Abstract
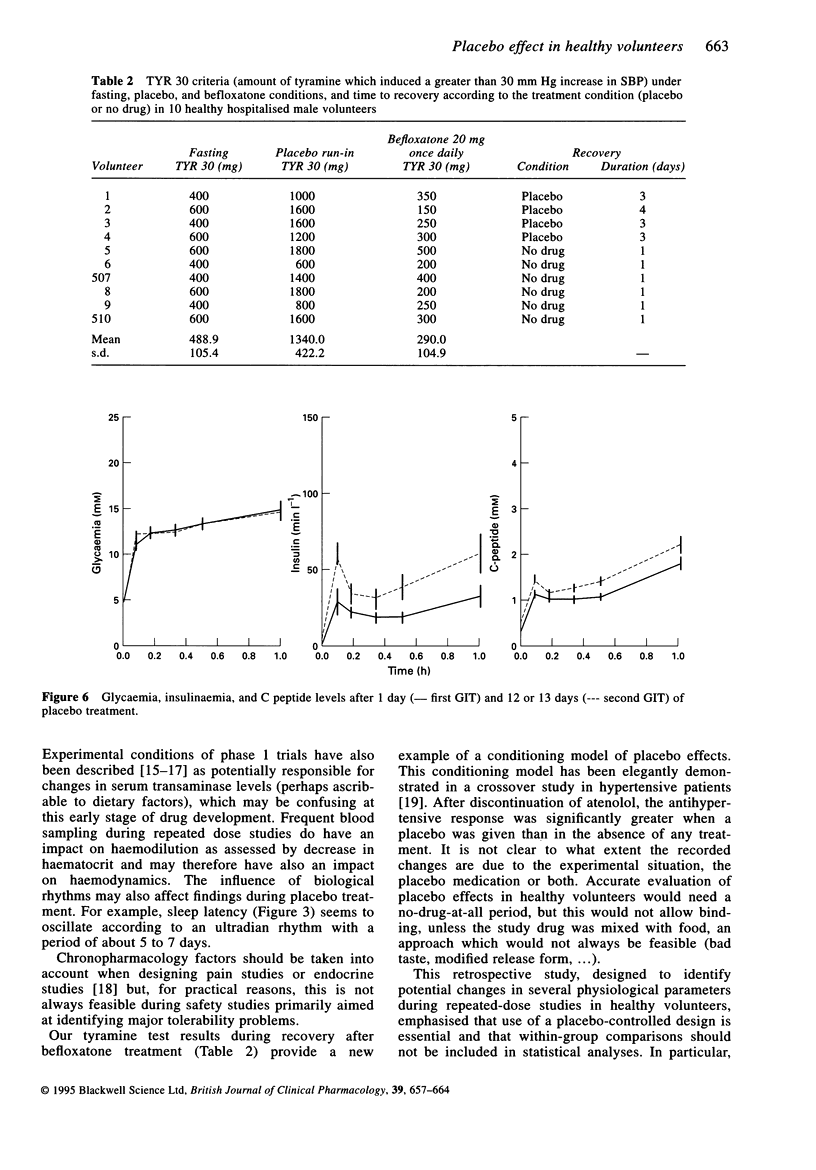
1. Although placebo administration is now commonly used as a control condition during clinical pharmacology studies conducted in healthy volunteers, data in placebo-treated subjects usually receive little attention. 2. The profile of several physiological and hormonal parameters was reviewed during the placebo sessions of five double-blind phase I studies involving hospitalisation of healthy volunteers for 2 weeks or more. 3. A clear trend towards an increase in heart rate, which culminated at about 10 beats min-1 at the end of the placebo period was observed, whereas both systolic and diastolic blood pressures remained globally unchanged. 4. Increases in the time to sleep initiation or in asthenia self-ratings during the placebo sessions suggested poor neuropsychiatric tolerability of experimental conditions in some of the studies. 5. In conclusion, all these data confirm that subjects change during the course of these studies. This is an important reason for conducting phase I studies under double-blind placebo-controlled conditions and to refrain from within-group comparisons (vs baseline, first-day effect).
