Abstract
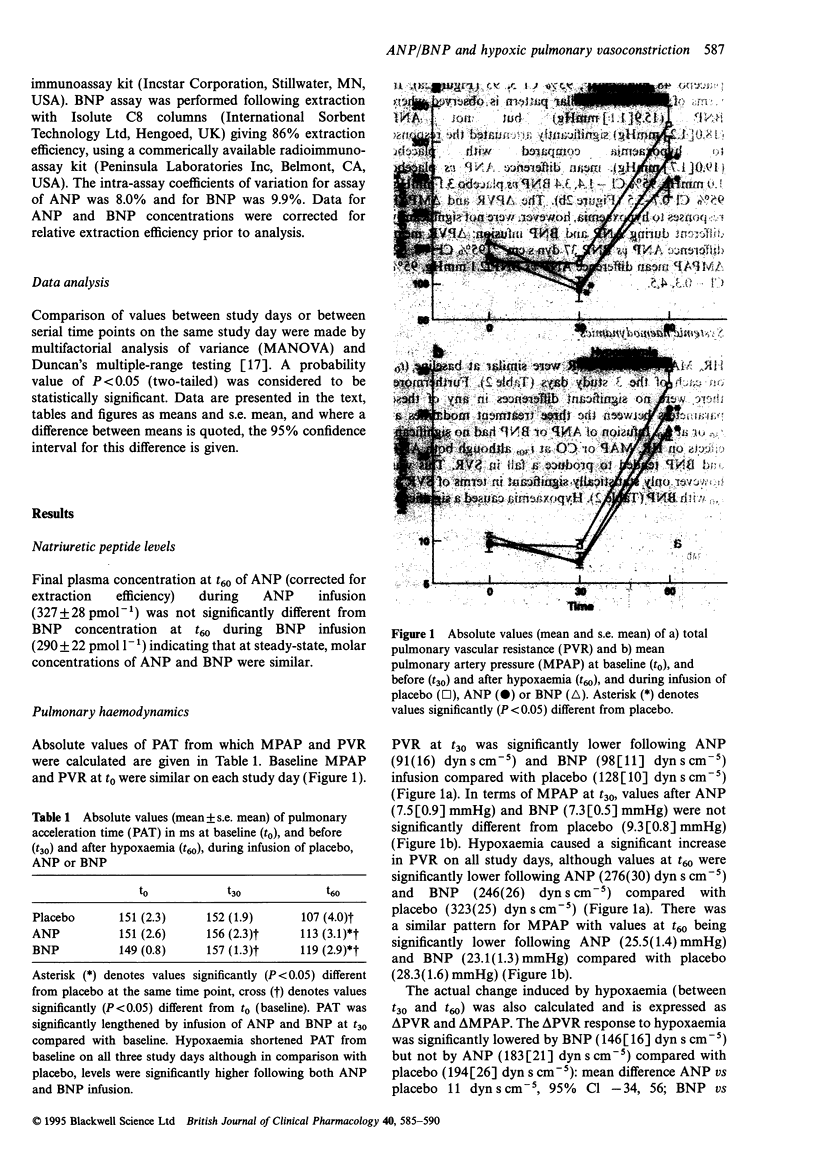
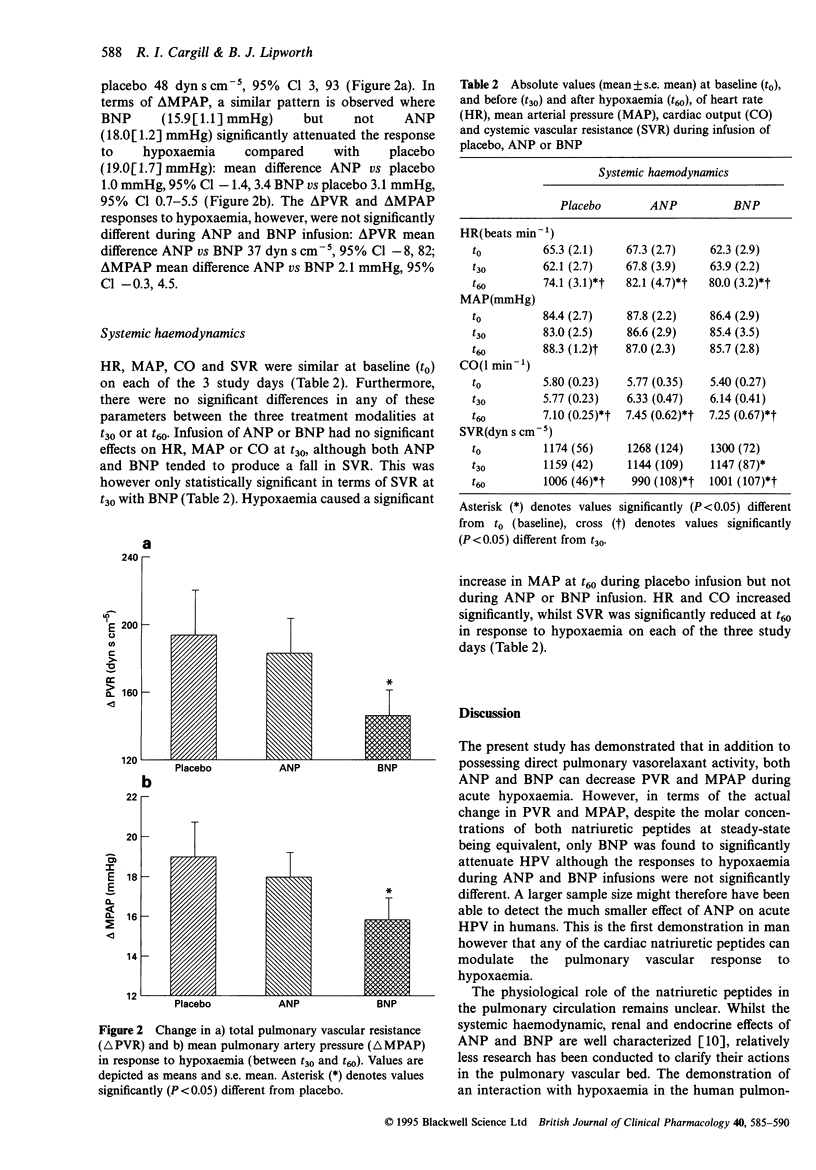
1. Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) have pulmonary vasorelaxant activity with plasma concentrations being elevated in patients with hypoxaemic pulmonary hypertension. However, their effects on acute hypoxic pulmonary vasoconstriction (HPV), the initiating stimulus for pulmonary hypertension have not to date been investigated. We have therefore studied the effects of ANP and BNP on acute HPV in humans. 2. Eight healthy volunteers were studied on three separate occasions. After reaching a resting haemodynamic state (t0), an infusion of either ANP (10 pmol kg-1 min-1), BNP (10 pmol kg-1 min-1) or placebo (5% dextrose) was commenced. This was given alone for 30 min (t30) before subjects were rendered hypoxaemic (SaO2 75-80%) for a further 30 min (t60), with the initial infusion continuing to t60. Pulsed-wave Doppler analysis of pulmonary artery flow was used to measure mean pulmonary arterial pressure (MPAP) and hence total pulmonary vascular resistance (PVR) was calculated. 3. MPAP and PVR both tended to decrease in response to ANP and BNP infusion, although compared with placebo, the difference at t30 was only statistically significant for PVR. Hypoxaemia increased MPAP and PVR, although values at t60 were significantly lower following both ANP and BNP compared with placebo. 4. In terms of the actual change in PVR (delta PVR) induced by hypoxaemia (from t30 to t60), BNP (146(16) dyn s cm-5), but not ANP (183(21) dyn s cm-5) significantly attenuated delta PVR compared with placebo (194(26) dyns s cm-5): mean difference BNP versus placebo 48 dyn s cm-5, 95% Cl 3-93. An identical pattern was observed for delta MPAP where BNP (15.9(1.1) mmHg), but not ANP (18.0(1.2) mmHg) significantly attenuated delta MPAP compared with placebo (19.0(1.7) mmHg): mean difference BNP versus placebo 3.1 mmHg, 95% Cl 0.7-5.5. 