Abstract
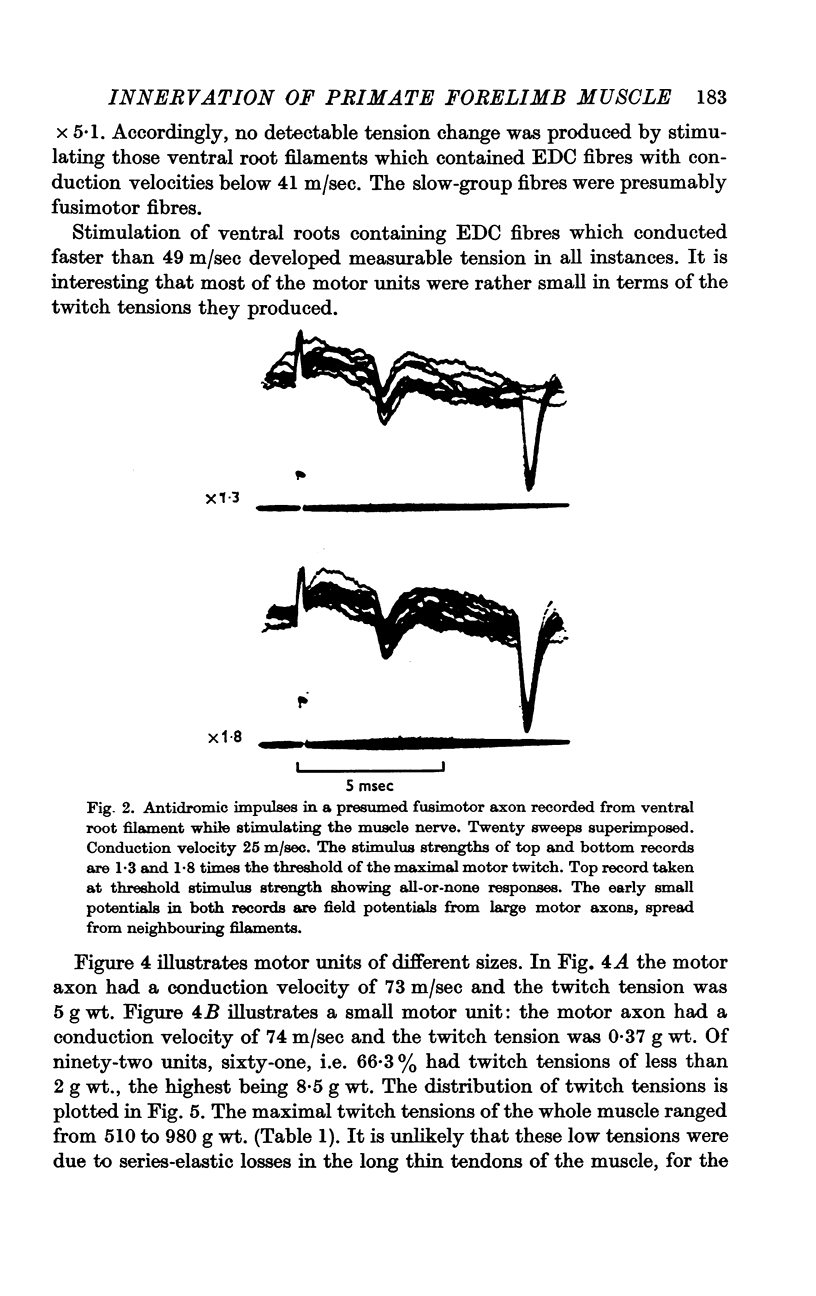
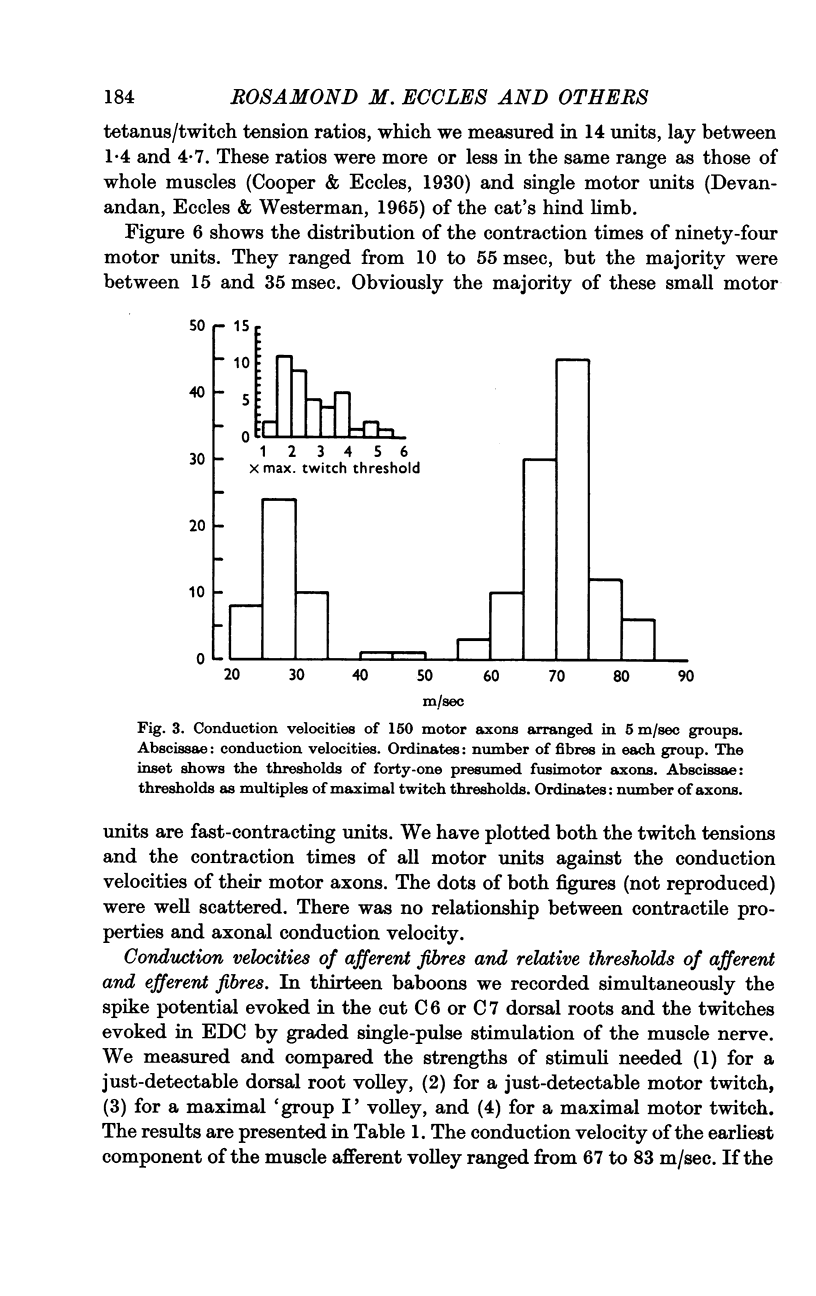
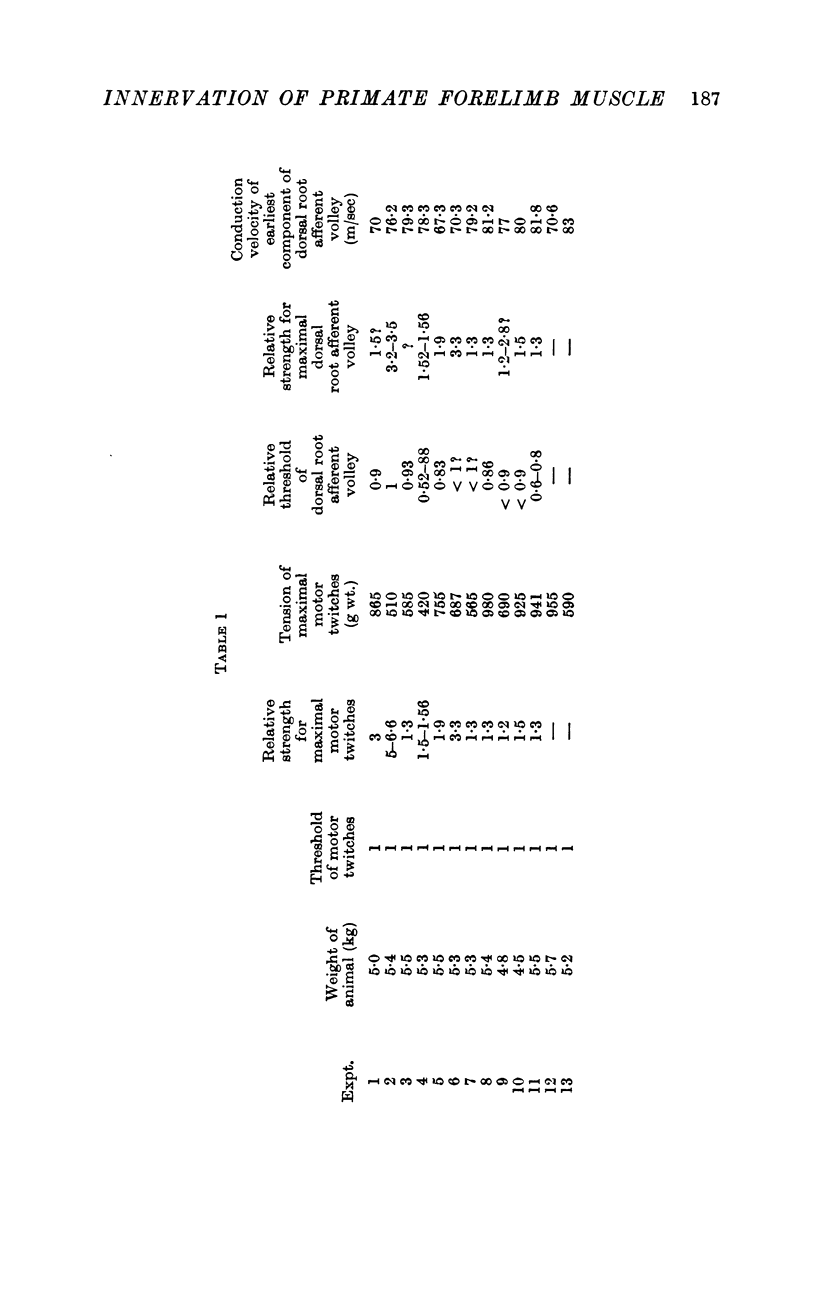
1. One hundred and fifty efferent axons innervating m. extensor digitorum communis (EDC) were isolated in filaments of C7 and C8 