Abstract
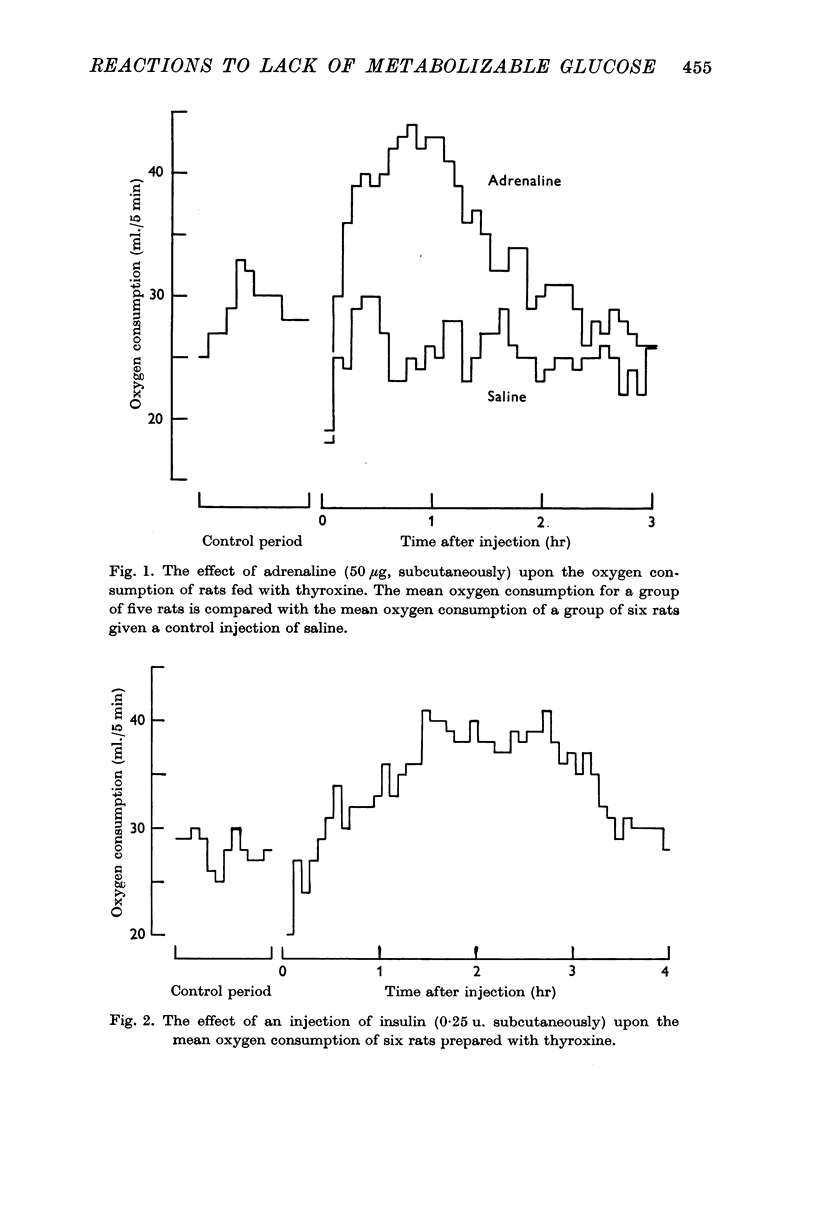
1. Following the injection of adrenaline into rats pre-treated with thyroxine, there is a pronounced and prolonged increase in their oxygen consumption. On this basis a method is described for following increases in the rate of secretion of adrenaline in response to physiological stimuli in rats.
2. Using this method the onset and the cessation of the increased rate of secretion of adrenaline during insulin induced hypoglycaemia has been found to correspond to a blood glucose concentration of approximately 40 mg/100 ml.
