Abstract
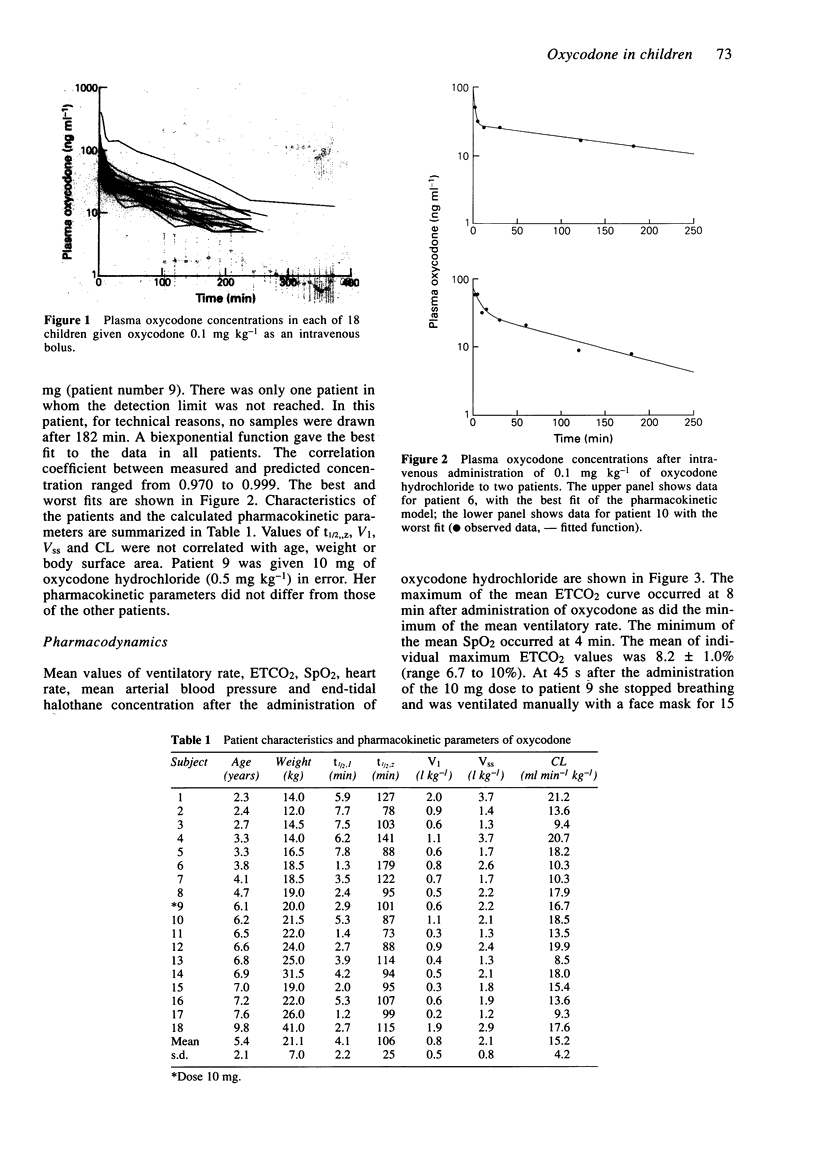
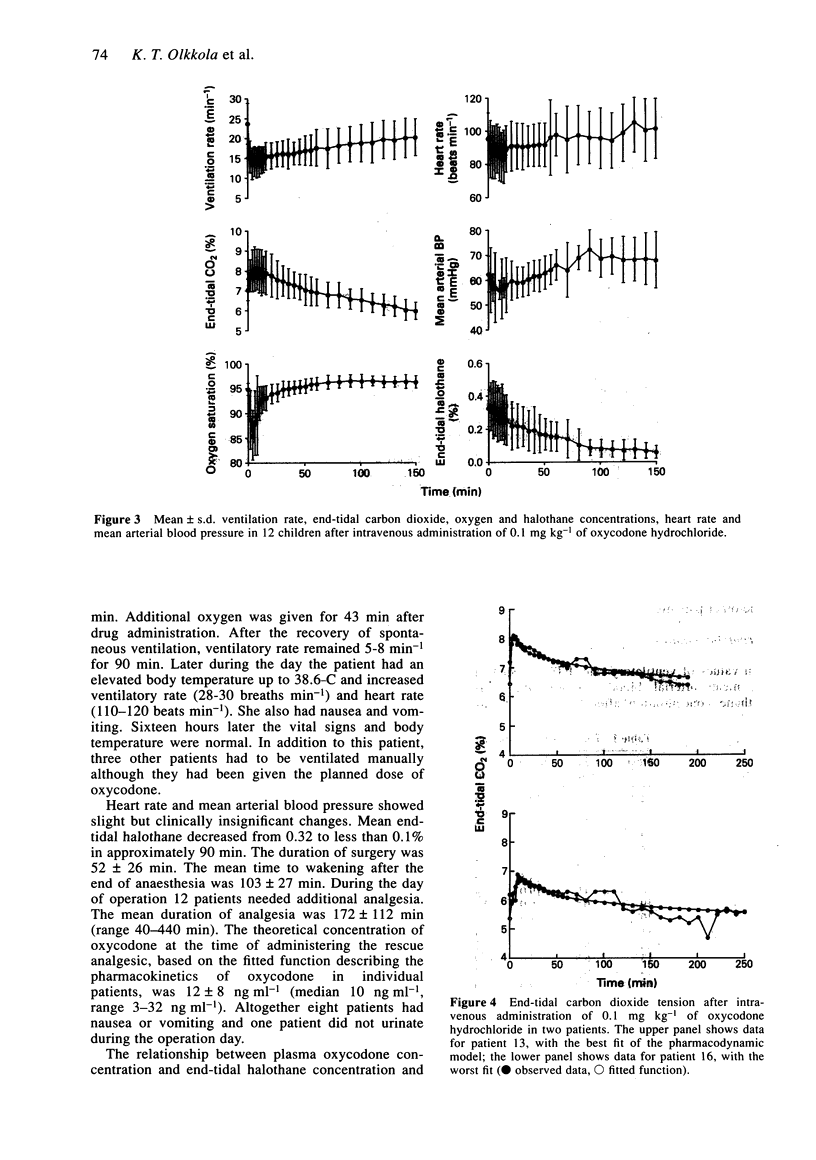
1. Oxycodone hydrochloride (0.1 mg kg-1) was given by intravenous bolus to 18 children after ophthalmic surgery. Plasma was sampled for up to 8 h. Blood pressure, heart rate, peripheral arteriolar oxygen saturation, end-tidal carbon dioxide and halothane concentrations and ventilatory rate were also recorded. 2. Mean (+/- s.d.) values of drug clearance and volume of distribution (Vss) were 15.2 +/- 4.2 ml min-1 kg-1 and 2.1 +/- 0.8 l kg-1. Maximum mean end-tidal carbon dioxide concentration and minimum mean ventilatory rate occurred 8 min after administration of oxycodone but the minimum mean peripheral arteriolar oxygen saturation occurred at 4 min. 3. Oxycodone (0.1 mg kg-1) appears to cause greater ventilatory depression than comparable analgesic doses of other opioids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hamunen K., Olkkola K. T., Maunuksela E. L. Comparison of the ventilatory effects of morphine and buprenorphine in children. Acta Anaesthesiol Scand. 1993 Jul;37(5):449–453. doi: 10.1111/j.1399-6576.1993.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hamunen K. Ventilatory effects of morphine, pethidine and methadone in children. Br J Anaesth. 1993 Apr;70(4):414–418. doi: 10.1093/bja/70.4.414. [DOI] [PubMed] [Google Scholar]
- Hartvig P., Tamsen A., Fagerlund C., Dahlström B. Pharmacokinetics of pethidine during anaesthesia and patient-controlled analgesic therapy. Acta Anaesthesiol Scand Suppl. 1982;74:52–54. doi: 10.1111/j.1399-6576.1982.tb01846.x. [DOI] [PubMed] [Google Scholar]
- Holford N. H., Sheiner L. B. Understanding the dose-effect relationship: clinical application of pharmacokinetic-pharmacodynamic models. Clin Pharmacokinet. 1981 Nov-Dec;6(6):429–453. doi: 10.2165/00003088-198106060-00002. [DOI] [PubMed] [Google Scholar]
- Holland C. T., Satchell P. M., Farrow B. R. Oesophageal compliance in naturally occurring canine megaoesophagus. Aust Vet J. 1993 Nov;70(11):414–420. doi: 10.1111/j.1751-0813.1993.tb06079.x. [DOI] [PubMed] [Google Scholar]
- Kalso E., Pöyhiä R., Onnela P., Linko K., Tigerstedt I., Tammisto T. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand. 1991 Oct;35(7):642–646. doi: 10.1111/j.1399-6576.1991.tb03364.x. [DOI] [PubMed] [Google Scholar]
