Abstract
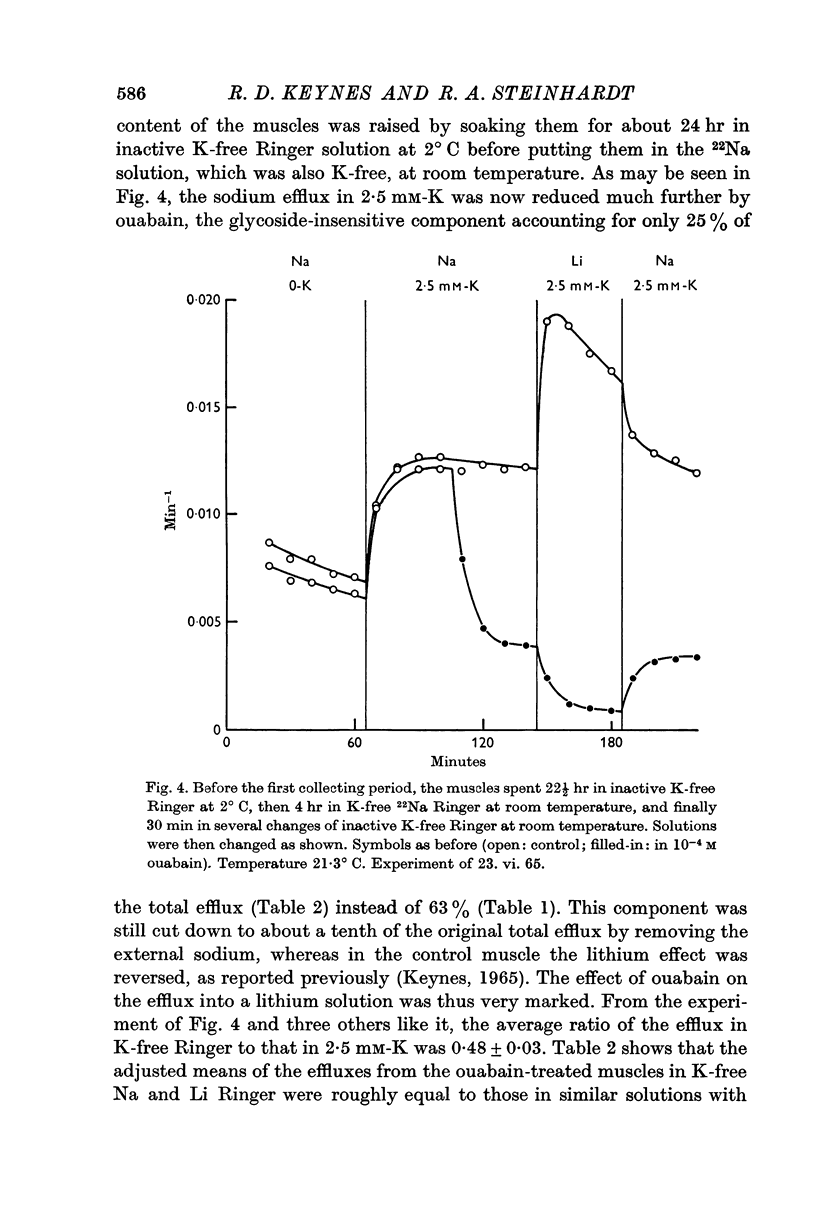
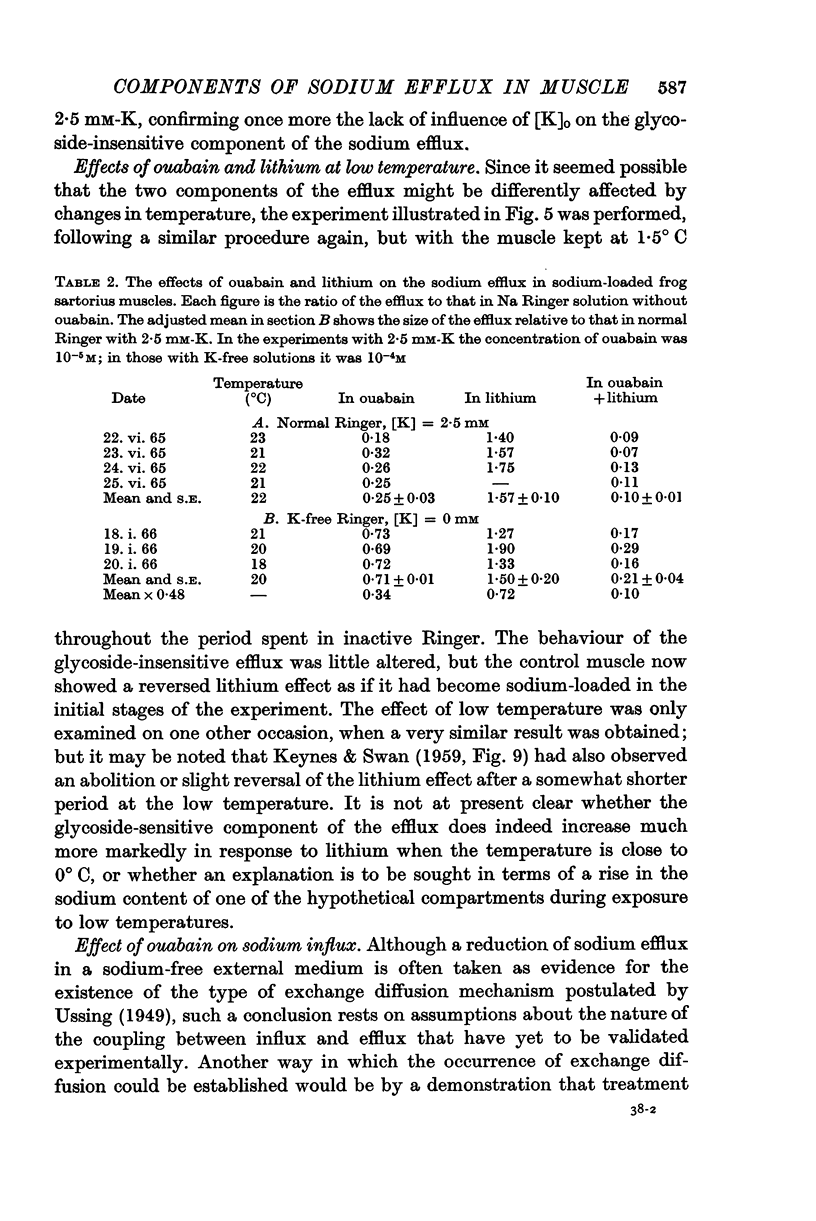
1. In normal Ringer solution containing 2·5 mM-K only 37% of the efflux of labelled sodium from a freshly dissected frog muscle is blocked by treatment with ouabain; in sodium-loaded muscles the ouabain-sensitive fraction of the efflux increases to 75%.
