Abstract
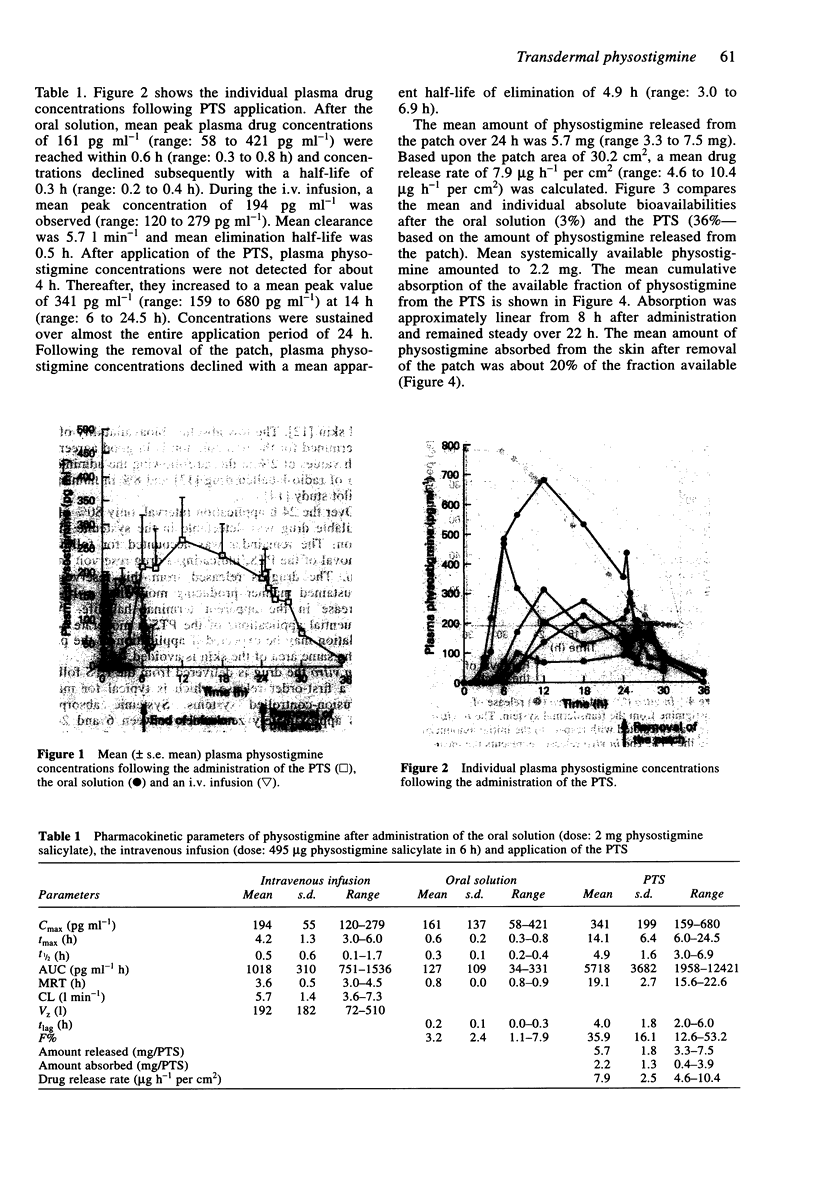
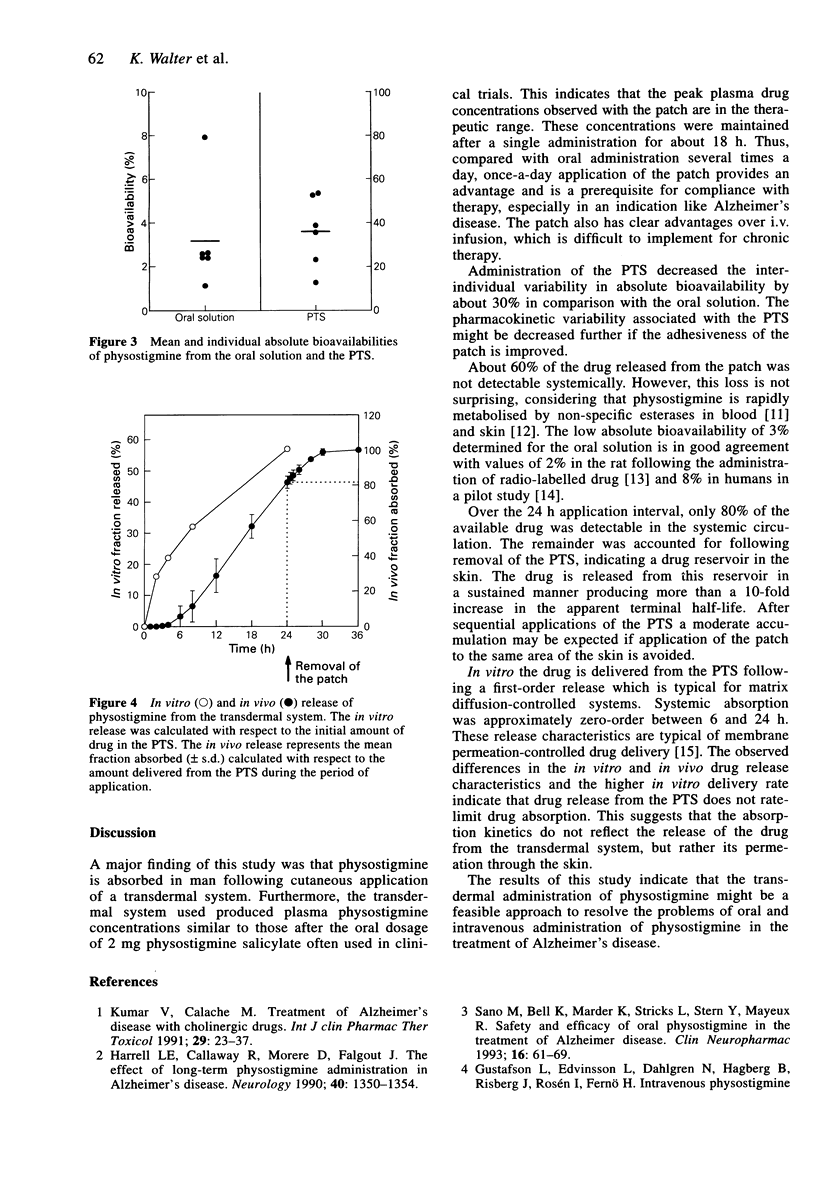
1. The pharmacokinetics of physostigmine were investigated in a three-way cross-over design in six healthy, male volunteers comparing a physostigmine transdermal system (PTS), an oral solution and an i.v. infusion. 2. A single application of the patch over 24 h produced detectable plasma drug concentrations after a mean lag-time of 4 h. Thereafter, the drug was absorbed continuously from the PTS and putative therapeutic plasma concentrations were measured over approximately 18 h. 3. A mean absolute bioavailability of 36% was determined for the transdermal system and 3% for the oral solution. In comparison with the oral solution, interindividual variability of pharmacokinetics was less with the PTS. 4. The mean amount of physostigmine released from the transdermal system after 24 h was 5.7 mg. Because of extensive metabolism, only 2.2 mg of physostigmine were detected systemically. 5. After removing the PTS, the mean apparent half-life of elimination was 4.9 h, compared with 0.5 h for the i.v. infusion. This indicates continued drug absorption from a skin depot. 6. Physostigmine was well tolerated by the volunteers. With the PTS, a mild erythema was observed at the area of application, disappearing within a few hours.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christie J. E., Shering A., Ferguson J., Glen A. I. Physostigmine and arecoline: effects of intravenous infusions in Alzheimer presenile dementia. Br J Psychiatry. 1981 Jan;138:46–50. doi: 10.1192/bjp.138.1.46. [DOI] [PubMed] [Google Scholar]
- Gustafson L., Edvinsson L., Dahlgren N., Hagberg B., Risberg J., Rosén I., Fernö H. Intravenous physostigmine treatment of Alzheimer's disease evaluated by psychometric testing, regional cerebral blood flow (rCBF) measurement, and EEG. Psychopharmacology (Berl) 1987;93(1):31–35. doi: 10.1007/BF02439583. [DOI] [PubMed] [Google Scholar]
