Abstract
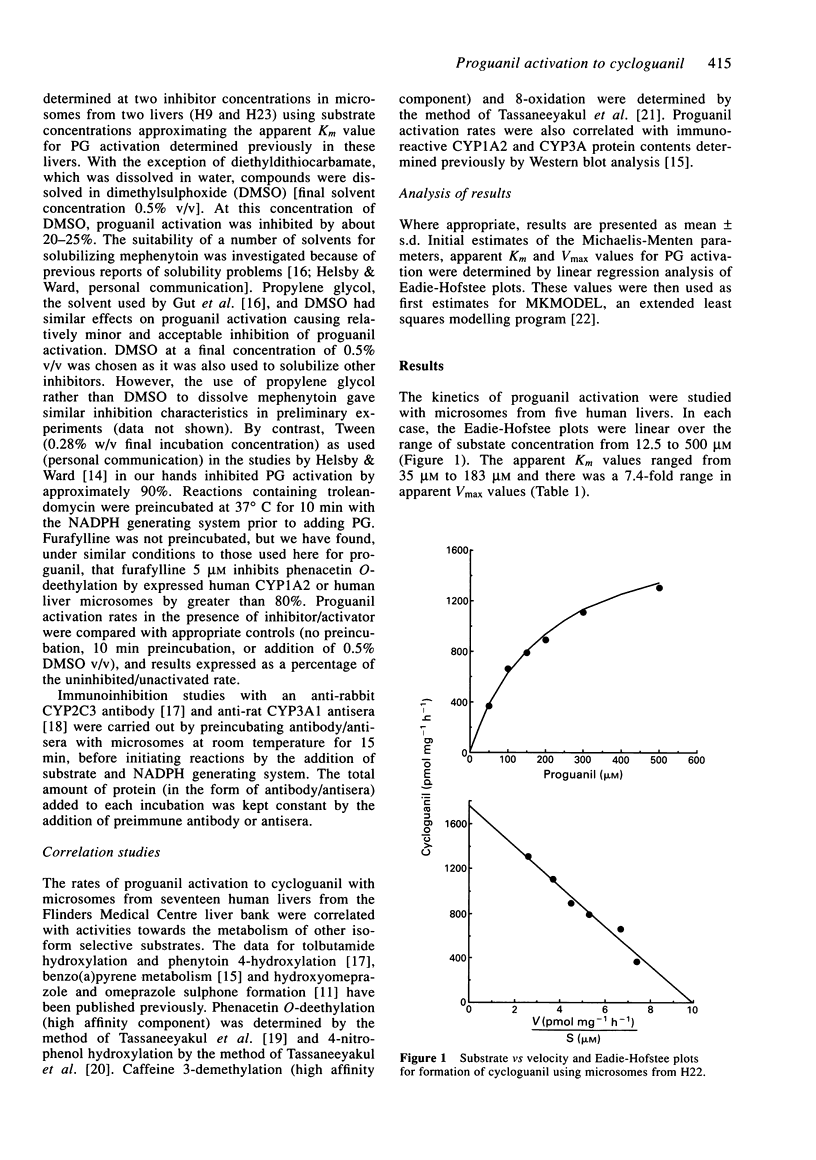
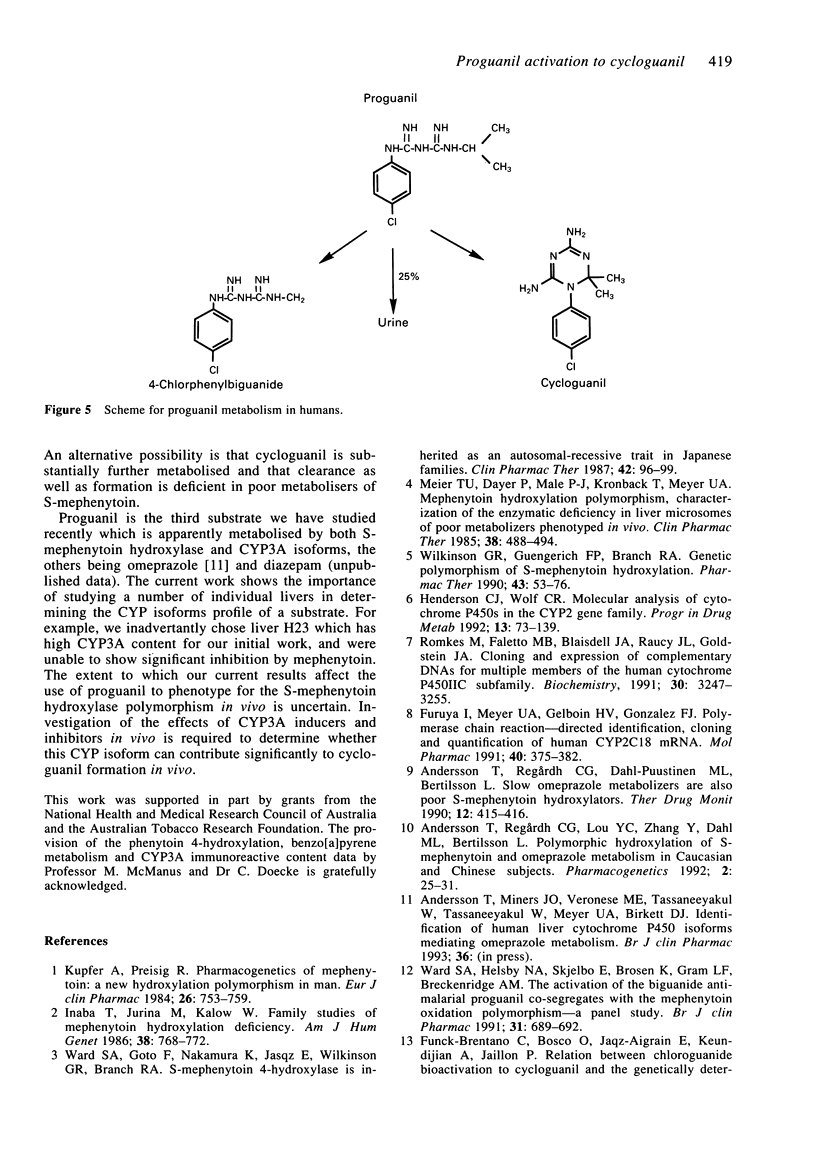
1. The activation of proguanil to cycloguanil by human liver microsomes was studied to define the cytochrome P450 (CYP) isoforms involved in this reaction. 2. Apparent Km values for proguanil ranged from 35 microM to 183 microM with microsomes from four human livers. 3. There was a 6.3-fold range of activity with microsomes from seventeen human livers. Rates of proguanil activation correlated significantly with CYP3A activities (benzo[a]pyrene metabolism, caffeine 8-oxidation and omeprazole sulphone formation) and CYP3A immunoreactive content. There was also a highly significant correlation with rates of hydroxyomeprazole formation. Correlations with activities selective for CYP1A2, CYP2C9/10 and CYP2E1, and with immunoreactive CYP1A2 content were not significant. 4. Proguanil activation was inhibited by R,S-mephenytoin, troleandomycin and by inhibitory anti-CYP3A antiserum and anti-CYP2C IgG and was activated by alpha-naphthoflavone. Inhibitors selective for CYP1A2, CYP2E1, CYP2A6 or CYP2C9/10 had little or no effect on proguanil activation. The extents of inhibition by R,S-mephenytoin, troleandomycin and the two antibodies varied with the immunoreactive CYP3A content of the microsomes used. 5. It is concluded that proguanil activation to cycloguanil by human liver microsomes is mediated both by S-mephenytoin hydroxylase and isoforms of the CYP3A subfamily. This has implications for the use of proguanil as an in vivo probe for the S-mephenytoin poor metaboliser phenotype.