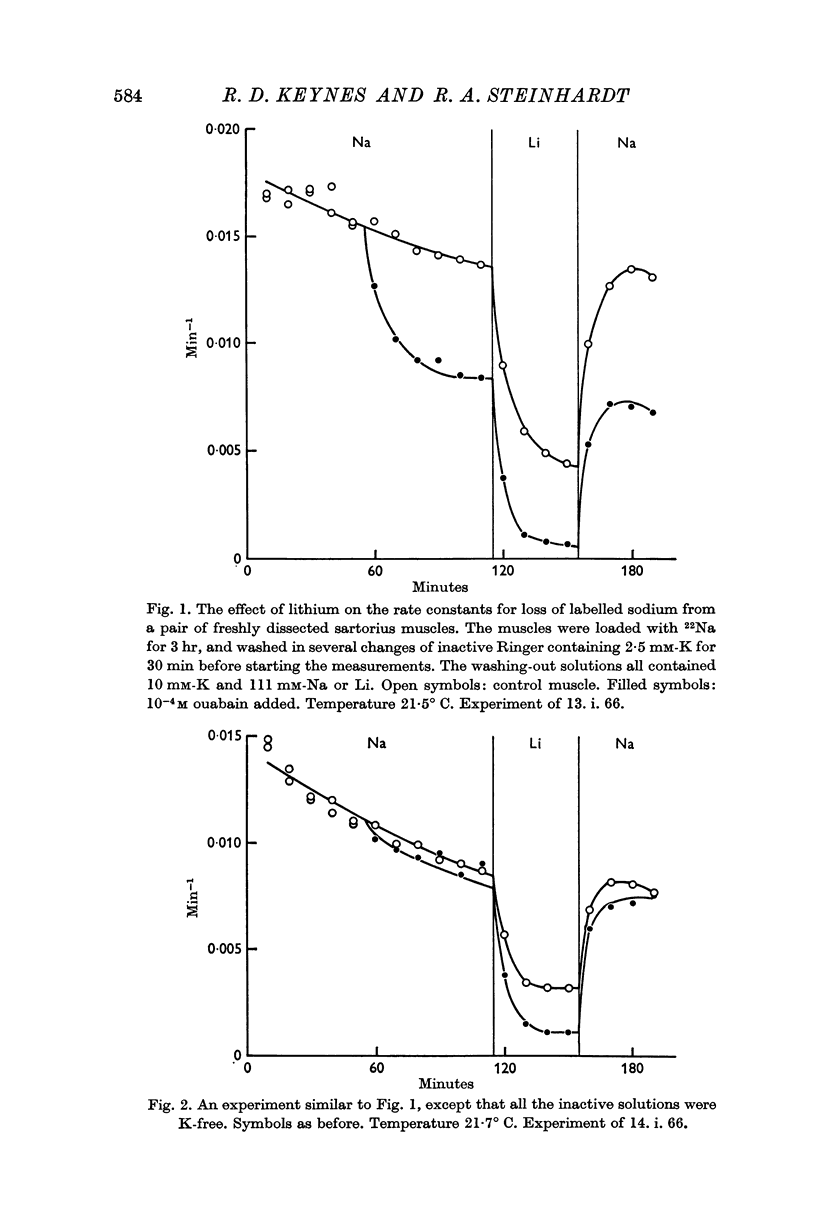
2. Under all conditions, the ouabain-insensitive component of the sodium efflux is markedly reduced if the sodium in the external medium is replaced by lithium; at least in sodium-loaded muscles, the ouabain-sensitive component is increased in lithium Ringer.
