Abstract
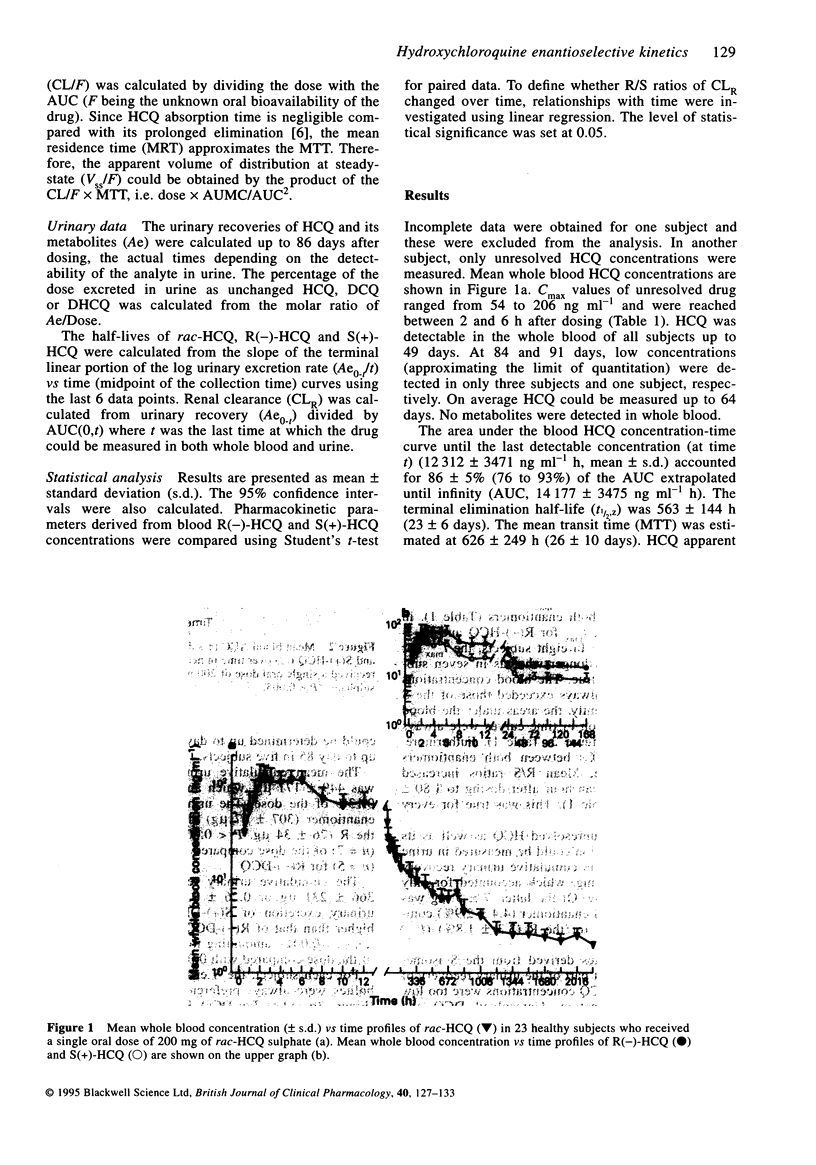
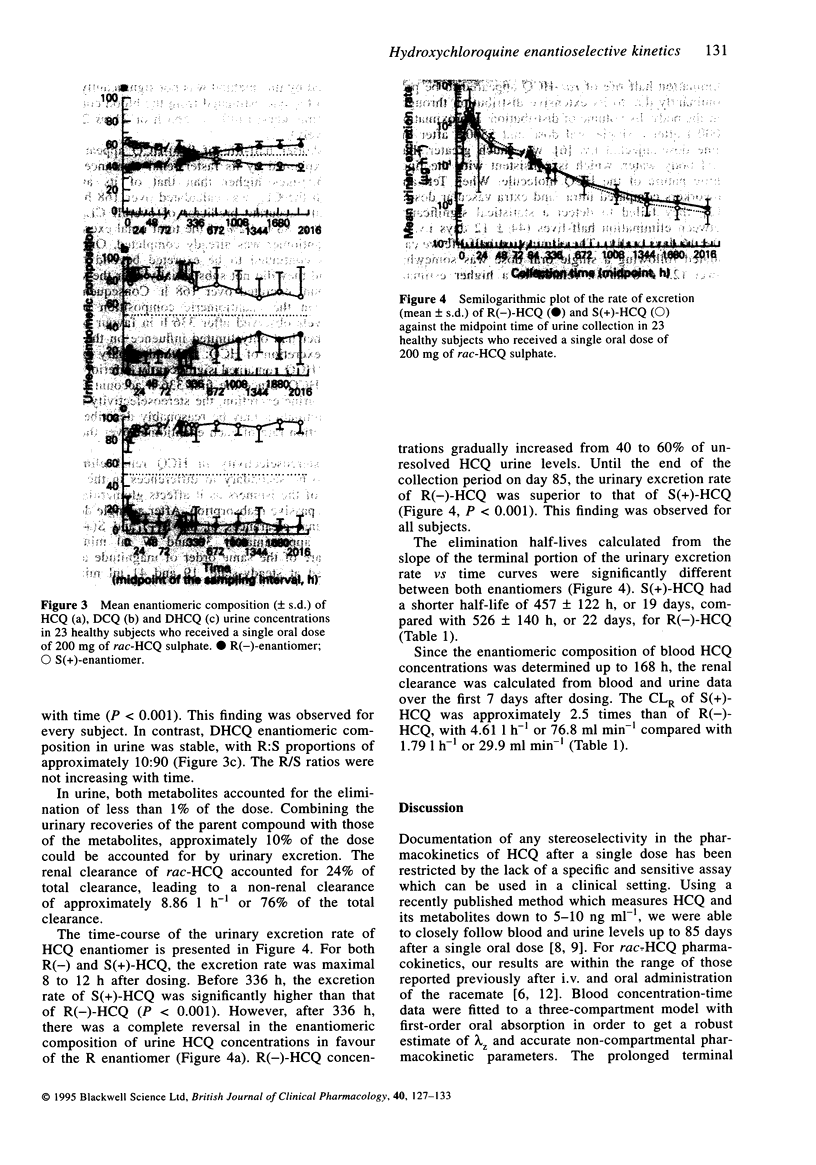
1. Stereoselectivity in the disposition of hydroxychloroquine was investigated in 23 healthy males following a single oral dose of 200 mg racemic HCQ (rac-HCQ) sulphate. Total concentrations (R+S) and R/S ratios of HCQ and its metabolites were measured by stereoselective h.p.l.c. 2. HCQ was detected in whole blood and urine, up to 91 and 85 days after dosing, respectively. Metabolites could not be detected in whole blood while in urine detectable concentrations were still present after 85 days. The blood concentrations of HCQ enantiomers were measurable until 168 h post-dose. 3. R(-)-HCQ accounted for 62 +/- 3% (mean +/- s.d.) of the AUC of rac-HCQ AUC. The elimination half-life of S(+)-HCQ (457 +/- 122 h) was significantly shorter than that of R(-)-HCQ (526 +/- 140 h), partly due to its faster urinary excretion and hepatic metabolism. Its renal clearance was twice that of R(-)-HCQ (4.61 +/- 4.01 vs 1.79 +/- 1.30 1 h-1), and metabolites derived from the S-isomer represented 80-90% of the urinary recovery of the dose. 4. Over 85 days, 4.4 +/- 2.9 and 3.3 +/- 1.8% of the dose was recovered in urine as unchanged S(+)-HCQ and R(-)-HCQ, respectively. For the first 2 weeks, S(+)-HCQ excretion rate clearly surpassed that of R(-)-HCQ whereas afterwards the inverse was observed. However, since the first 2 weeks account for 95% of rac-HCQ renal excretion, the total urinary excretion of S(+)-HCQ clearly surpassed that of R(-)-HCQ.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ducharme J., Wainer I. W., Parenteau H. I., Rodman J. H. Stereoselective distribution of hydroxychloroquine in the rabbit following single and multiple oral doses of the racemate and the separate enantiomers. Chirality. 1994;6(4):337–346. doi: 10.1002/chir.530060418. [DOI] [PubMed] [Google Scholar]
- Fieger H., Iredale J., Wainer I. W. Enantioselective determination of hydroxychloroquine and its major metabolites in urine and the observation of a reversal in the (+)/(-)-hydroxychloroquine ratio. Chirality. 1993;5(2):65–70. doi: 10.1002/chir.530050205. [DOI] [PubMed] [Google Scholar]
- García-Cases C., Duque A., Borja J., Izquierdo I., de la Fuente V., Torrent J., Jané F. Evaluation of the methodological quality of clinical trial protocols. A preliminary experience in Spain. Eur J Clin Pharmacol. 1993;44(4):401–402. doi: 10.1007/BF00316482. [DOI] [PubMed] [Google Scholar]