Full text
PDF

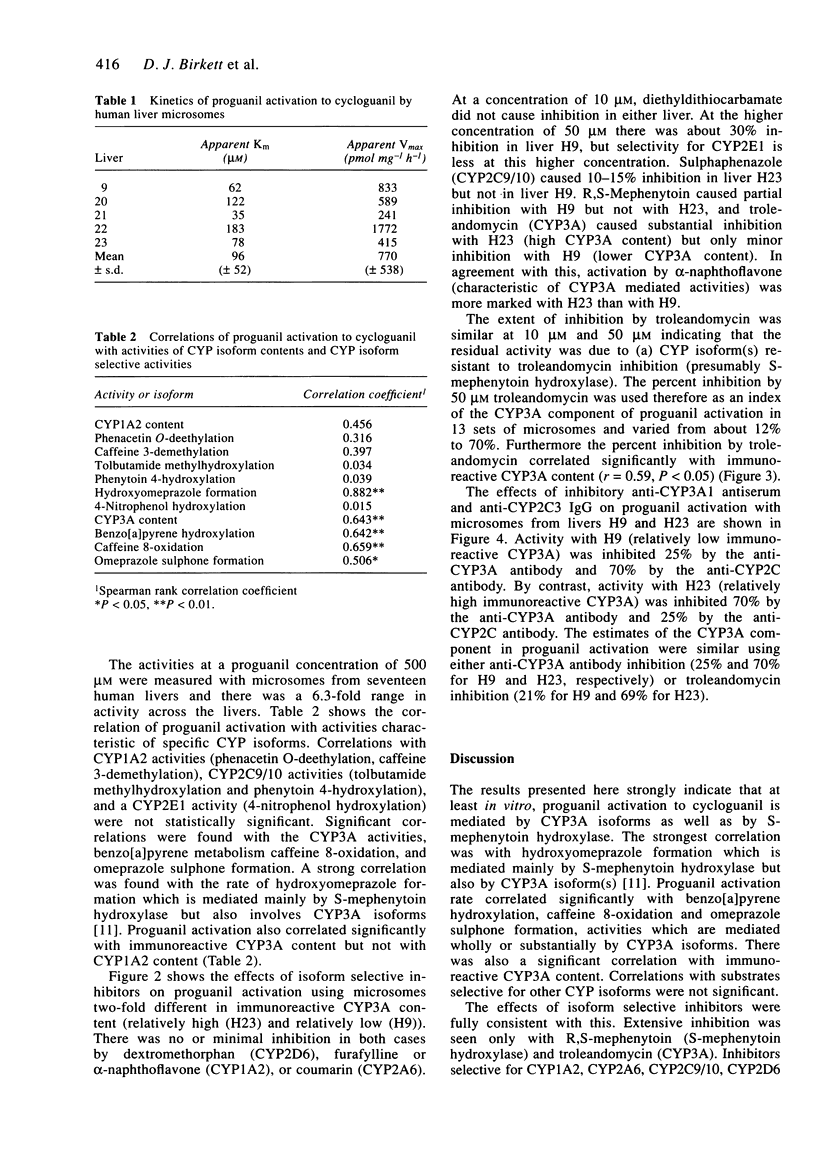
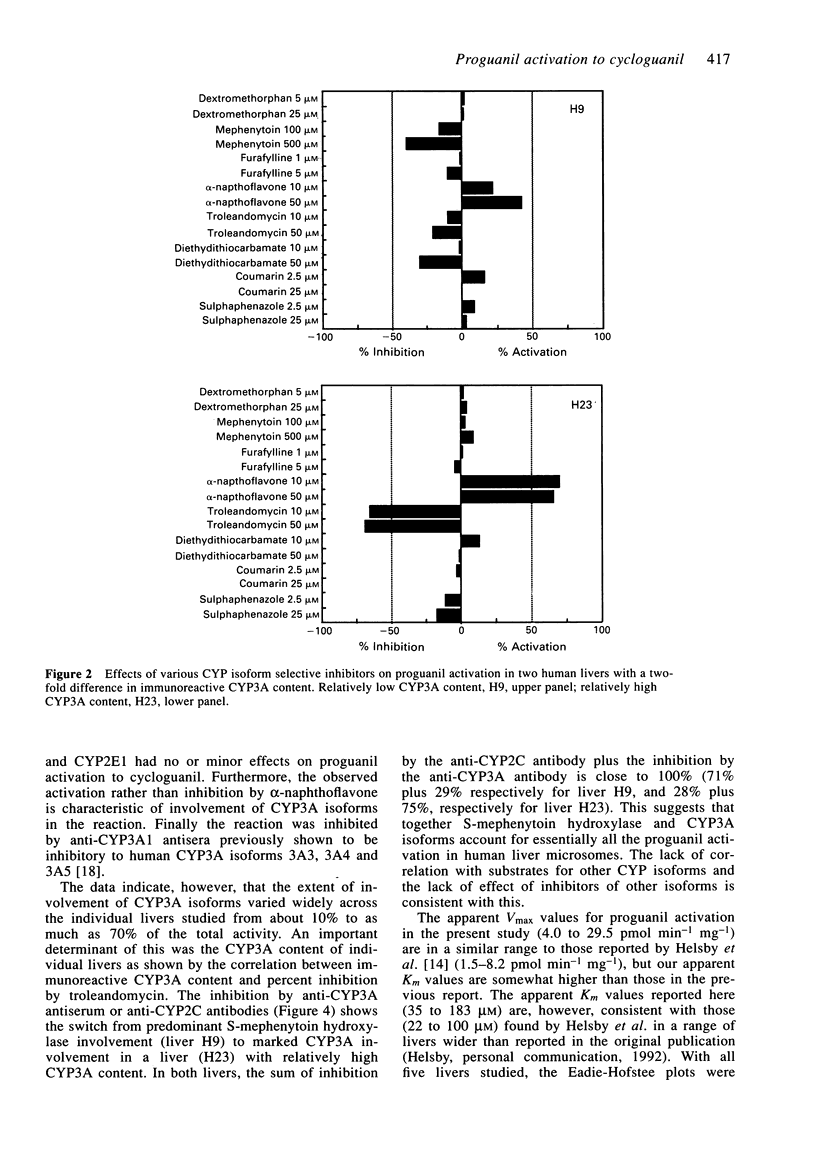
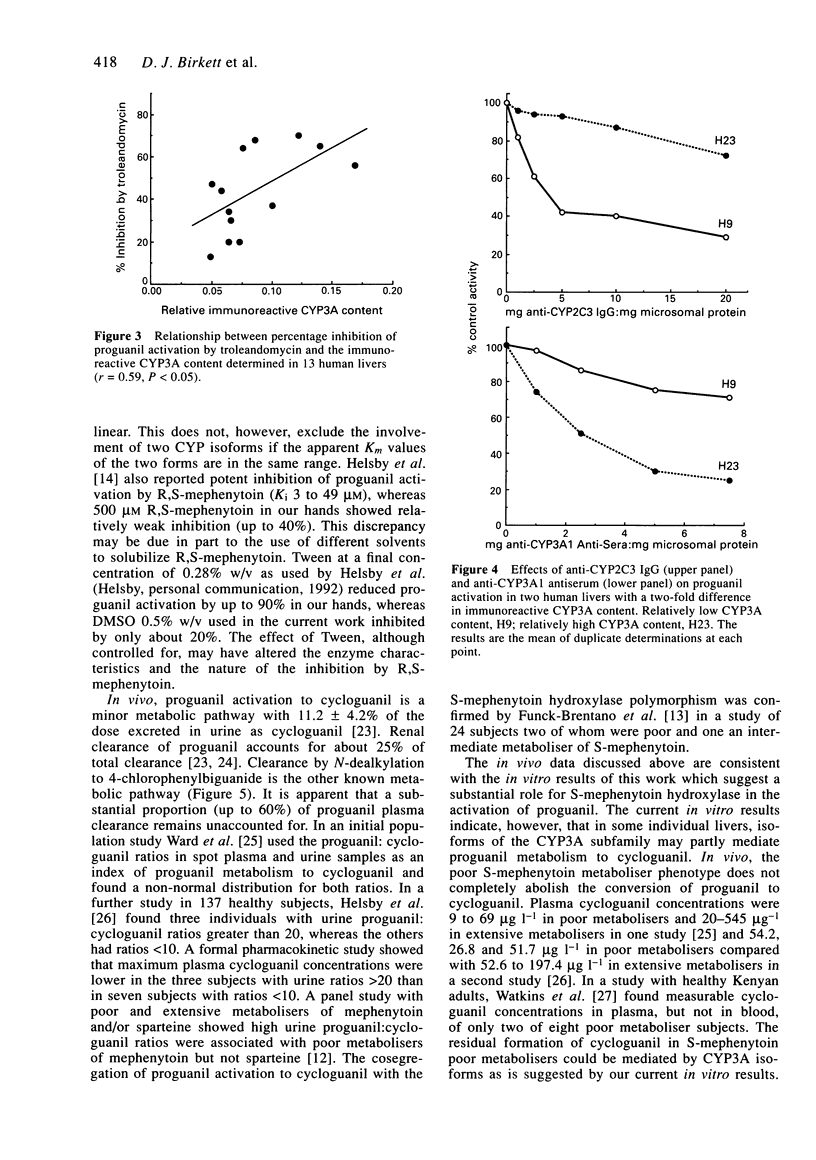

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Regårdh C. G., Dahl-Puustinen M. L., Bertilsson L. Slow omeprazole metabolizers are also poor S-mephenytoin hydroxylators. Ther Drug Monit. 1990 Jul;12(4):415–416. doi: 10.1097/00007691-199007000-00020. [DOI] [PubMed] [Google Scholar]
- Andersson T., Regårdh C. G., Lou Y. C., Zhang Y., Dahl M. L., Bertilsson L. Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics. 1992 Feb;2(1):25–31. doi: 10.1097/00008571-199202000-00005. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Korzekwa K., Nagata K., Gillette J., Gelboin H. V., Gonzalez F. J. Estradiol metabolism by complementary deoxyribonucleic acid-expressed human cytochrome P450s. Endocrinology. 1990 Jun;126(6):3101–3106. doi: 10.1210/endo-126-6-3101. [DOI] [PubMed] [Google Scholar]
- Doecke C. J., Veronese M. E., Pond S. M., Miners J. O., Birkett D. J., Sansom L. N., McManus M. E. Relationship between phenytoin and tolbutamide hydroxylations in human liver microsomes. Br J Clin Pharmacol. 1991 Feb;31(2):125–130. doi: 10.1111/j.1365-2125.1991.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstein M. D., Veenendaal J. R., Scott H. V., Rieckmann K. H. Steady-state kinetics of proguanil and its active metabolite, cycloguanil, in man. Chemotherapy. 1988;34(5):385–392. doi: 10.1159/000238597. [DOI] [PubMed] [Google Scholar]
- Funck-Brentano C., Bosco O., Jacqz-Aigrain E., Keundjian A., Jaillon P. Relation between chloroguanide bioactivation to cycloguanil and the genetically determined metabolism of mephenytoin in humans. Clin Pharmacol Ther. 1992 May;51(5):507–512. doi: 10.1038/clpt.1992.55. [DOI] [PubMed] [Google Scholar]