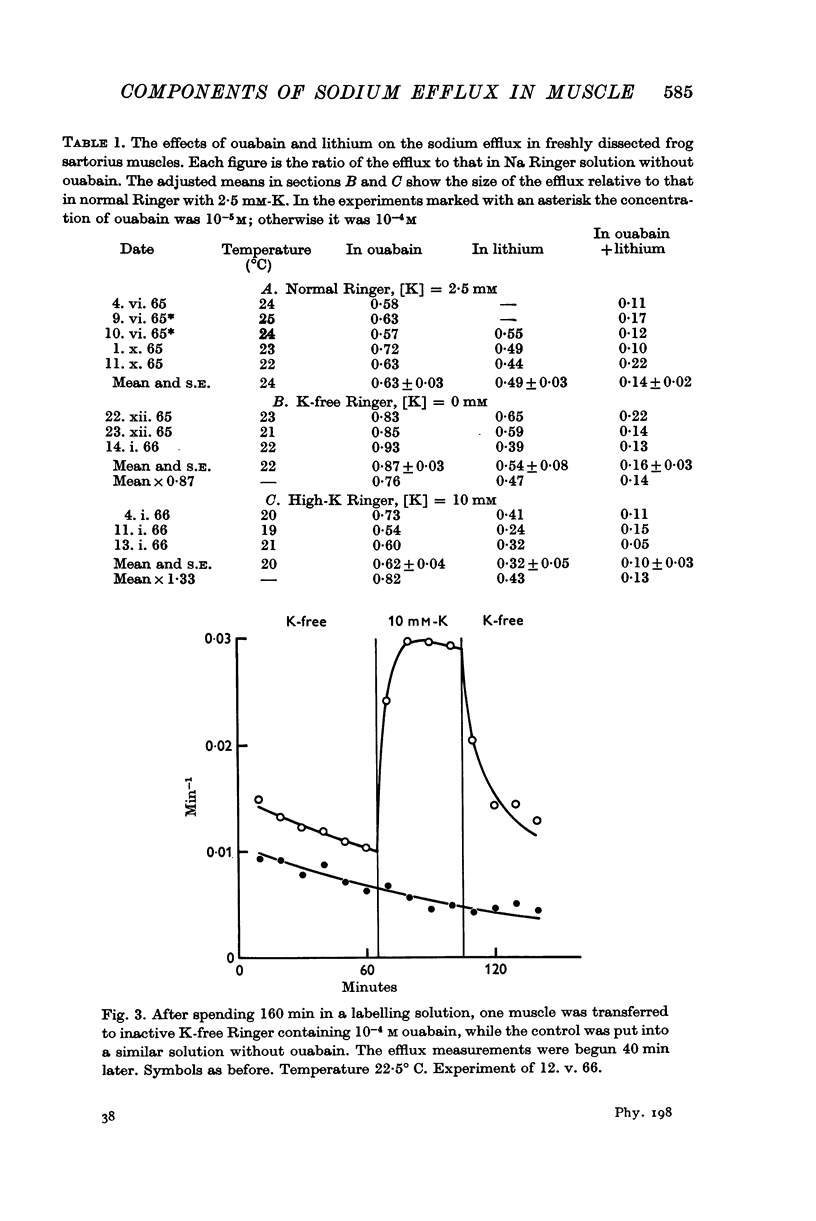
3. Only the ouabain-sensitive component of the efflux is affected by the external potassium concentration.
4. In the presence of 2·5 mM-K the sodium influx in a freshly dissected muscle is not significantly altered by ouabain, but in a K-free medium the influx and the efflux are both reduced by nearly 20%.
