Abstract
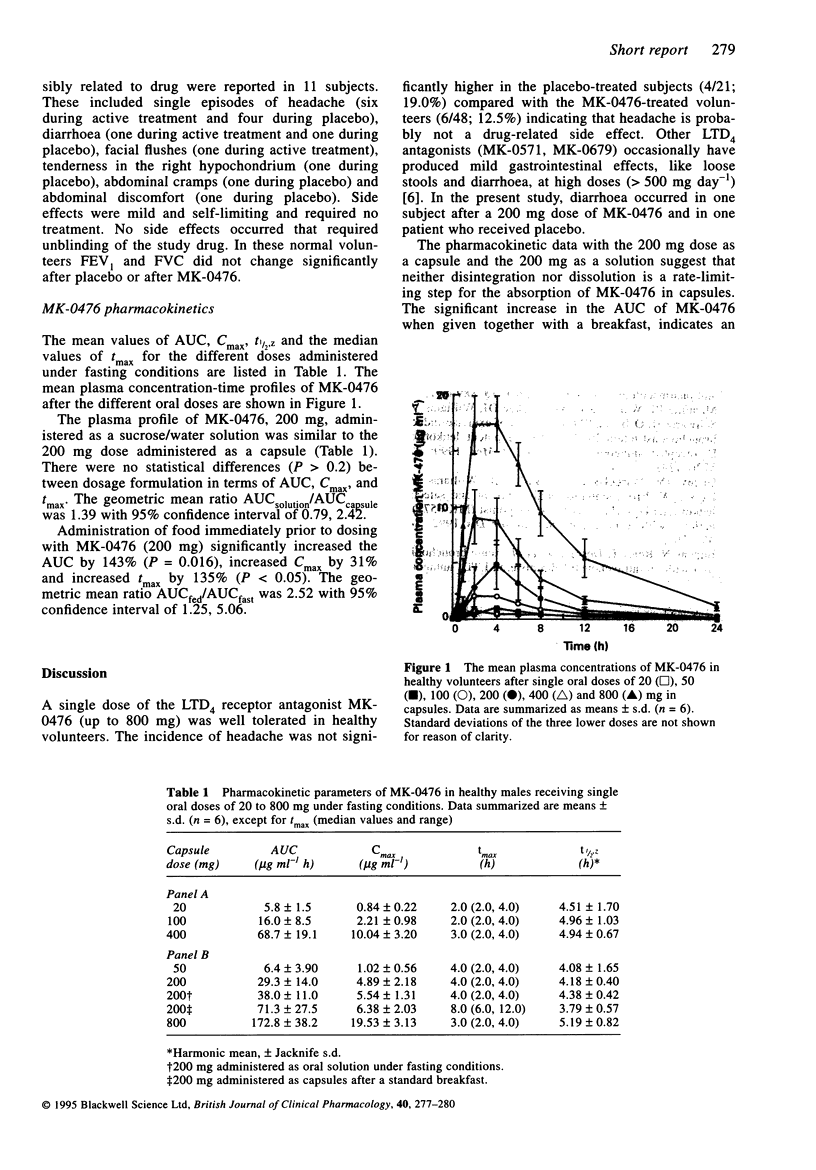
MK-4076 or sodium 1-(1(R)-(3-(2-(7-chloro-2-quinolinyl)-(E)- ethenyl)phenyl) 3-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl) cycloproprane) acetate is a novel, potent, and specific LTD4-receptor antagonist. The safety, tolerability and plasma drug profiles of single oral doses of MK-0476 (capsules) were evaluated in 18 healthy male volunteers assigned to one of the two parallel 9-subject panels. Under fasting conditions, increasing single doses of 20 to 800 mg were administered in a first part of the study and in a second part, 200 mg MK-0476 was given either as a solution, under fasting conditions, or as capsules, after a standard breakfast. All volunteers completed the study. Side effects, reported by the investigator to be related to study drug, were mild and transient. No laboratory abnormalities were noted. In the evaluated dose range of MK-0476 (20 to 800 mg) the median value of tmax ranged from 2 to 4 h, while the apparent t1/2 value averaged 4 to 5 h. The median tmax value of the 200 mg capsule dose was not significantly different from the median tmax of the 200 mg oral solution dose indicating that neither disintegration nor dissolution is a rate-limiting step for the absorption of MK-0476 from capsules. There was a statistically significant increase in the AUC (geometric mean ratio of fed/fast was 2.52 with 95% confidence interval of 1.25, 5.06) and in Cmax (geometric mean ratio of fed/fast was 1.36 with 95% confidence interval of 0.60, 3.04) when MK-0476 was given together with a breakfast, suggesting an increase in bioavailability.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahlén S. E., Hedqvist P., Hammarström S., Samuelsson B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980 Dec 4;288(5790):484–486. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- Gaddy J. N., Margolskee D. J., Bush R. K., Williams V. C., Busse W. W. Bronchodilation with a potent and selective leukotriene D4 (LTD4) receptor antagonist (MK-571) in patients with asthma. Am Rev Respir Dis. 1992 Aug;146(2):358–363. doi: 10.1164/ajrccm/146.2.358. [DOI] [PubMed] [Google Scholar]
- Impens N., Reiss T. F., Teahan J. A., Desmet M., Rossing T. H., Shingo S., Zhang J., Schandevyl W., Verbesselt R., Dupont A. G. Acute bronchodilation with an intravenously administered leukotriene D4 antagonist, MK-679. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1442–1446. doi: 10.1164/ajrccm/147.6_Pt_1.1442. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. J., Watson R. M., Margolskee D. J., Williams V. C., Schwartz J. I., O'Byrne P. M. Inhibition of exercise-induced bronchoconstriction by MK-571, a potent leukotriene D4-receptor antagonist. N Engl J Med. 1990 Dec 20;323(25):1736–1739. doi: 10.1056/NEJM199012203232504. [DOI] [PubMed] [Google Scholar]
- Olanoff L. S., Walle T., Cowart T. D., Walle U. K., Oexmann M. J., Conradi E. C. Food effects on propranolol systemic and oral clearance: support for a blood flow hypothesis. Clin Pharmacol Ther. 1986 Oct;40(4):408–414. doi: 10.1038/clpt.1986.198. [DOI] [PubMed] [Google Scholar]
- Rocci M. L., Jr, Jusko W. J. LAGRAN program for area and moments in pharmacokinetic analysis. Comput Programs Biomed. 1983 Jun;16(3):203–216. doi: 10.1016/0010-468x(83)90082-x. [DOI] [PubMed] [Google Scholar]