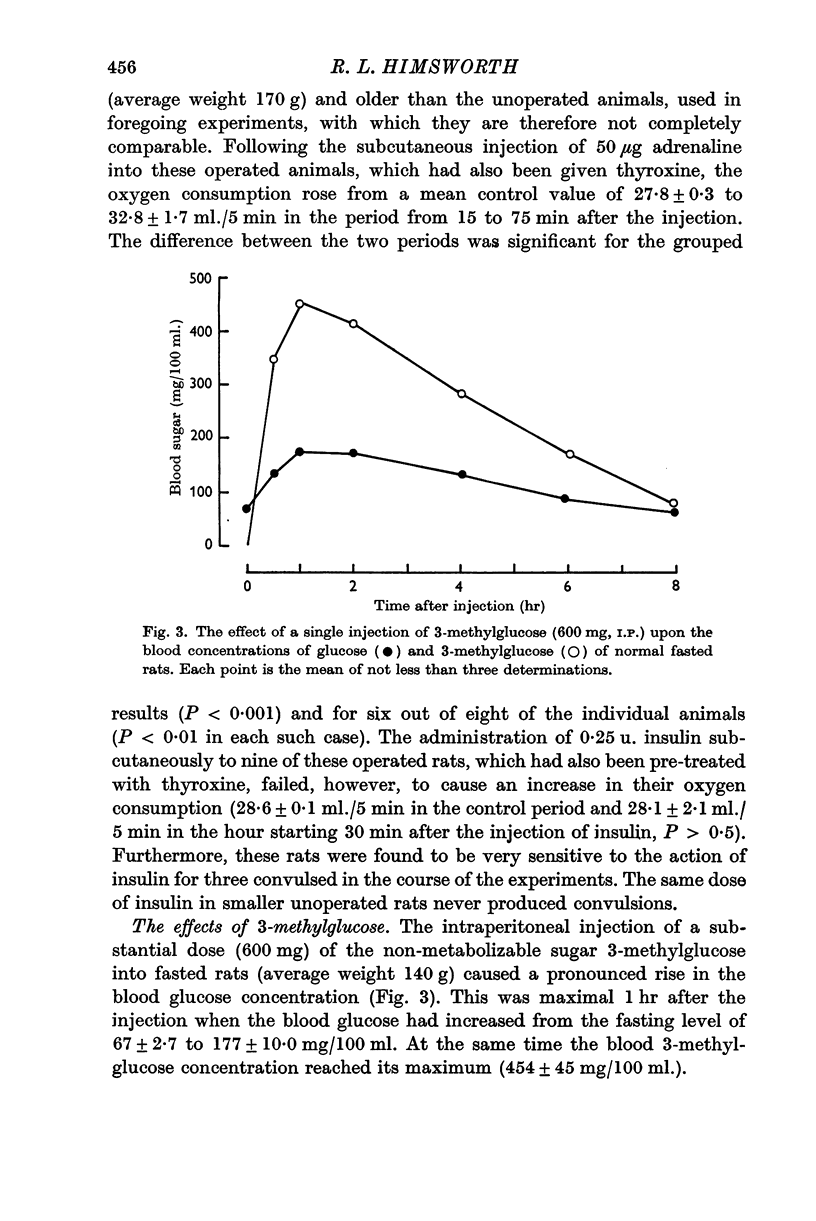
3. 3-Methylglucose (3-O-methyl-D-glucopyranose) is shown to cause a marked and sustained increase in the blood glucose concentration.
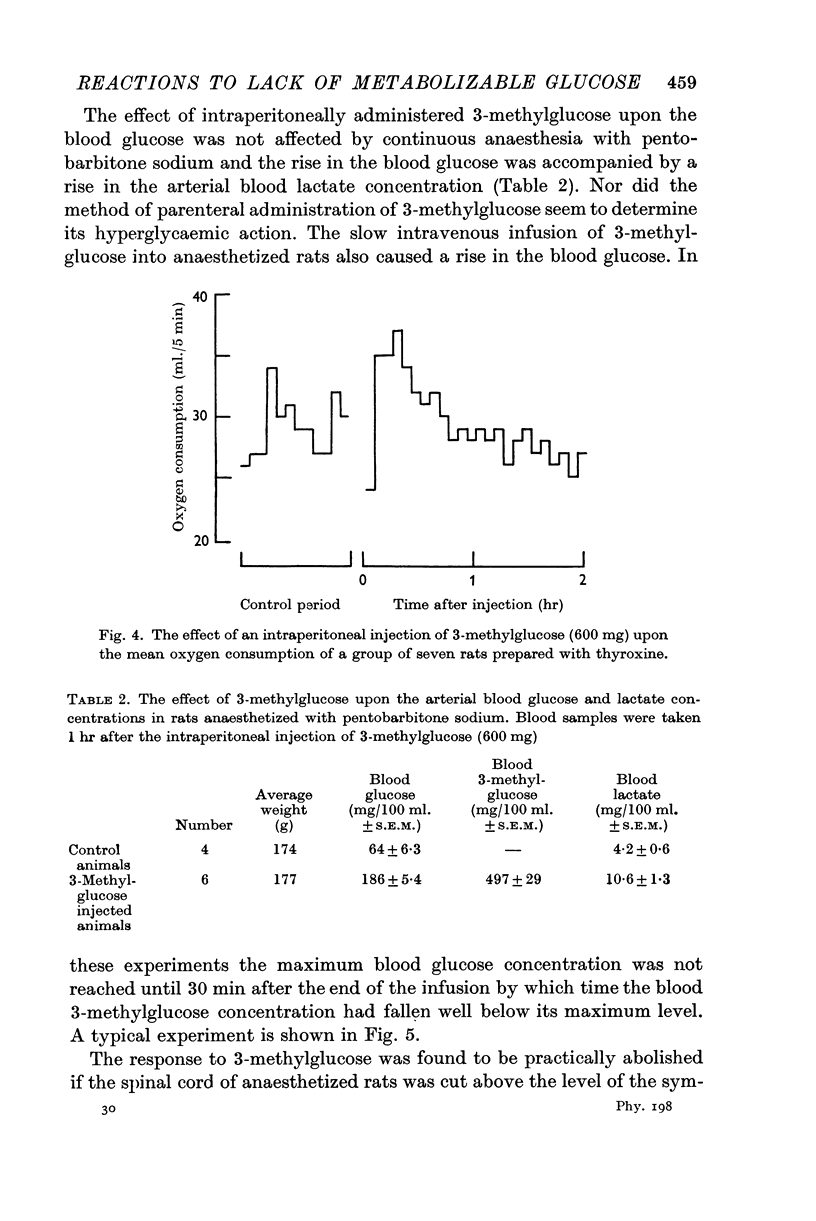
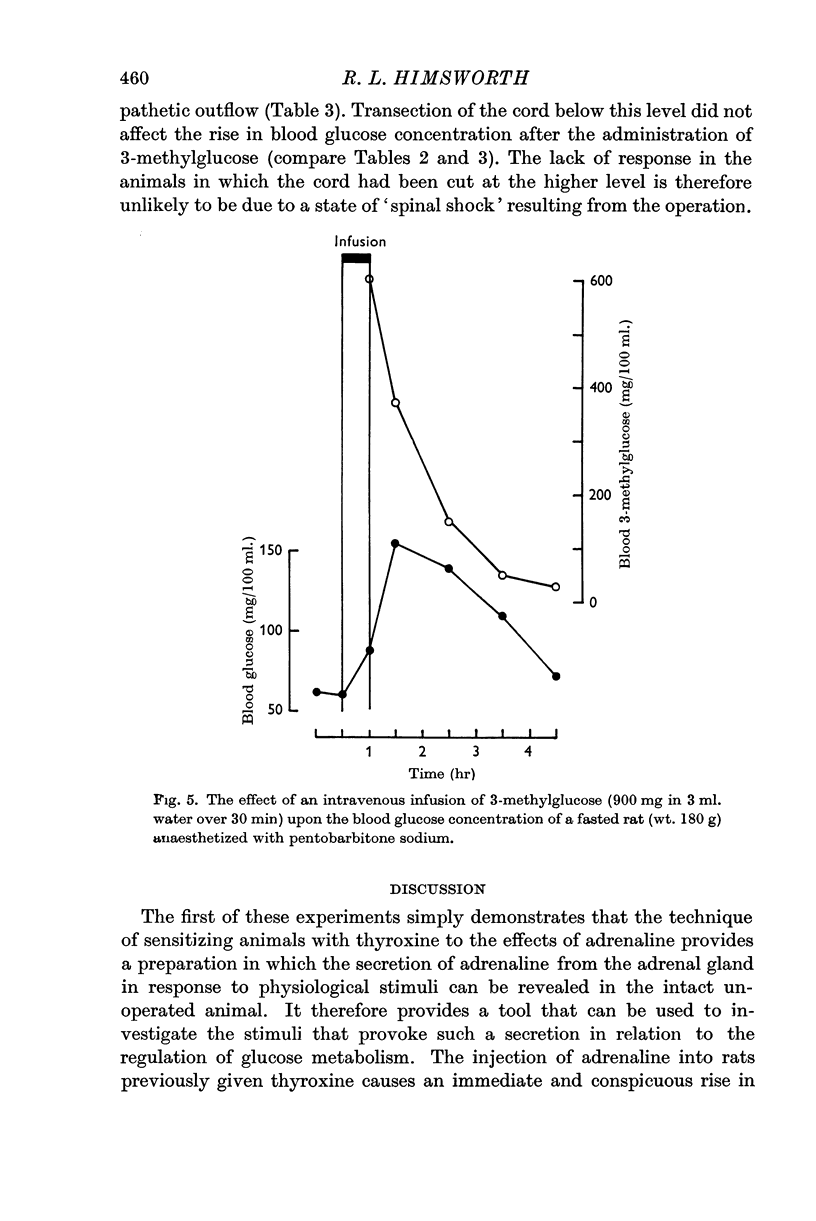
4. This action of 3-methylglucose can be accounted for by an increase in the rate of secretion of adrenaline, as it is accompanied by a rise in oxygen consumption in thyroid treated rats and is prevented by interruption of the central nervous connexions of the adrenal medulla.