5. The sodium efflux can therefore be regarded as consisting of (1) a sodium-potassium coupled component that is blocked by ouabain and involves a sodium-sodium exchange in the absence of external potassium, and (2) a potassium-insensitive component that is unaffected by ouabain and tends to reach saturation at relatively lower internal sodium concentrations.
6. The evidence is considered for attributing component (1) to an efflux of sodium from the sarcoplasm proper, and component (2) to an efflux from the sarcoplasmic reticulum. Although such an interpretation is consistent with many of the observations, a definite identification of the possible sodium compartments in frog muscle cannot yet be made.
Full text
PDF


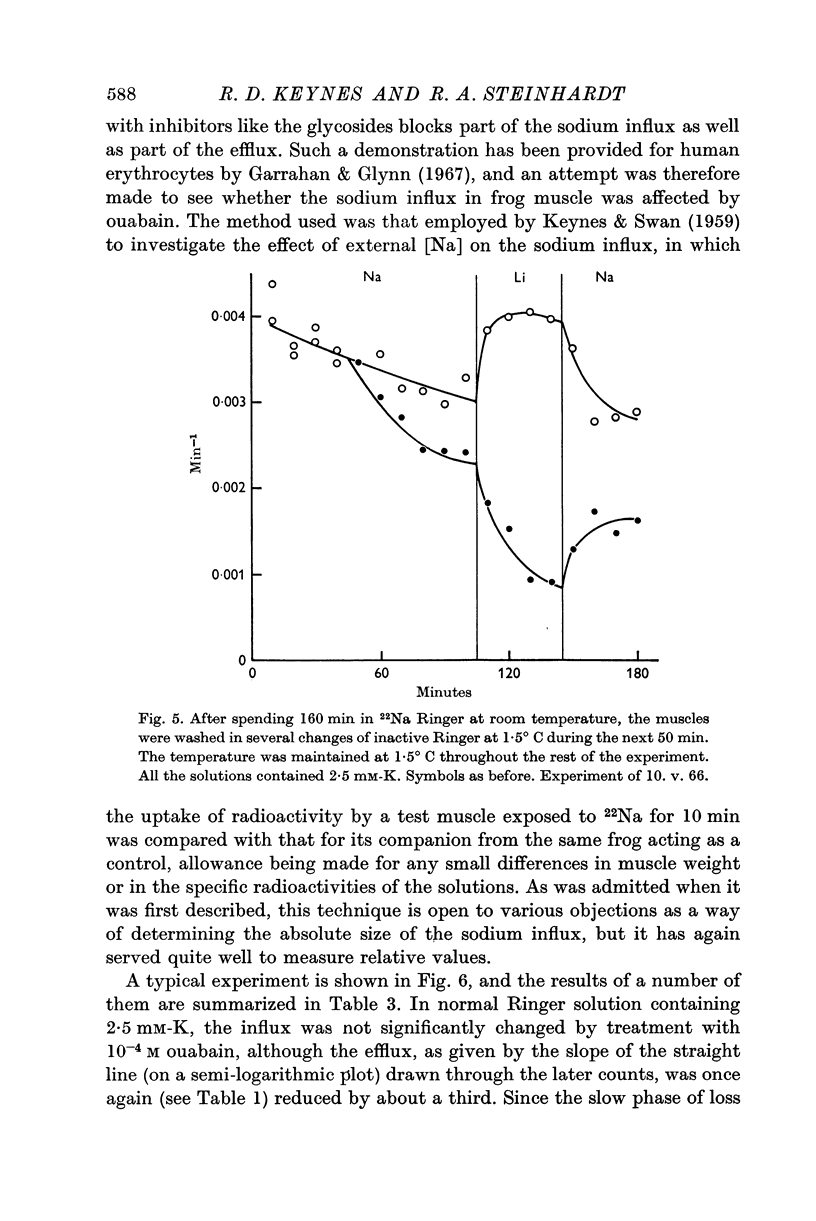
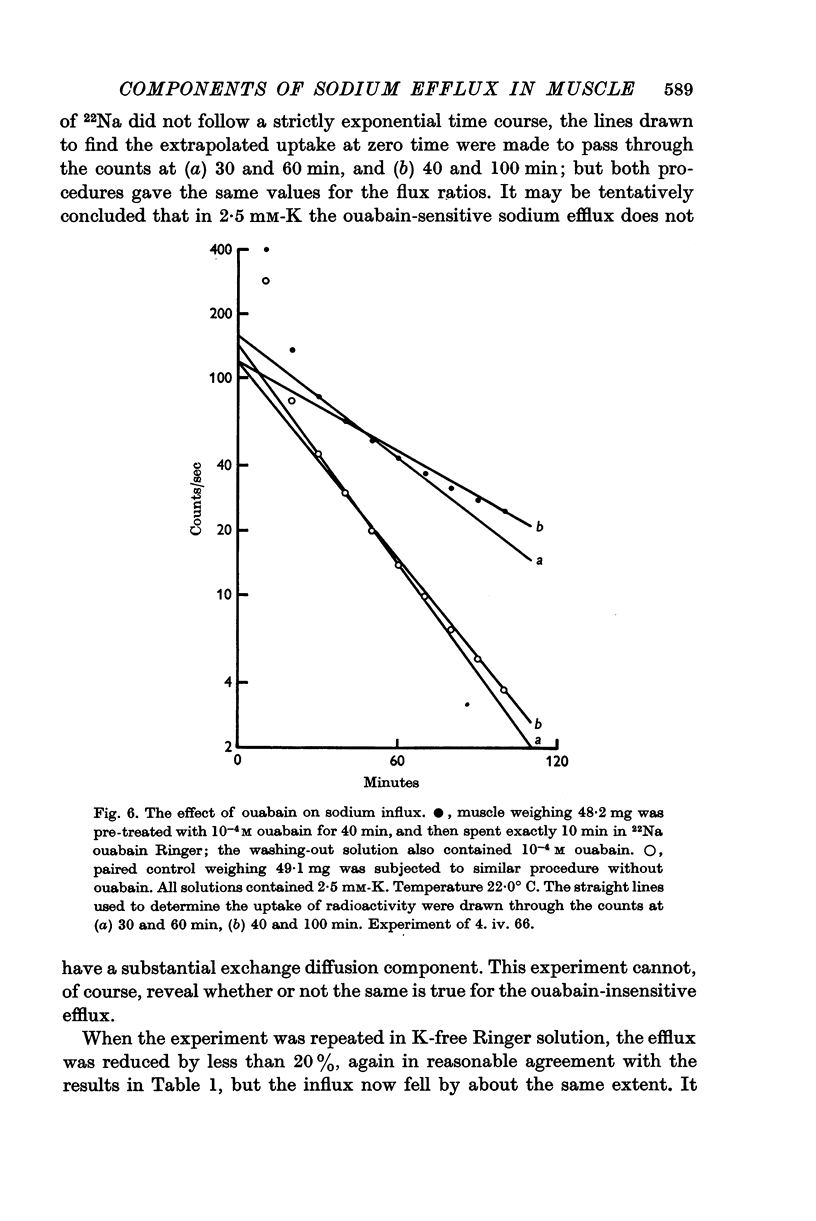
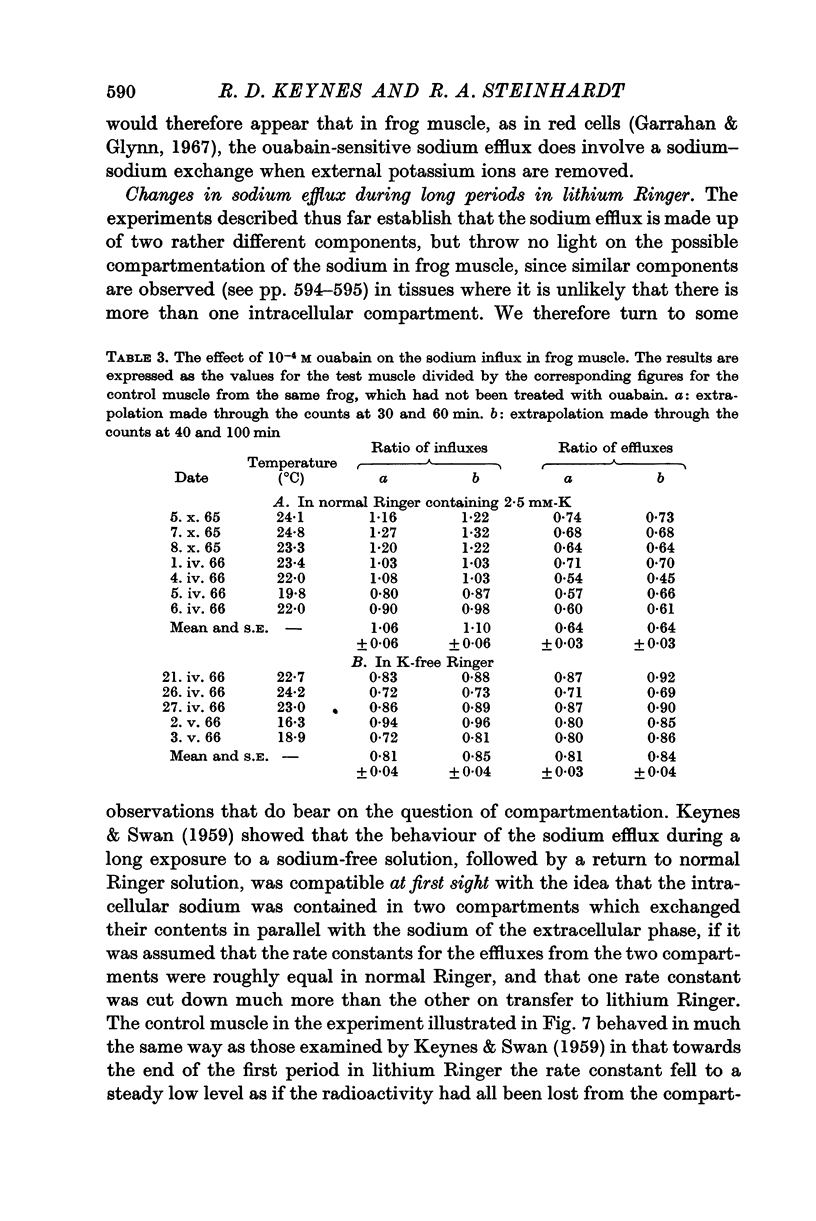
