Abstract
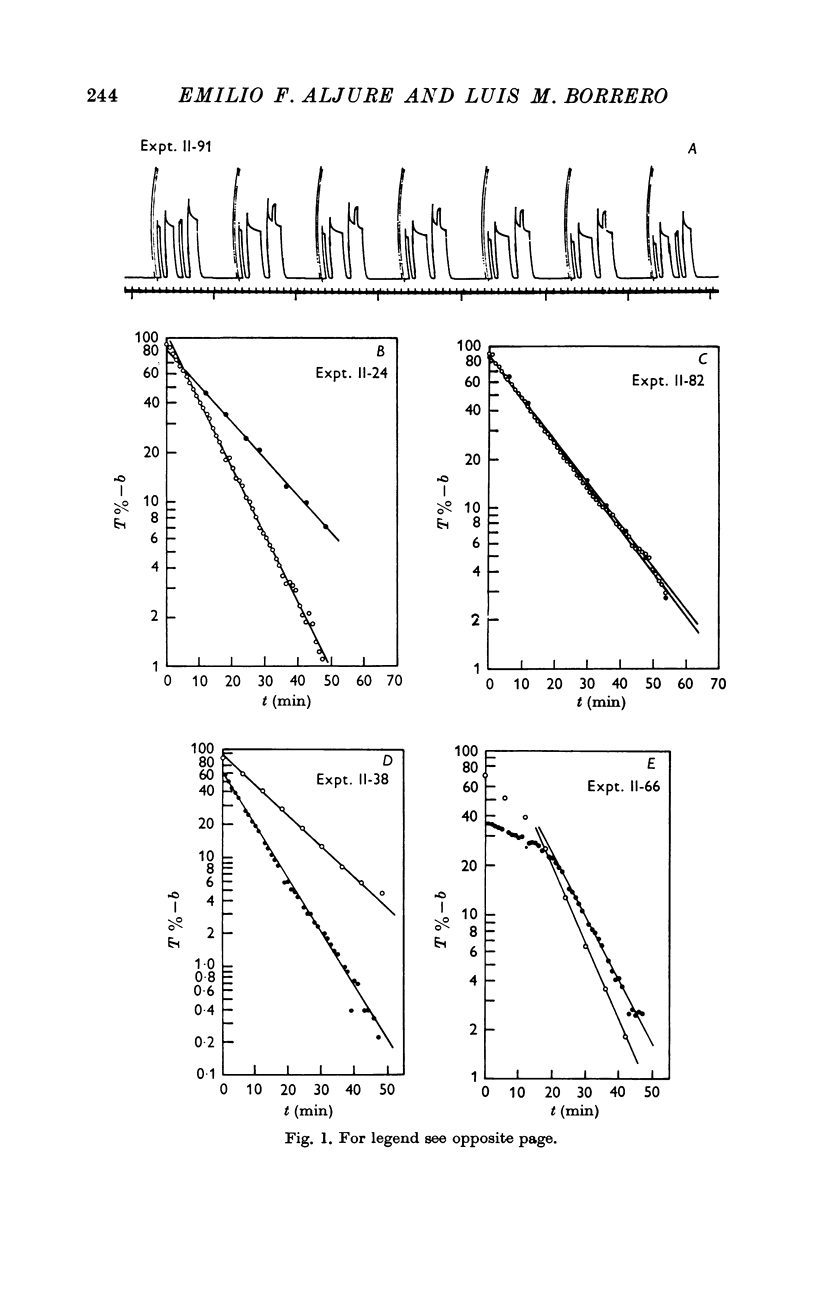
1. Fatigue curves were obtained on muscles contracting at length (L) different from the optimal (Lo), and performing isometric tetani of fixed duration at regular intervals. Every sixth tetanus occurred at Lo, the remainder took place at L. The parameters of the fatigue curve were compared with those of controls contracting always at Lo. Fatigability was measured as the coefficient of exponential decrease of tension with time.
