma-derived heavy-chain ferritin are dependent on stimulation of IL-10 production. Int. J. Cancer 92:843-850. [DOI] [PubMed] [Google Scholar]
- 13.Iwai, K., S. K. Drake, N. B. Wehr, A. M. Weissman, T. LaVaute, N. Minato, R. D. Klausner, R. L. Levine, and T. A. Rouault. 1998. Iron-dependent oxidation, ubiquitination, and degradation of iron regulatory protein 2: implications for degradation of oxidized proteins. Proc. Natl. Acad. Sci. USA 95:4924-4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwak, E. L., D. A. Larochelle, C. Beaumont, S. V. Torti, and F. M. Torti. 1995. Role for NF-κB in the regulation of ferritin H by tumor necrosis factor-alpha. J. Biol. Chem. 270:15285-15293. [DOI] [PubMed] [Google Scholar]
- 15.Li, N., and M. Karin. 1999. Is NF-κB the sensor of oxidative stress? FASEB J. 13:1137-1143. [PubMed] [Google Scholar]
- 16.Morel, Y., and R. Barouki. 1999. Repression of gene expression by oxidative stress. Biochem. J. 342:481-496. [PMC free article] [PubMed] [Google Scholar]
- 17.Mulvey, M. R., H. Fang, C. F. Holmes, and D. G. Scraba. 1994. The cellular U-particle, whose synthesis is induced by mengovirus infection, is homologous to apoferritin. Virology 198:81-91. [DOI] [PubMed] [Google Scholar]
- 18.Mulvey, M. R., L. C. Kuhn, and D. G. Scraba. 1996. Induction of ferritin synthesis in cells infected with Mengo virus. J. Biol. Chem. 271:9851-9857. [DOI] [PubMed] [Google Scholar]
- 19.Orino, K., L. Lehman, Y. Tsuji, H. Ayaki, S. V. Torti, and F. M. Torti. 2001. Ferritin and the response to oxidative stress. Biochem. J. 357:241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmenberg, A. C. 1990. Proteolytic processing of picornaviral polyprotein. Annu. Rev. Microbiol. 44:603-623. [DOI] [PubMed] [Google Scholar]
- 21.Pinero, D. J., J. Hu, B. M. Cook, R. C. J. Scaduto, and J. R. Connor. 2000. Interleukin-1β increases binding of the iron regulatory protein and the synthesis of ferritin by increasing the labile iron pool. Biochim. Biophys. Acta 1497:279-288. [DOI] [PubMed] [Google Scholar]
- 22.Pinna, L. A. 1990. Casein kinase 2: an ‘eminence grise’ in cellular regulation? Biochim. Biophys. Acta 1054:267-284. [DOI] [PubMed] [Google Scholar]
- 23.Reed, L. J., and H. A. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 24.Rueckert, R. 1996. Picornaviridae and their replication, p. 609-654. In B. N. Fields (ed.), Virology. Raven Press, New York, N.Y.
- 25.Schmidt, N. J. 1979. Cell culture techniques for diagnostic virology, p. 65-139. In E. H. Lennette and N. J. Schmidt (ed.), Diagnostic procedures for viral, rickettsial, and chlamydial infections. American Public Health Association, Washington, D.C.
- 26.Thannickal, V. J., and B. L. Fanburg. 2000. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L1005-L1028. [DOI] [PubMed] [Google Scholar]
- 27.van Pesch, V., O. van Eyll, and T. Michiels. 2001. The leader protein of Theiler's virus inhibits immediate-early alpha/beta interferon production. J. Virol. 75:7811-7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilcek, J., and G. S. Sen. 1996. Interferons and other cytokines, p. 375-399. In B. N. Fields (ed.), Virology. Raven Press, New York, N.Y.
- 29.Vile, G. F., and R. M. Tyrrell. 1993. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J. Biol. Chem. 268:14678-14681. [PubMed] [Google Scholar]
- 30.Vlahopoulos, S., I. Boldogh, A. Casola, and A. R. Brasier. 1999. Nuclear factor-κB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 94:1878-1889. [PubMed] [Google Scholar]
- 31.Wu, C. C., and C. Thiemermann. 1996. Biological control and inhibition of induction of nitric oxide synthase. Methods Enzymol. 268:408-420. [DOI] [PubMed] [Google Scholar]
- 32.Zinn, K., A. Keller, L. A. Whittemore, and T. Maniatis. 1988. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science 240:210-213. [DOI] [PubMed] [Google Scholar]
- 33.Zoll, J., J. M. Galama, F. J. van Kuppeveld, and W. J. Melchers. 1996. Mengovirus leader is involved in the inhibition of host cell protein synthesis. J. Virol. 70:4948-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoll, J., F. J. van Kuppeveld, J. M. Galama, and W. J. Melchers. 1998. Genetic analysis of mengovirus protein 2A: its function in polyprotein processing and virus reproduction. J. Gen. Virol. 79:17-25. [DOI] [PubMed] [Google Scholar]