- Harrell L. E., Callaway R., Morere D., Falgout J. The effect of long-term physostigmine administration in Alzheimer's disease. Neurology. 1990 Sep;40(9):1350–1354. doi: 10.1212/wnl.40.9.1350. [DOI] [PubMed] [Google Scholar]
- Kark R. A., Budelli M. M., Wachsner R. Double-blind, triple-crossover trial of low doses of oral physostigmine in inherited ataxias. Neurology. 1981 Mar;31(3):288–292. doi: 10.1212/wnl.31.3.288. [DOI] [PubMed] [Google Scholar]
- Knapp S., Wardlow M. L., Thal L. J. Sensitive analysis of plasma physostigmine levels using dual-cell electrochemistry in the redox mode. J Chromatogr. 1990 Mar 16;526(1):97–107. doi: 10.1016/s0378-4347(00)82487-1. [DOI] [PubMed] [Google Scholar]
- Kumar V., Calache M. Treatment of Alzheimer's disease with cholinergic drugs. Int J Clin Pharmacol Ther Toxicol. 1991 Jan;29(1):23–37. [PubMed] [Google Scholar]
- Sano M., Bell K., Marder K., Stricks L., Stern Y., Mayeux R. Safety and efficacy of oral physostigmine in the treatment of Alzheimer disease. Clin Neuropharmacol. 1993 Feb;16(1):61–69. doi: 10.1097/00002826-199302000-00007. [DOI] [PubMed] [Google Scholar]
- Somani S. M., Dube S. N. Physostigmine--an overview as pretreatment drug for organophosphate intoxication. Int J Clin Pharmacol Ther Toxicol. 1989 Aug;27(8):367–387. [PubMed] [Google Scholar]
- Somani S. M. Pharmacokinetics and pharmacodynamics of physostigmine in the rat after oral administration. Biopharm Drug Dispos. 1989 Mar-Apr;10(2):187–203. doi: 10.1002/bdd.2510100208. [DOI] [PubMed] [Google Scholar]
- WAGNER J. G., NELSON E. KINETIC ANALYSIS OF BLOOD LEVELS AND URINARY EXCRETION IN THE ABSORPTIVE PHASE AFTER SINGLE DOSES OF DRUG. J Pharm Sci. 1964 Nov;53:1392–1403. doi: 10.1002/jps.2600531126. [DOI] [PubMed] [Google Scholar]
- Whelpton R., Hurst P. Bioavailability of oral physostigmine. N Engl J Med. 1985 Nov 14;313(20):1293–1294. doi: 10.1056/NEJM198511143132016. [DOI] [PubMed] [Google Scholar]
- Williams F. M. Serum enzymes of drug metabolism. Pharmacol Ther. 1987;34(1):99–109. doi: 10.1016/0163-7258(87)90094-5. [DOI] [PubMed] [Google Scholar]