- Furuya H., Meyer U. A., Gelboin H. V., Gonzalez F. J. Polymerase chain reaction-directed identification, cloning, and quantification of human CYP2C18 mRNA. Mol Pharmacol. 1991 Sep;40(3):375–382. [PubMed] [Google Scholar]
- Gut J., Meier U. T., Catin T., Meyer U. A. Mephenytoin-type polymorphism of drug oxidation: purification and characterization of a human liver cytochrome P-450 isozyme catalyzing microsomal mephenytoin hydroxylation. Biochim Biophys Acta. 1986 Dec 10;884(3):435–447. doi: 10.1016/0304-4165(86)90194-7. [DOI] [PubMed] [Google Scholar]
- Helsby N. A., Ward S. A., Edwards G., Howells R. E., Breckenridge A. M. The pharmacokinetics and activation of proguanil in man: consequences of variability in drug metabolism. Br J Clin Pharmacol. 1990 Oct;30(4):593–598. doi: 10.1111/j.1365-2125.1990.tb03818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsby N. A., Ward S. A., Howells R. E., Breckenridge A. M. In vitro metabolism of the biguanide antimalarials in human liver microsomes: evidence for a role of the mephenytoin hydroxylase (P450 MP) enzyme. Br J Clin Pharmacol. 1990 Aug;30(2):287–291. doi: 10.1111/j.1365-2125.1990.tb03777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba T., Jurima M., Kalow W. Family studies of mephenytoin hydroxylation deficiency. Am J Hum Genet. 1986 May;38(5):768–772. [PMC free article] [PubMed] [Google Scholar]
- Küpfer A., Preisig R. Pharmacogenetics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur J Clin Pharmacol. 1984;26(6):753–759. doi: 10.1007/BF00541938. [DOI] [PubMed] [Google Scholar]