5. Thus, although both ANP and BNP exhibit pulmonary vasorelaxant activity, only BNP significantly attenuated the MPAP and PVR responses to acute hypoxaemia. This suggests that the natriuretic peptides may have a role in attenuating pulmonary hypertension secondary to hypoxaemia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adnot S., Andrivet P., Chabrier P. E., Piquet J., Plas P., Braquet P., Roudot-Thoraval F., Brun-Buisson C. Atrial natriuretic factor in chronic obstructive lung disease with pulmonary hypertension. Physiological correlates and response to peptide infusion. J Clin Invest. 1989 Mar;83(3):986–993. doi: 10.1172/JCI113985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnot S., Chabrier P. E., Brun-Buisson C., Viossat I., Braquet P. Atrial natriuretic factor attenuates the pulmonary pressor response to hypoxia. J Appl Physiol (1985) 1988 Nov;65(5):1975–1983. doi: 10.1152/jappl.1988.65.5.1975. [DOI] [PubMed] [Google Scholar]
- Anderson J. V., Donckier J., McKenna W. J., Bloom S. R. The plasma release of atrial natriuretic peptide in man. Clin Sci (Lond) 1986 Aug;71(2):151–155. doi: 10.1042/cs0710151. [DOI] [PubMed] [Google Scholar]
- Beard J. T., 2nd, Newman J. H., Loyd J. E., Byrd B. F., 3rd Doppler estimation of changes in pulmonary artery pressure during hypoxic breathing. J Am Soc Echocardiogr. 1991 Mar-Apr;4(2):121–130. doi: 10.1016/s0894-7317(14)80523-3. [DOI] [PubMed] [Google Scholar]
- Cargill R. I., Coutie W. J., Lipworth B. J. The effects of angiotensin II on circulating levels of natriuretic peptides. Br J Clin Pharmacol. 1994 Aug;38(2):139–142. doi: 10.1111/j.1365-2125.1994.tb04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill R. I., Lipworth B. J. Pulmonary vasorelaxant activity of atrial natriuretic peptide and brain natriuretic peptide in humans. Thorax. 1995 Feb;50(2):183–185. doi: 10.1136/thx.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T., Barrett P. Q., Rasmussen H. Ca channels in adrenal glomerulosa cells: K+ and angiotensin II increase T-type Ca channel current. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2412–2416. doi: 10.1073/pnas.85.7.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutaia M., Rounds S. Hypoxic pulmonary vasoconstriction. Physiologic significance, mechanism, and clinical relevance. Chest. 1990 Mar;97(3):706–718. doi: 10.1378/chest.97.3.706. [DOI] [PubMed] [Google Scholar]