ventral roots of baboons. Conduction velocities were measured antidromically by stimulating the muscle nerve and recording from the filaments, and fell into two groups: a fast (49-84 m/sec) and a slow (22-41m/sec), presumably fusimotor group. The threshold for these latter axons exceeded the strength needed to elicit the maximal motor twitch.
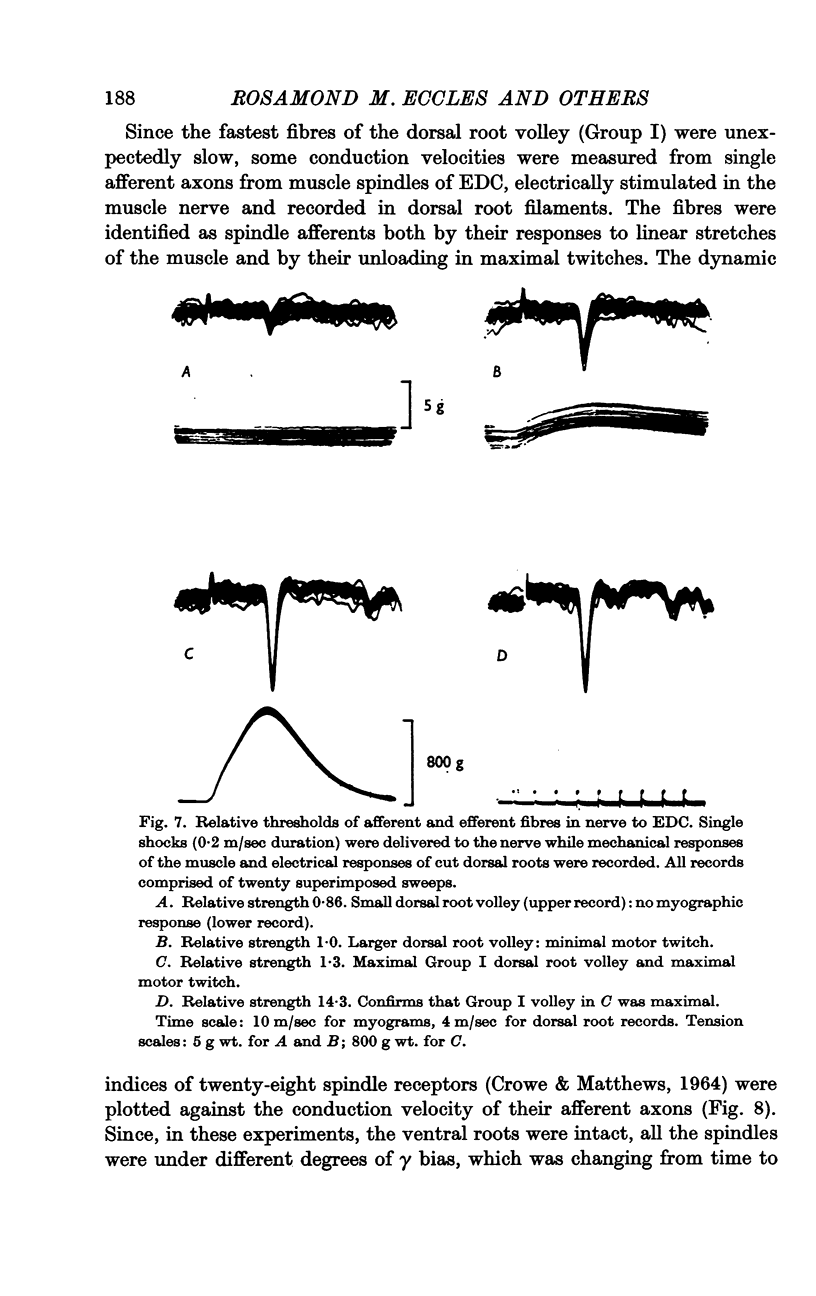
2. Stimulation of ventral root filaments containing slow axons produced no contractile tension in EDC.
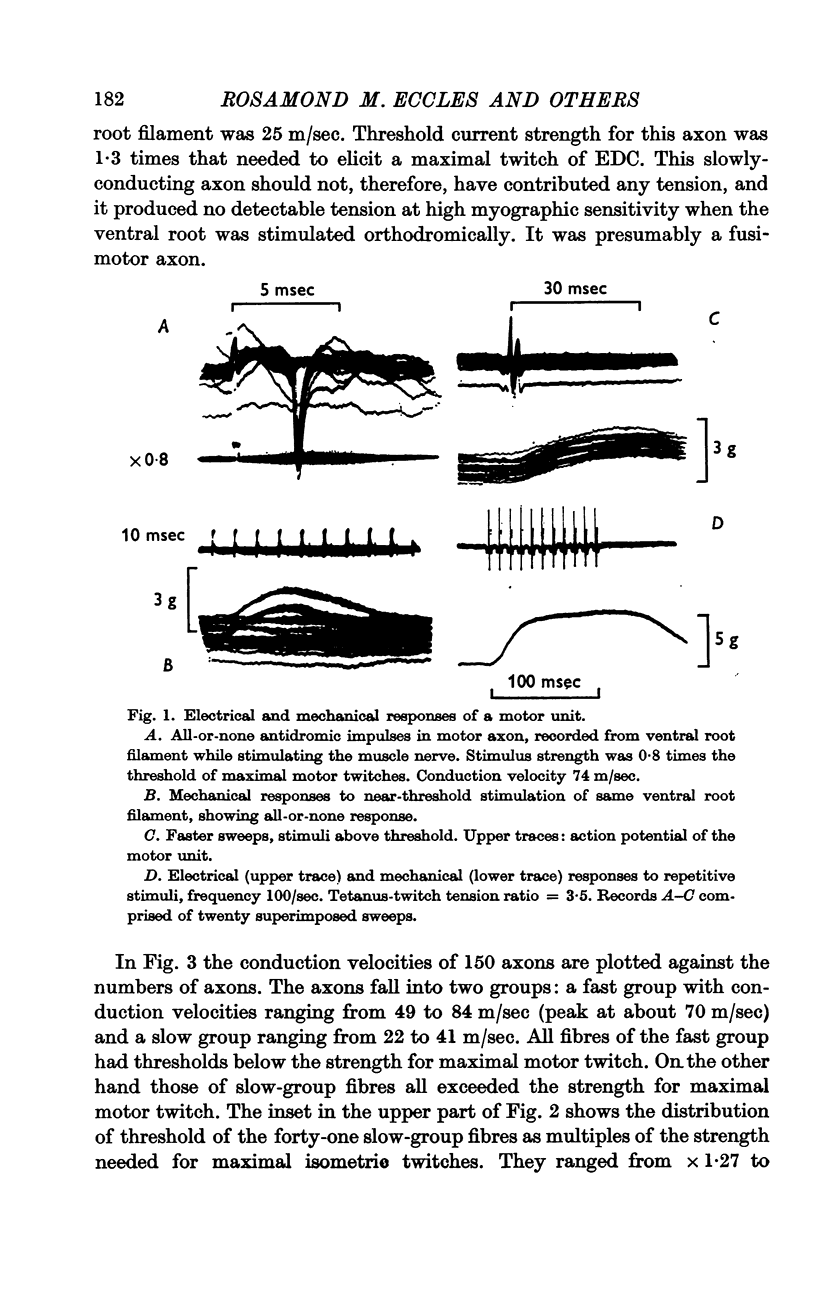