- McManus M. E., Burgess W. M., Veronese M. E., Huggett A., Quattrochi L. C., Tukey R. H. Metabolism of 2-acetylaminofluorene and benzo(a)pyrene and activation of food-derived heterocyclic amine mutagens by human cytochromes P-450. Cancer Res. 1990 Jun 1;50(11):3367–3376. [PubMed] [Google Scholar]
- Meier U. T., Dayer P., Malè P. J., Kronbach T., Meyer U. A. Mephenytoin hydroxylation polymorphism: characterization of the enzymatic deficiency in liver microsomes of poor metabolizers phenotyped in vivo. Clin Pharmacol Ther. 1985 Nov;38(5):488–494. doi: 10.1038/clpt.1985.213. [DOI] [PubMed] [Google Scholar]
- Romkes M., Faletto M. B., Blaisdell J. A., Raucy J. L., Goldstein J. A. Cloning and expression of complementary DNAs for multiple members of the human cytochrome P450IIC subfamily. Biochemistry. 1991 Apr 2;30(13):3247–3255. doi: 10.1021/bi00227a012. [DOI] [PubMed] [Google Scholar]
- Tassaneeyakul W., Birkett D. J., Veronese M. E., McManus M. E., Tukey R. H., Quattrochi L. C., Gelboin H. V., Miners J. O. Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J Pharmacol Exp Ther. 1993 Apr;265(1):401–407. [PubMed] [Google Scholar]
- Tassaneeyakul W., Mohamed Z., Birkett D. J., McManus M. E., Veronese M. E., Tukey R. H., Quattrochi L. C., Gonzalez F. J., Miners J. O. Caffeine as a probe for human cytochromes P450: validation using cDNA-expression, immunoinhibition and microsomal kinetic and inhibitor techniques. Pharmacogenetics. 1992 Aug;2(4):173–183. doi: 10.1097/00008571-199208000-00004. [DOI] [PubMed] [Google Scholar]
- Tassaneeyakul W., Veronese M. E., Birkett D. J., Miners J. O. High-performance liquid chromatographic assay for 4-nitrophenol hydroxylation, a putative cytochrome P-4502E1 activity, in human liver microsomes. J Chromatogr. 1993 Jun 23;616(1):73–78. doi: 10.1016/0378-4347(93)80473-h. [DOI] [PubMed] [Google Scholar]