- Kalso E., Vainio A., Mattila M. J., Rosenberg P. H., Seppälä T. Morphine and oxycodone in the management of cancer pain: plasma levels determined by chemical and radioreceptor assays. Pharmacol Toxicol. 1990 Oct;67(4):322–328. doi: 10.1111/j.1600-0773.1990.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Kalso E., Vainio A. Morphine and oxycodone hydrochloride in the management of cancer pain. Clin Pharmacol Ther. 1990 May;47(5):639–646. doi: 10.1038/clpt.1990.85. [DOI] [PubMed] [Google Scholar]
- Leow K. P., Smith M. T., Williams B., Cramond T. Single-dose and steady-state pharmacokinetics and pharmacodynamics of oxycodone in patients with cancer. Clin Pharmacol Ther. 1992 Nov;52(5):487–495. doi: 10.1038/clpt.1992.176. [DOI] [PubMed] [Google Scholar]
- Maunuksela E. L., Olkkola K. T., Korpela R. Measurement of pain in children with self-reporting and behavioral assessment. Clin Pharmacol Ther. 1987 Aug;42(2):137–141. doi: 10.1038/clpt.1987.123. [DOI] [PubMed] [Google Scholar]
- Pöyhiä R., Olkkola K. T., Seppälä T., Kalso E. The pharmacokinetics of oxycodone after intravenous injection in adults. Br J Clin Pharmacol. 1991 Oct;32(4):516–518. doi: 10.1111/j.1365-2125.1991.tb03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöyhiä R., Seppälä T., Olkkola K. T., Kalso E. The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol. 1992 Jun;33(6):617–621. doi: 10.1111/j.1365-2125.1992.tb04090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C. S., Wood A. J., Koshakji R. P., Wood M. The effect of halothane on drug disposition: contribution of changes in intrinsic drug metabolizing capacity and hepatic blood flow. Anesthesiology. 1985 Jul;63(1):70–76. doi: 10.1097/00000542-198507000-00011. [DOI] [PubMed] [Google Scholar]
- Todd P. A., Sorkin E. M. Diclofenac sodium. A reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1988 Mar;35(3):244–285. doi: 10.2165/00003495-198835030-00004. [DOI] [PubMed] [Google Scholar]
- Wagner J. G. Linear pharmacokinetic equations allowing direct calculation of many needed pharmacokinetic parameters from the coefficients and exponents of polyexponential equations which have been fitted to the data. J Pharmacokinet Biopharm. 1976 Oct;4(5):443–467. doi: 10.1007/BF01062831. [DOI] [PubMed] [Google Scholar]