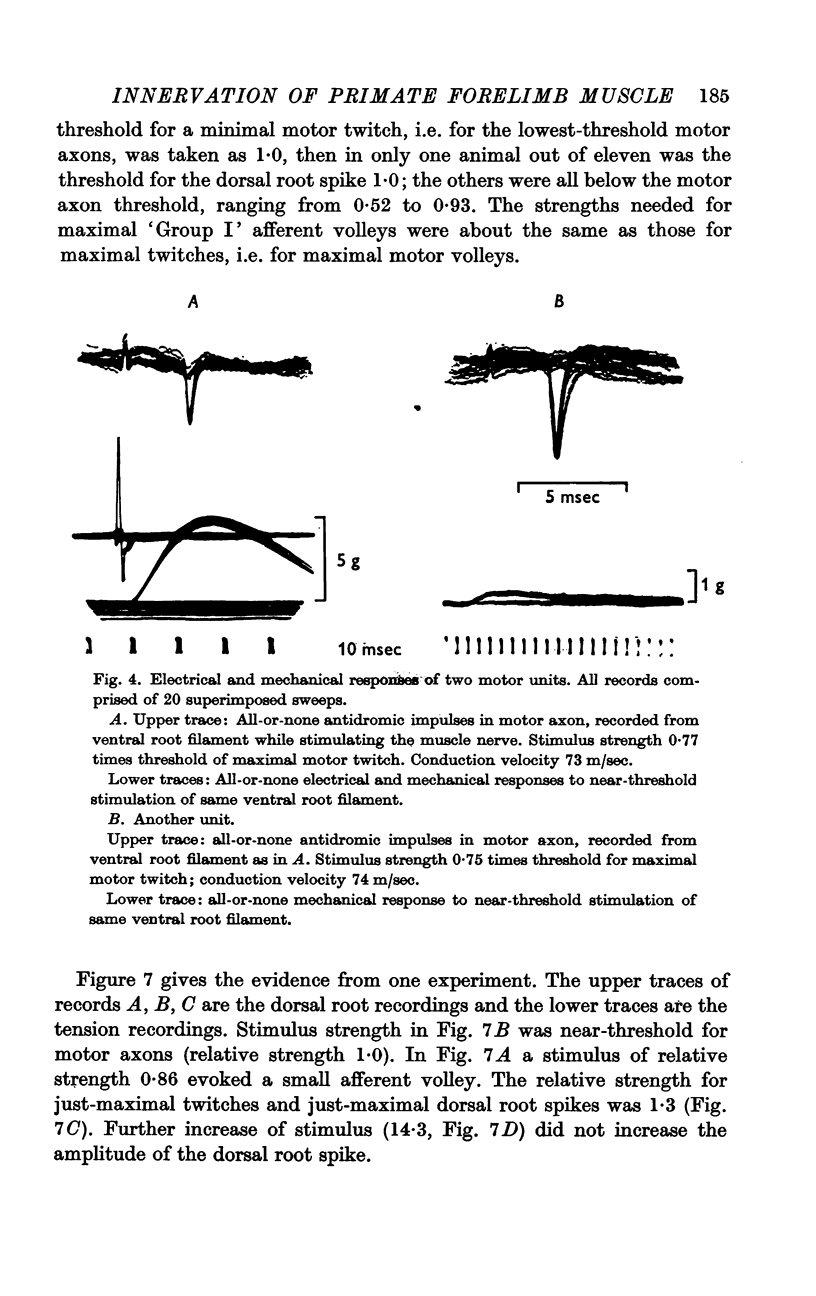
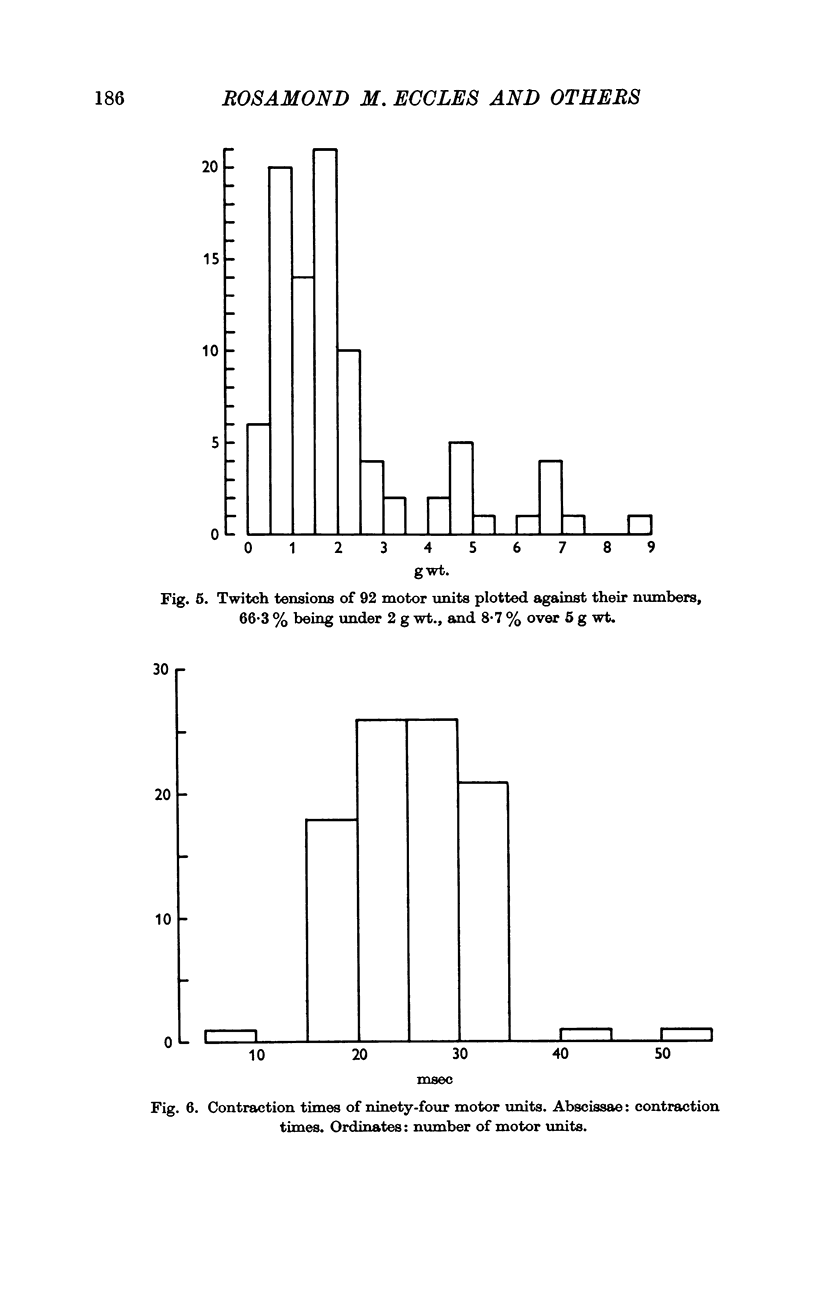
3. Stimulation of ventral root filaments containing fast-group axons elicited all-or-nothing twitches of motor units of EDC. The twitch tensions of 66·3% of the units were < 2·0 g wt.; only 8·7% were > 5·0 g wt. Tetanus-twitch ratios were 1·4-4·7 in a sample of 14 units. Contraction times were between 15 and 35 msec in 97% of the units. There was no correlation between contractile properties and axonal conduction velocity.
4. Afferent volleys from the stimulated EDC nerve were recorded from C6 or C7 dorsal roots. The threshold was below the threshold for a just-detectable motor twitch in ten out of eleven baboons. Conduction velocity of the earliest component of the muscle afferent volley was 67-83 m/sec.