- Dabestani A., Mahan G., Gardin J. M., Takenaka K., Burn C., Allfie A., Henry W. L. Evaluation of pulmonary artery pressure and resistance by pulsed Doppler echocardiography. Am J Cardiol. 1987 Mar 1;59(6):662–668. doi: 10.1016/0002-9149(87)91189-1. [DOI] [PubMed] [Google Scholar]
- Graettinger W. F., Greene E. R., Voyles W. F. Doppler predictions of pulmonary artery pressure, flow, and resistance in adults. Am Heart J. 1987 Jun;113(6):1426–1437. doi: 10.1016/0002-8703(87)90658-2. [DOI] [PubMed] [Google Scholar]
- Kawashima A., Kubo K., Hirai K., Yoshikawa S., Matsuzawa Y., Kobayashi T. Plasma levels of atrial natriuretic peptide under acute hypoxia in normal subjects. Respir Physiol. 1989 Apr;76(1):79–91. doi: 10.1016/0034-5687(89)90019-4. [DOI] [PubMed] [Google Scholar]
- Kitabatake A., Inoue M., Asao M., Masuyama T., Tanouchi J., Morita T., Mishima M., Uematsu M., Shimazu T., Hori M. Noninvasive evaluation of pulmonary hypertension by a pulsed Doppler technique. Circulation. 1983 Aug;68(2):302–309. doi: 10.1161/01.cir.68.2.302. [DOI] [PubMed] [Google Scholar]
- Klinger J. R., Hill N. S. Right ventricular dysfunction in chronic obstructive pulmonary disease. Evaluation and management. Chest. 1991 Mar;99(3):715–723. doi: 10.1378/chest.99.3.715. [DOI] [PubMed] [Google Scholar]
- Lang C. C., Choy A. M., Struthers A. D. Atrial and brain natriuretic peptides: a dual natriuretic peptide system potentially involved in circulatory homeostasis. Clin Sci (Lond) 1992 Nov;83(5):519–527. doi: 10.1042/cs0830519. [DOI] [PubMed] [Google Scholar]
- Lang C. C., Choy A. M., Turner K., Tobin R., Coutie W., Struthers A. D. The effect of intravenous saline loading on plasma levels of brain natriuretic peptide in man. J Hypertens. 1993 Jul;11(7):737–741. doi: 10.1097/00004872-199307000-00009. [DOI] [PubMed] [Google Scholar]
- Lang C. C., Coutie W. J., Struthers A. D., Dhillon D. P., Winter J. H., Lipworth B. J. Elevated levels of brain natriuretic peptide in acute hypoxaemic chronic obstructive pulmonary disease. Clin Sci (Lond) 1992 Nov;83(5):529–533. doi: 10.1042/cs0830529. [DOI] [PubMed] [Google Scholar]