Full text
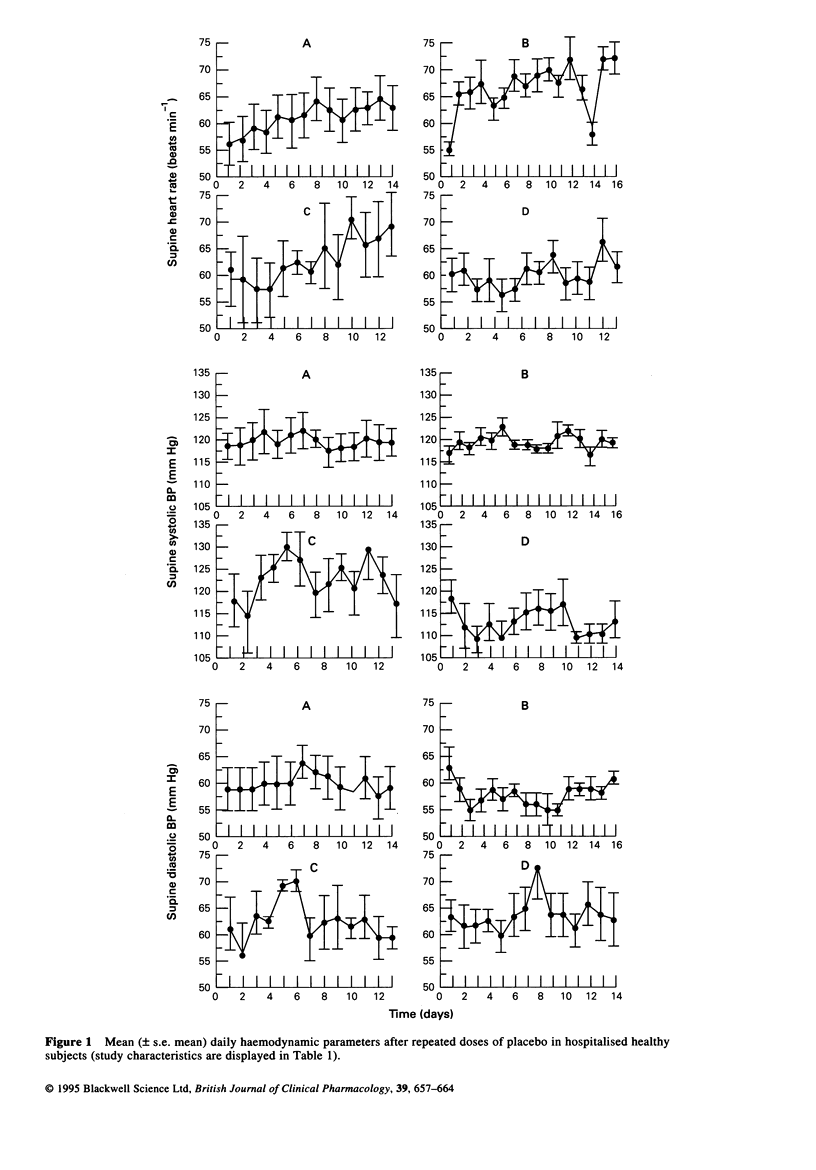
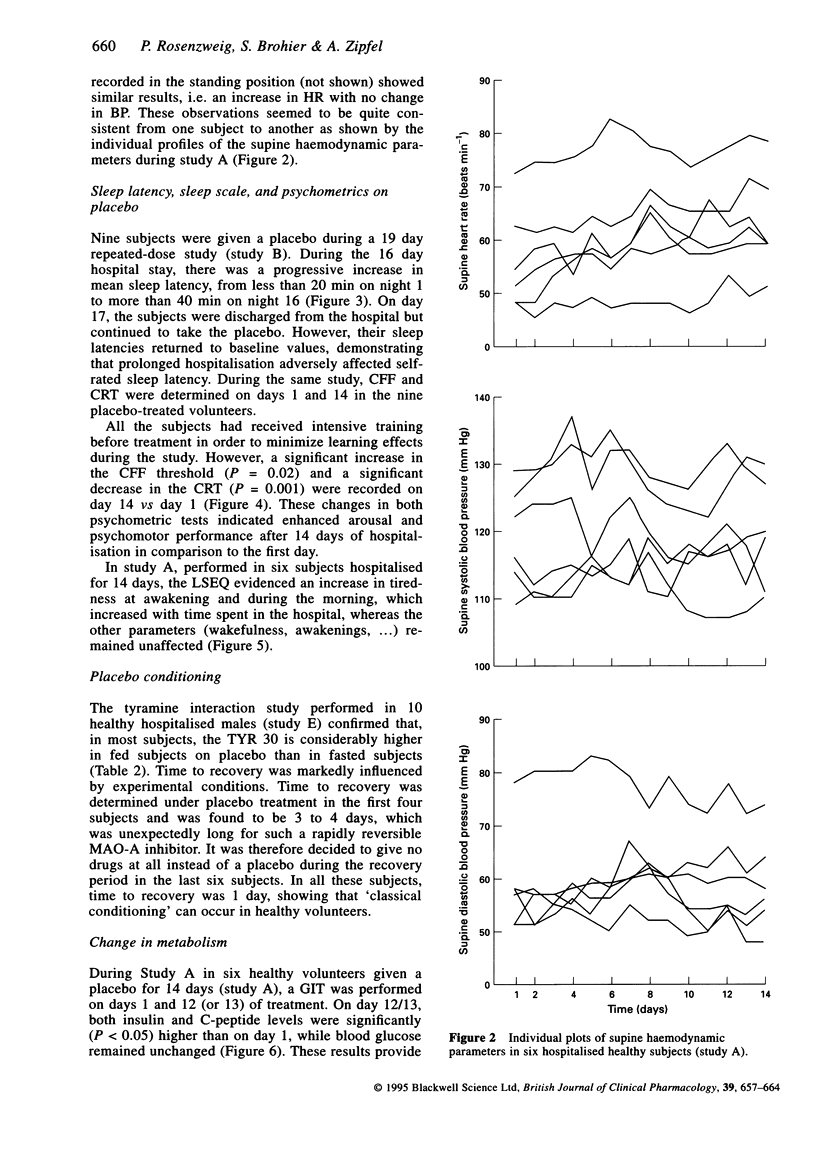
PDF

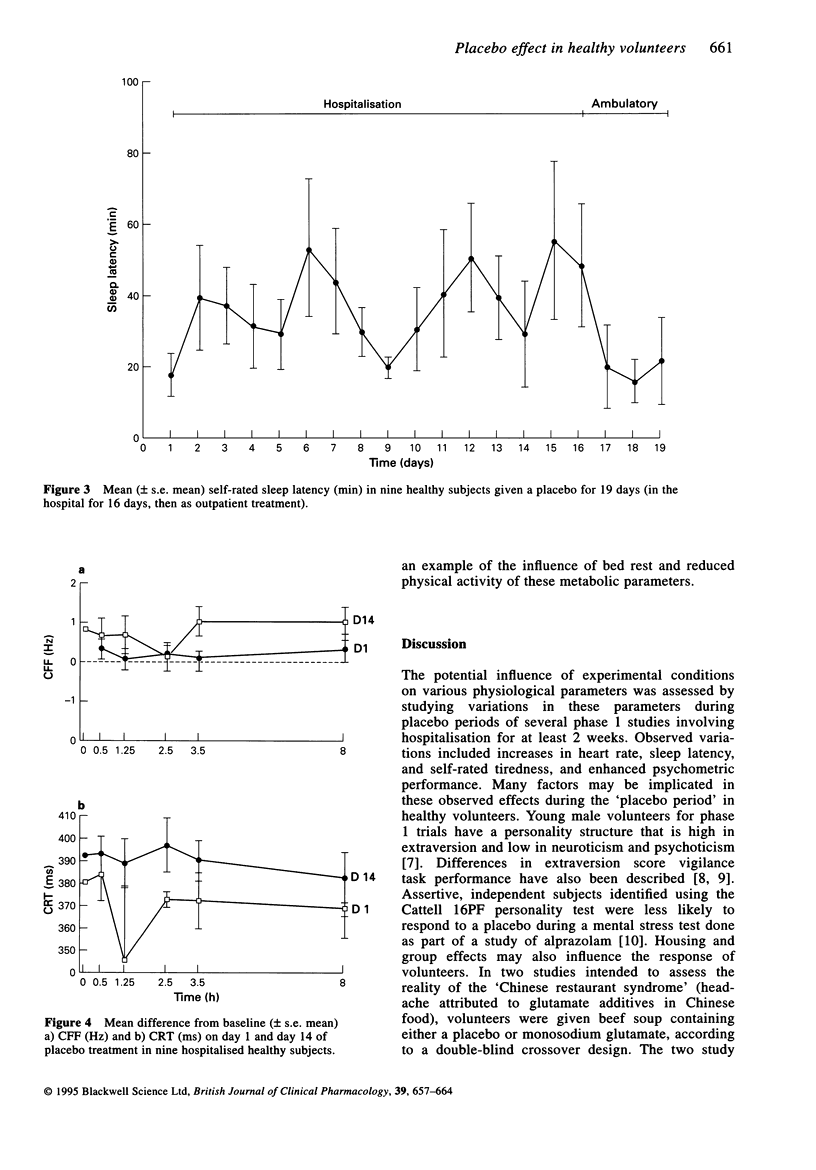
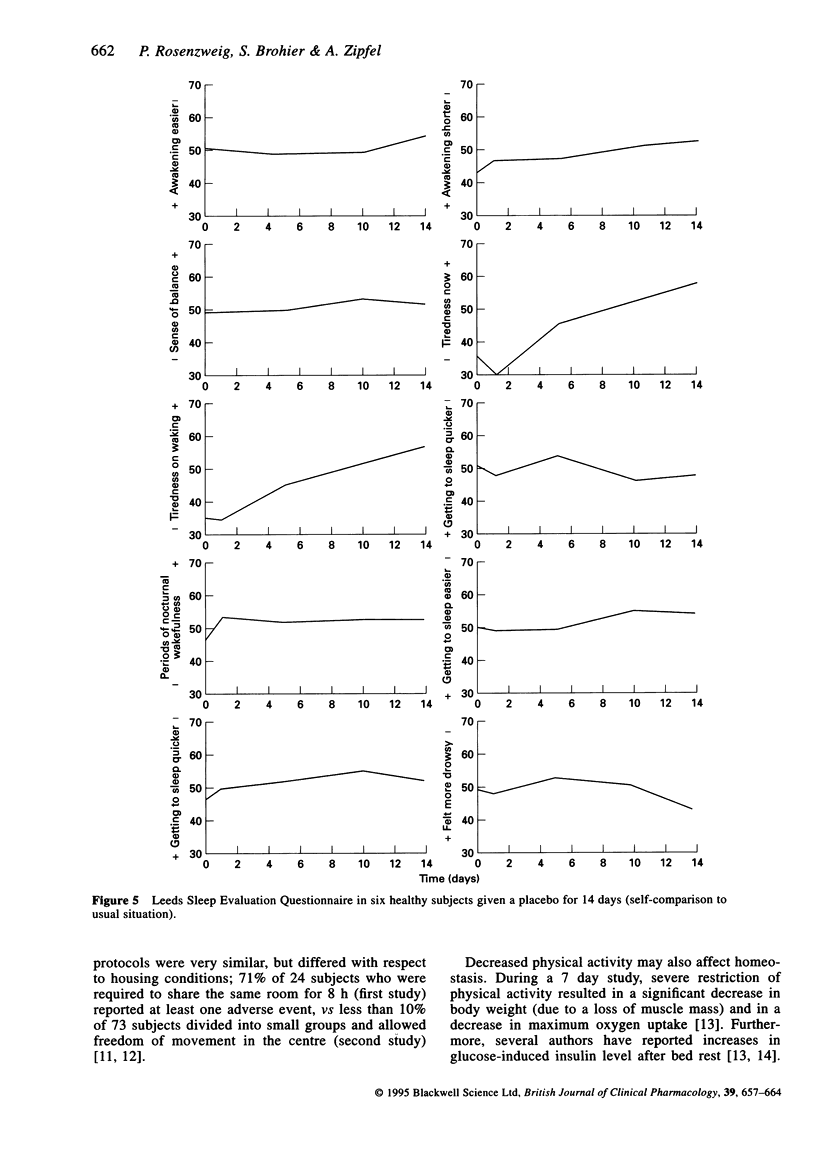

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball C. J., McLaren P. M., Morrison P. J. The personality structure of 'normal' volunteers. Br J Clin Pharmacol. 1993 Oct;36(4):369–371. doi: 10.1111/j.1365-2125.1993.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. B. Extraversion and variety-seeking in a monotonous task. Br J Psychol. 1975 Feb;66(1):9–13. doi: 10.1111/j.2044-8295.1975.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Hindmarch I., Gudgeon A. C. The effects of clobazam and lorazepam on aspects of psychomotor performance and car handling ability. Br J Clin Pharmacol. 