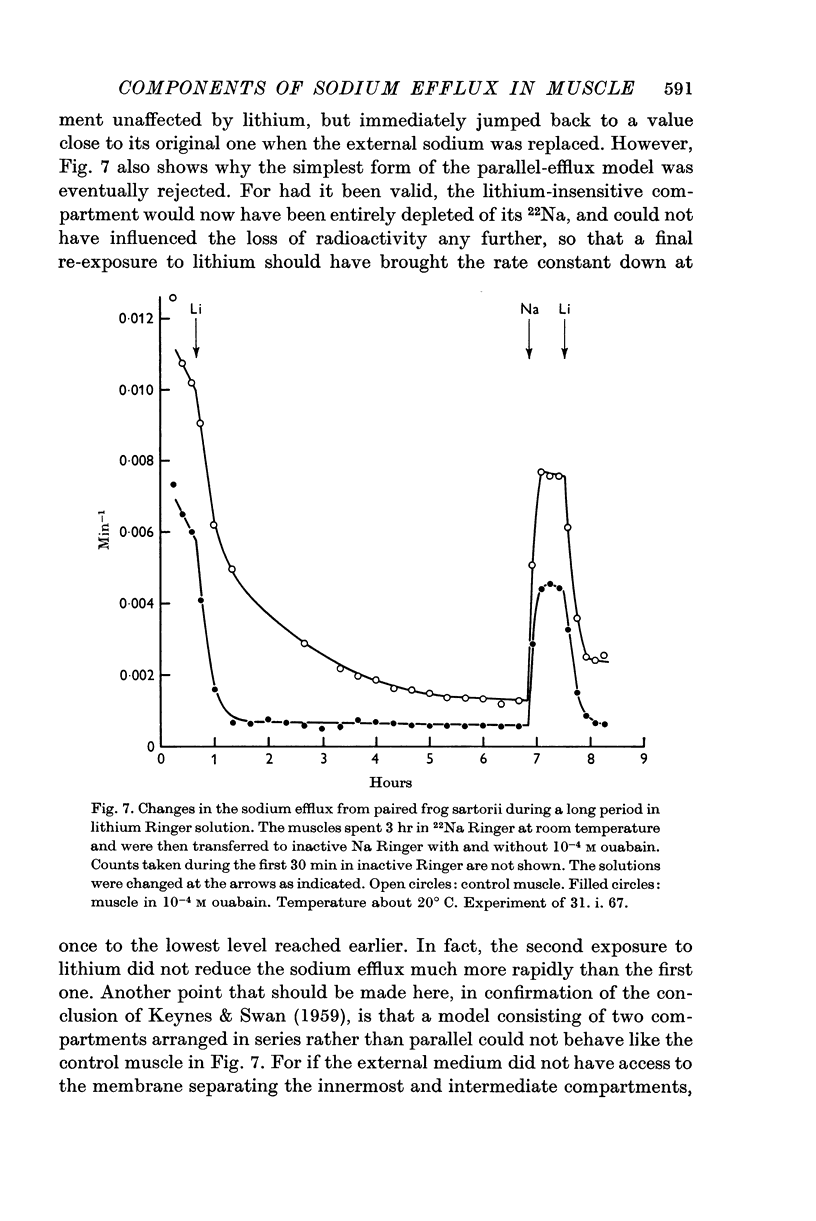

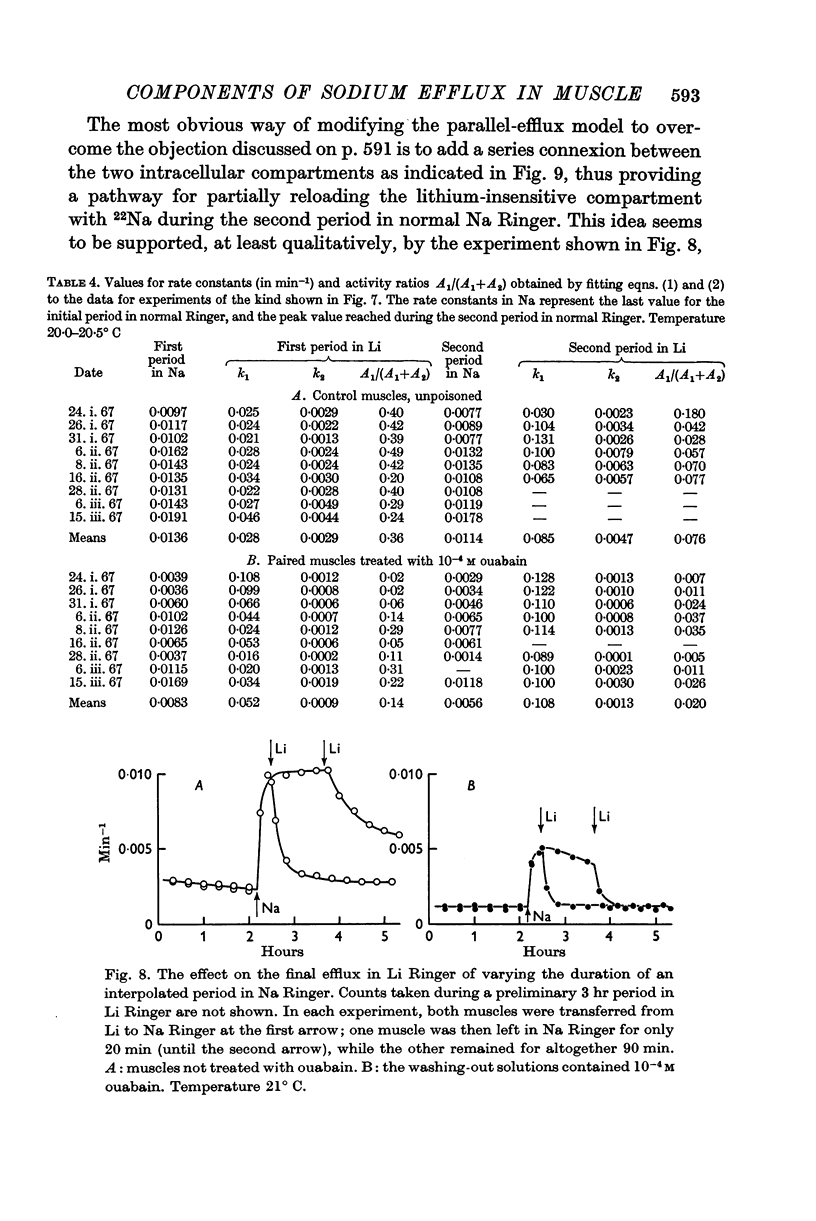


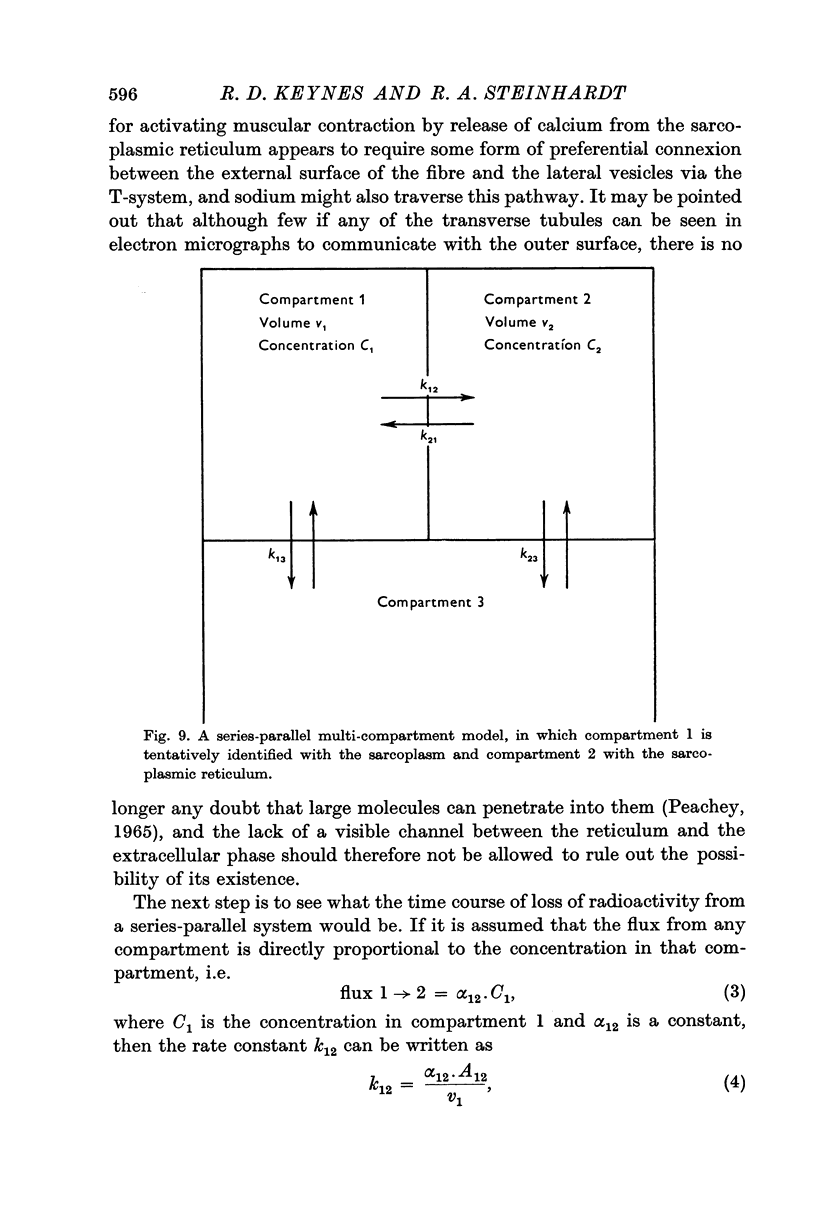



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Manil J., Steinhardt R. A. A ouabain-insensitive, calcium-sensitive sodium efflux from giant axons of Loligo. J Physiol. 1967 Jul;191(2):100P–102P. [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. C. Factors governing movement and distribution of inorganic ions in nerve and muscle. Physiol Rev. 1968 Jan;48(1):1–64. doi: 10.1152/physrev.1968.48.1.1. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Gage P. W. Frog skeletal muscle fibers: changes in electrical properties after disruption of transverse tubular system. Science. 1967 Dec 29;158(3809):1700–1701. doi: 10.1126/science.158.3809.1700. [DOI] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. SOME FURTHER OBSERVATIONS ON THE SODIUM EFFLUX IN FROG MUSCLE. J Physiol. 1965 May;178:305–325. doi: 10.1113/jphysiol.1965.sp007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEV A. A. DETERMINATION OF ACTIVITY AND ACTIVITY COEFFICIENTS OF POTASSIUM AND SODIUM IONS IN FROG MUSCLE FIBRES. Nature. 1964 Mar 14;201:1132–1134. doi: 10.1038/2011132a0. [DOI] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]