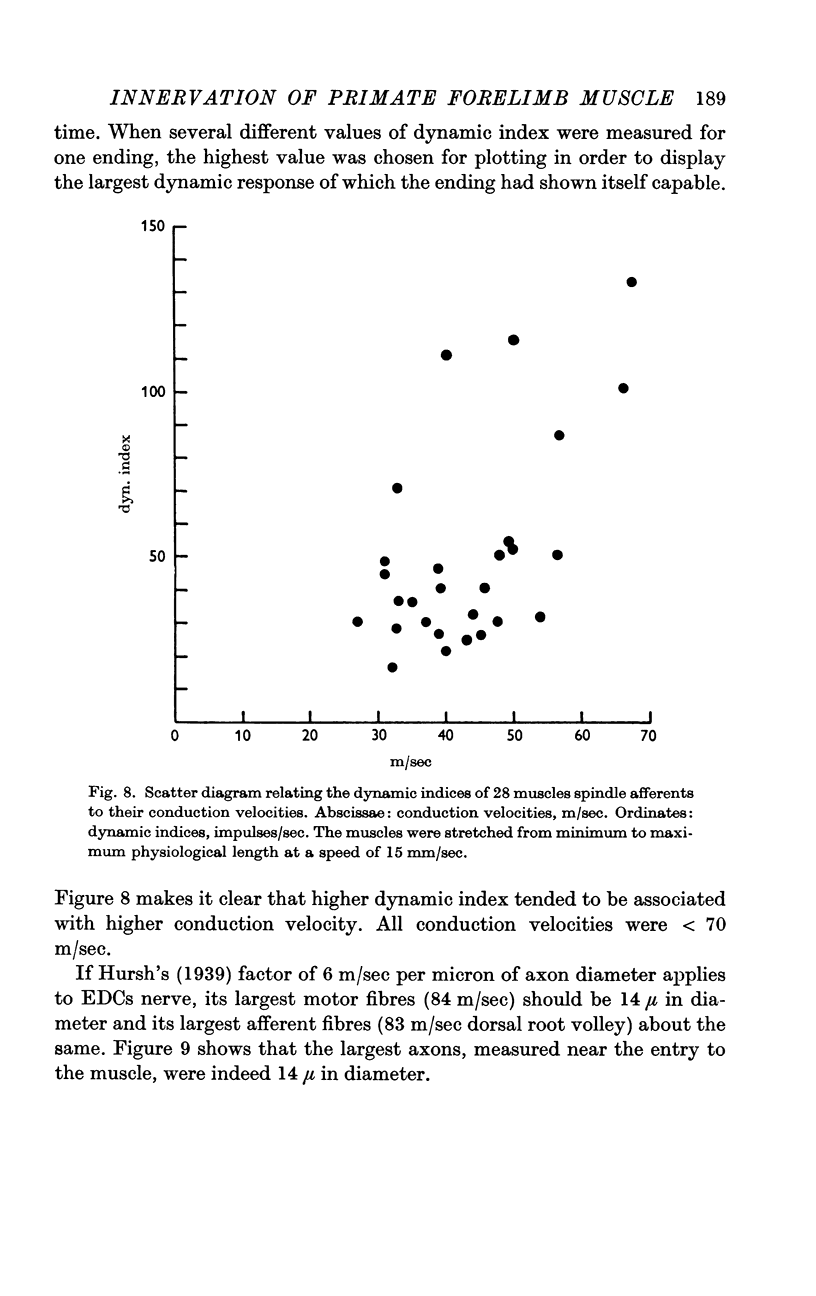
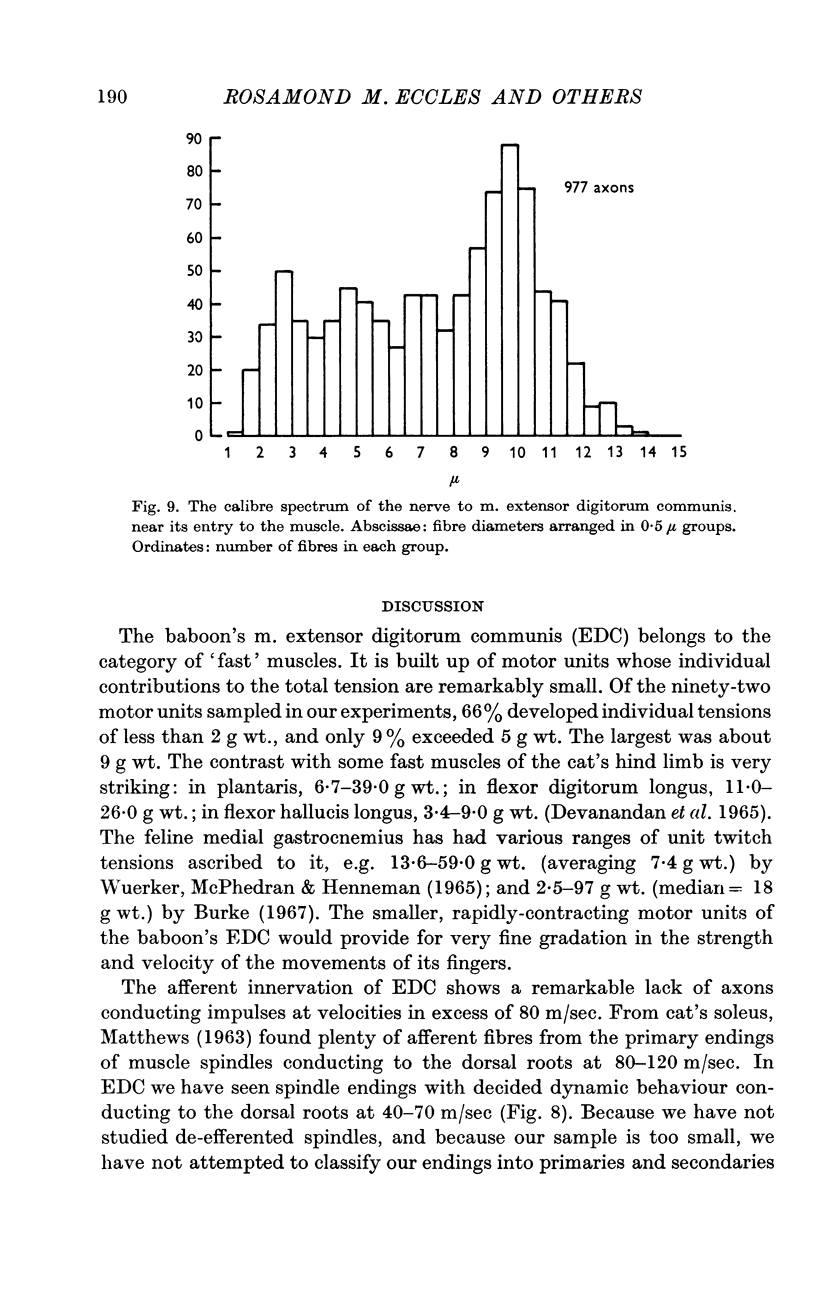
5. The conduction velocities of twenty-eight spindle afferents, identified by their responses to linear stretches of EDC and by their unloading by maximal twitches, were all < 70 m/sec. Higher dynamic sensitivity tended to be associated with higher conduction velocity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. Conduction velocity in proximal and distal portions of forelimb axons in the baboon. J Physiol. 1968 Sep;198(1):167–178. doi: 10.1113/jphysiol.1968.sp008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. The isometric responses of mammalian muscles. J Physiol. 1930 Jun 27;69(4):377–385. doi: 10.1113/jphysiol.1930.sp002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANANDAN M. S., ECCLES R. M., WESTERMAN R. A. SINGLE MOTOR UNITS OF MAMMALIAN MUSCLE. J Physiol. 1965 May;178:359–367. doi: 10.1113/jphysiol.1965.sp007632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERN J. E., LANDGREN S., PHILLIPS C. G., PORTER R. Selective excitation of corticofugal neurones by surface-anodal stimulation of the baboon's motor cortex. J Physiol. 1962 Apr;161:73–90. doi: 10.1113/jphysiol.1962.sp006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeze T. H., Phillips C. G., Sheridan J. D. Thresholds of cortical activation of muslce spindles and alpha motoneurones of the baboon's hand. J Physiol. 1968 Mar;195(2):419–449. doi: 10.1113/jphysiol.1968.sp008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIDDELL E. G. T., PHILLIPS C. G. The cortical representation of motor units. Brain. 1952 Dec;75(4):510–525. doi: 10.1093/brain/75.4.510. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HETEROGENEOUS PALE MUSCLE (M. GASTROCNEMIUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:85–99. doi: 10.1152/jn.1965.28.1.85. [DOI] [PubMed] [Google Scholar]
- McLeod J. G., Wray S. H. Conduction velocity and fibre diameter of the median and ulnar nerves of the baboon. J Neurol Neurosurg Psychiatry. 1967 Jun;30(3):240–247. doi: 10.1136/jnnp.30.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]