- Iredale J., Wainer I. W. Determination of hydroxychloroquine and its major metabolites in plasma using sequential achiral-chiral high-performance liquid chromatography. J Chromatogr. 1992 Jan 17;573(2):253–258. doi: 10.1016/0378-4347(92)80126-b. [DOI] [PubMed] [Google Scholar]
- McChesney E. W. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983 Jul 18;75(1A):11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- McLachlan A. J., Tett S. E., Cutler D. J., Day R. O. Disposition and absorption of hydroxychloroquine enantiomers following a single dose of the racemate. Chirality. 1994;6(4):360–364. doi: 10.1002/chir.530060421. [DOI] [PubMed] [Google Scholar]
- McLachlan A. J., Tett S. E., Cutler D. J., Day R. O. Disposition of the enantiomers of hydroxychloroquine in patients with rheumatoid arthritis following multiple doses of the racemate. Br J Clin Pharmacol. 1993 Jul;36(1):78–81. doi: 10.1111/j.1365-2125.1993.tb05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar T. Disease modifying drugs for rheumatoid arthritis: yesterday's treatment today or today's treatment tomorrow? Br J Clin Pharmacol. 1990 Oct;30(4):501–510. doi: 10.1111/j.1365-2125.1990.tb03807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Day R. O., Brown K. F. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988 Sep;26(3):303–313. doi: 10.1111/j.1365-2125.1988.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett S. E., Cutler D. J., Day R. O., Brown K. F. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989 Jun;27(6):771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett S. E., Day R. O., Cutler D. J. Concentration-effect relationship of hydroxychloroquine in rheumatoid arthritis--a cross sectional study. J Rheumatol. 1993 Nov;20(11):1874–1879. [PubMed] [Google Scholar]
- Tett S. E., McLachlan A. J., Cutler D. J., Day R. O. Pharmacokinetics and pharmacodynamics of hydroxychloroquine enantiomers in patients with rheumatoid arthritis receiving multiple doses of racemate. Chirality. 1994;6(4):355–359. doi: 10.1002/chir.530060420. [DOI] [PubMed] [Google Scholar]
- Wainer I. W., Chen J. C., Parenteau H., Abdullah S., Ducharme J., Fieger H., Iredale J. Distribution of the enantiomers of hydroxychloroquine and its metabolites in ocular tissues of the rabbit after oral administration of racemic-hydroxychloroquine. Chirality. 1994;6(4):347–354. doi: 10.1002/chir.530060419. [DOI] [PubMed] [Google Scholar]