1980 Aug;10(2):145–150. doi: 10.1111/j.1365-2125.1980.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I. Psychomotor function and psychoactive drugs. Br J Clin Pharmacol. 1980 Sep;10(3):189–209. doi: 10.1111/j.1365-2125.1980.tb01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. L., Raskin P., Love T., Triebwasser J., Lecocq F. R., Schnure J. J. Glucose intolerance during decreased physical activity in man. Diabetes. 1972 Feb;21(2):101–107. doi: 10.2337/diab.21.2.101. [DOI] [PubMed] [Google Scholar]
- McCann C. C., Goldfarb B., Frisk M., Quera-Salva M. A., Meyer P. The role of personality factors and suggestion in placebo effect during mental stress test. Br J Clin Pharmacol. 1992 Jan;33(1):107–110. doi: 10.1111/j.1365-2125.1992.tb04008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikines K. J., Dela F., Tronier B., Galbo H. Effect of 7 days of bed rest on dose-response relation between plasma glucose and insulin secretion. Am J Physiol. 1989 Jul;257(1 Pt 1):E43–E48. doi: 10.1152/ajpendo.1989.257.1.E43. [DOI] [PubMed] [Google Scholar]
- Morselli P. L., Garattini S. Monosodium glutamate and the Chinese restaurant syndrome. Nature. 1970 Aug 8;227(5258):611–612. doi: 10.1038/227611a0. [DOI] [PubMed] [Google Scholar]
- Parrott A. C., Hindmarch I. Factor analysis of a sleep evaluation questionnaire. Psychol Med. 1978 May;8(2):325–329. doi: 10.1017/s0033291700014379. [DOI] [PubMed] [Google Scholar]
- Porikos K. P., Van Itallie T. B. Diet-induced changes in serum transaminase and triglyceride levels in healthy adult men. Role of sucrose and excess calories. Am J Med. 1983 Oct;75(4):624–630. doi: 10.1016/0002-9343(83)90444-8. [DOI] [PubMed] [Google Scholar]
- Pöllmann L. Circadian changes in the duration of local anaesthesia. Int J Oral Surg. 1982 Feb;11(1):36–39. doi: 10.1016/s0300-9785(82)80046-x. [DOI] [PubMed] [Google Scholar]
- Rosenzweig P., Brohier S., Zipfel A. The placebo effect in healthy volunteers: influence of experimental conditions on the adverse events profile during phase I studies. Clin Pharmacol Ther. 1993 Nov;54(5):578–583. doi: 10.1038/clpt.1993.190. [DOI] [PubMed] [Google Scholar]
- Suchman A. L., Ader R. Classic conditioning and placebo effects in crossover studies. Clin Pharmacol Ther. 1992 Oct;52(4):372–377. doi: 10.1038/clpt.1992.157. [DOI] [PubMed] [Google Scholar]