5. These findings can be explained by 3-methylglucose's acting upon the receptor which reacts to insulin hypoglycaemia by bringing about the secretion of adrenaline. It is shown that the effective stimulus to this receptor is not a low blood glucose concentration. The stimulus is possibly a depressed rate of utilization of glucose by the receptor tissue.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMIN J., GRANT R. T. Adrenaline release during insulin hypoglycaemia in the rabbit. J Physiol. 1959 Dec;149:228–249. doi: 10.1113/jphysiol.1959.sp006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism. 1962 Oct;11:1098–1112. [PubMed] [Google Scholar]
- CAMPBELL P. N., YOUNG F. G. Metabolic studies with 3-methyl glucose. I. Its fate in the animal body. Biochem J. 1952 Nov;52(3):439–444. doi: 10.1042/bj0520439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIU C. C., HSIEH A. C. A comparative study of four means of expressing the metabolic rate of rats. J Physiol. 1960 Mar;150:694–706. doi: 10.1113/jphysiol.1960.sp006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROFFORD O. B., RENOLD A. E. GLUCOSE UPTAKE BY INCUBATED RAT EPIDIDYMAL ADIPOSE TISSUE. CHARACTERISTICS OF THE GLUCOSE TRANSPORT SYSTEM AND ACTION OF INSULIN. J Biol Chem. 1965 Aug;240:3237–3244. [PubMed] [Google Scholar]
- CSAKY T. Z., GLENN J. E. Urinary recovery of 3-methylglucose administered to rats. Am J Physiol. 1957 Jan;188(1):159–162. doi: 10.1152/ajplegacy.1956.188.1.159. [DOI] [PubMed] [Google Scholar]
- Crone C. Facilitated transfer of glucose from blood into brain tissue. J Physiol. 1965 Nov;181(1):103–113. doi: 10.1113/jphysiol.1965.sp007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker S. E. The effects of progressive nutritional hypoproteinaemia of the extracellular-fluid phase and plasma colloid osmotic pressure in rats. Biochem J. 1948;43(3):444–453. [PMC free article] [PubMed] [Google Scholar]
- HIMSWORTH R. L. Effect of adrenaline and insulin upon the oxygen consumption of hyperthyroid rats. Nature. 1960 Mar 5;185:694–694. doi: 10.1038/185694a0. [DOI] [PubMed] [Google Scholar]
- HOKFELT B., BYDGEMAN S. Increased adrenaline production following administration of 2-deoxy-D-glucose in the rat. Proc Soc Exp Biol Med. 1961 Mar;106:537–539. doi: 10.3181/00379727-106-26394. [DOI] [PubMed] [Google Scholar]
- HOLTKAMP D. E., OCHS S., PFEIFFER C. C., HEMING A. E. Determination of the oxygen consumption of groups of rats. Endocrinology. 1955 Jan;56(1):93–104. doi: 10.1210/endo-56-1-93. [DOI] [PubMed] [Google Scholar]
- HYVARINEN A., NIKKILA E. A. Specific determination of blood glucose with o-toluidine. Clin Chim Acta. 1962 Jan;7:140–143. doi: 10.1016/0009-8981(62)90133-x. [DOI] [PubMed] [Google Scholar]
- Himsworth R. L. Interference with the metabolism of glucose by a non-metabolizable hexose (3-methylglucose). J Physiol. 1968 Sep;198(2):467–477. doi: 10.1113/jphysiol.1968.sp008617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of glucose oxidase (notatin): Addendum. Sedimentation and diffusion of glucose oxidase (notatin). Biochem J. 1948;42(2):221–229. [PMC free article] [PubMed] [Google Scholar]
- MORGAN H. E., REGEN D. M., PARK C. R. IDENTIFICATION OF A MOBILE CARRIER-MEDIATED SUGAR TRANSPORT SYSTEM IN MUSCLE. J Biol Chem. 1964 Feb;239:369–374. [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- VON EULER U. S., LUFT R. Effect of insulin on urinary excretion of adrenalin and noradrenalin; studies in ten healthy subjects and in six cases of acromegaly. Metabolism. 1952 Nov;1(6):528–532. [PubMed] [Google Scholar]