- Ward S. A., Goto F., Nakamura K., Jacqz E., Wilkinson G. R., Branch R. A. S-mephenytoin 4-hydroxylase is inherited as an autosomal-recessive trait in Japanese families. Clin Pharmacol Ther. 1987 Jul;42(1):96–99. doi: 10.1038/clpt.1987.114. [DOI] [PubMed] [Google Scholar]
- Ward S. A., Helsby N. A., Skjelbo E., Brøsen K., Gram L. F., Breckenridge A. M. The activation of the biguanide antimalarial proguanil co-segregates with the mephenytoin oxidation polymorphism--a panel study. Br J Clin Pharmacol. 1991 Jun;31(6):689–692. doi: 10.1111/j.1365-2125.1991.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. A., Watkins W. M., Mberu E., Saunders J. E., Koech D. K., Gilles H. M., Howells R. E., Breckenridge A. M. Inter-subject variability in the metabolism of proguanil to the active metabolite cycloguanil in man. Br J Clin Pharmacol. 1989 Jun;27(6):781–787. doi: 10.1111/j.1365-2125.1989.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins W. M., Mberu E. K., Nevill C. G., Ward S. A., Breckenridge A. M., Koech D. K. Variability in the metabolism of proguanil to the active metabolite cycloguanil in healthy Kenyan adults. Trans R Soc Trop Med Hyg. 1990 Jul-Aug;84(4):492–495. doi: 10.1016/0035-9203(90)90010-c. [DOI] [PubMed] [Google Scholar]
- Wattanagoon Y., Taylor R. B., Moody R. R., Ochekpe N. A., Looareesuwan S., White N. J. Single dose pharmacokinetics of proguanil and its metabolites in healthy subjects. Br J Clin Pharmacol. 1987 Dec;24(6):775–780. doi: 10.1111/j.1365-2125.1987.tb03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. R., Guengerich F. P., Branch R. A. Genetic polymorphism of S-mephenytoin hydroxylation. Pharmacol Ther. 1989;43(1):53–76. doi: 10.1016/0163-7258(89)90047-8. [DOI] [PubMed] [Google Scholar]