- Lang C. C., Motwani J., Coutie W. J., Struthers A. D. Influence of candoxatril on plasma brain natriuretic peptide in heart failure. Lancet. 1991 Jul 27;338(8761):255–255. doi: 10.1016/0140-6736(91)90397-8. [DOI] [PubMed] [Google Scholar]
- Lipworth B. J., Dagg K. D. Comparative effects of angiotensin II on Doppler parameters of left and right heart systolic and diastolic blood flow. Br J Clin Pharmacol. 1994 Mar;37(3):273–278. doi: 10.1111/j.1365-2125.1994.tb04275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukoyama M., Nakao K., Hosoda K., Suga S., Saito Y., Ogawa Y., Shirakami G., Jougasaki M., Obata K., Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991 Apr;87(4):1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post J. M., Hume J. R., Archer S. L., Weir E. K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992 Apr;262(4 Pt 1):C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Sato K., Murakami K., Nishimura K., Ito K., Kangawa K., Matsuo H. Possible involvement of sodium pump in the relaxation of rat aorta induced by alpha-human atrial natriuretic polypeptide. Jpn J Physiol. 1988;38(2):187–198. doi: 10.2170/jjphysiol.38.187. [DOI] [PubMed] [Google Scholar]
- Stewart A. G., Sheedy W., Thompson J. S., Morice A. H. Effects of SCH 34826, a neutral endopeptidase inhibitor, on hypoxic pulmonary vascular remodelling. Pulm Pharmacol. 1992 Jun;5(2):111–114. doi: 10.1016/0952-0600(92)90027-e. [DOI] [PubMed] [Google Scholar]
- Suga S., Nakao K., Hosoda K., Mukoyama M., Ogawa Y., Shirakami G., Arai H., Saito Y., Kambayashi Y., Inouye K. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992 Jan;130(1):229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- Weitzenblum E., Loiseau A., Hirth C., Mirhom R., Rasaholinjanahary J. Course of pulmonary hemodynamics in patients with chronic obstructive pulmonary disease. Chest. 1979 Jun;75(6):656–662. doi: 10.1378/chest.75.6.656. [DOI] [PubMed] [Google Scholar]
- Zhao L., Winter R. J., Krausz T., Hughes J. M. Effects of continuous infusion of atrial natriuretic peptide on the pulmonary hypertension induced by chronic hypoxia in rats. Clin Sci (Lond) 1991 Sep;81(3):379–385. doi: 10.1042/cs0810379. [DOI] [PubMed] [Google Scholar]