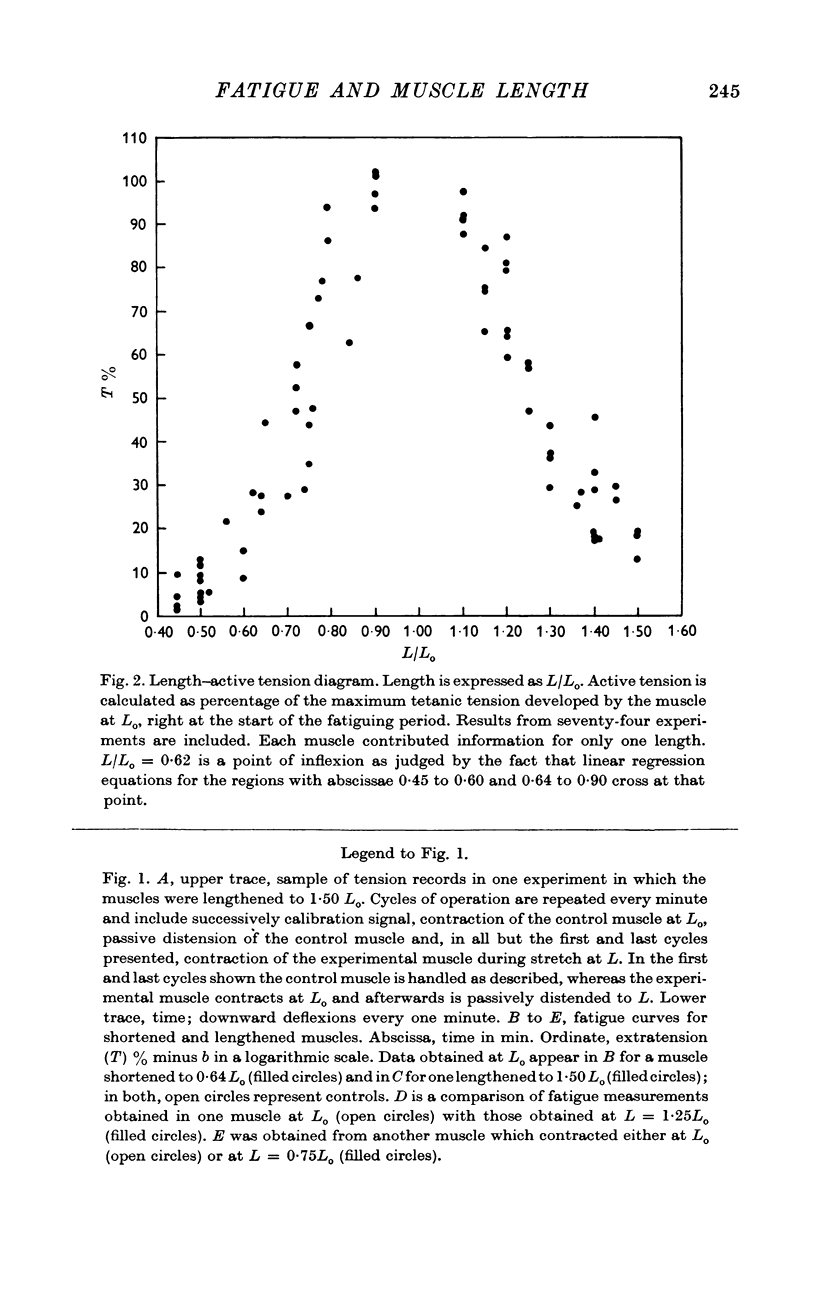
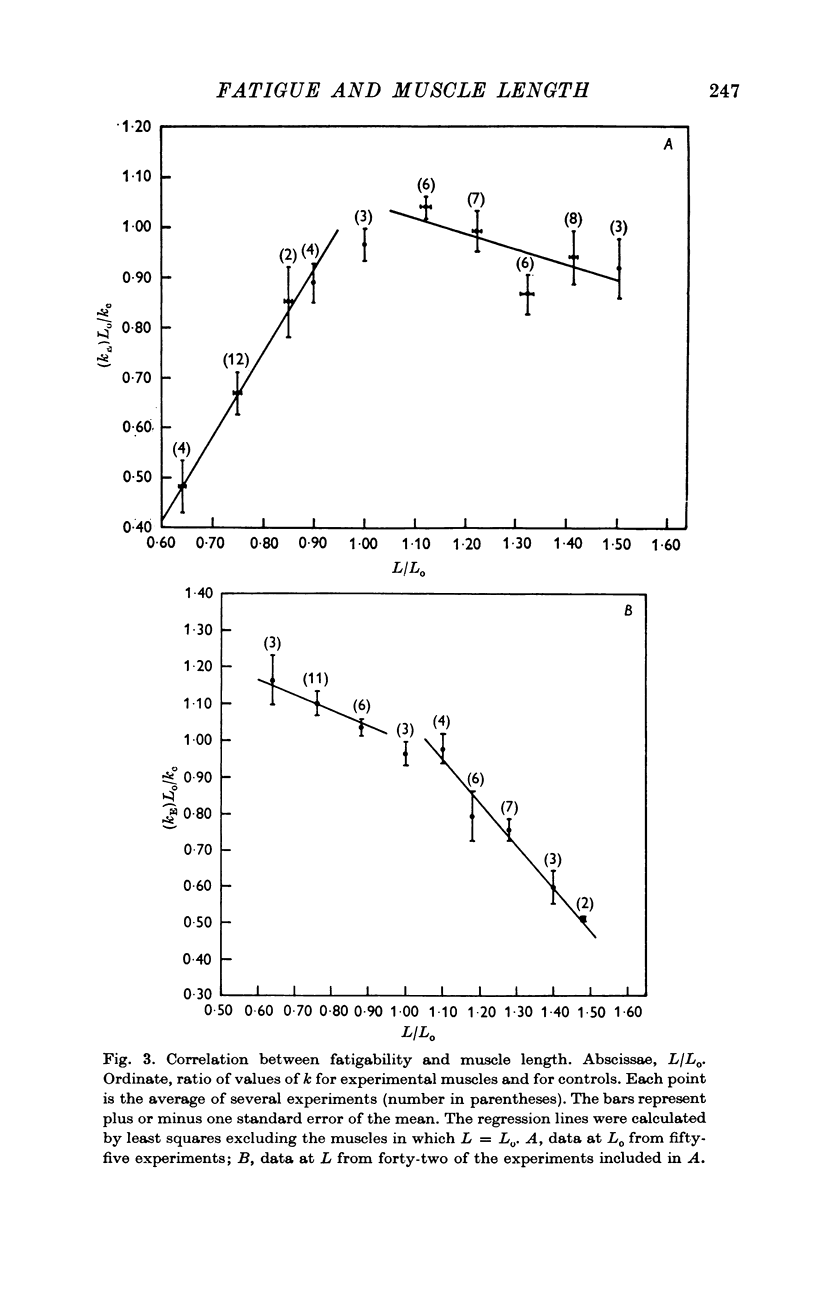
2. Fatigability varied linearly with length in shortened and lengthened muscles, but the slopes of the relations were significantly different for the two groups.
3. The tension values from single experimental muscles fell on two independent curves, one for those obtained at Lo, another for data at L. The same muscle showed, therefore, two different fatigue processes which followed independent temporal evolutions.
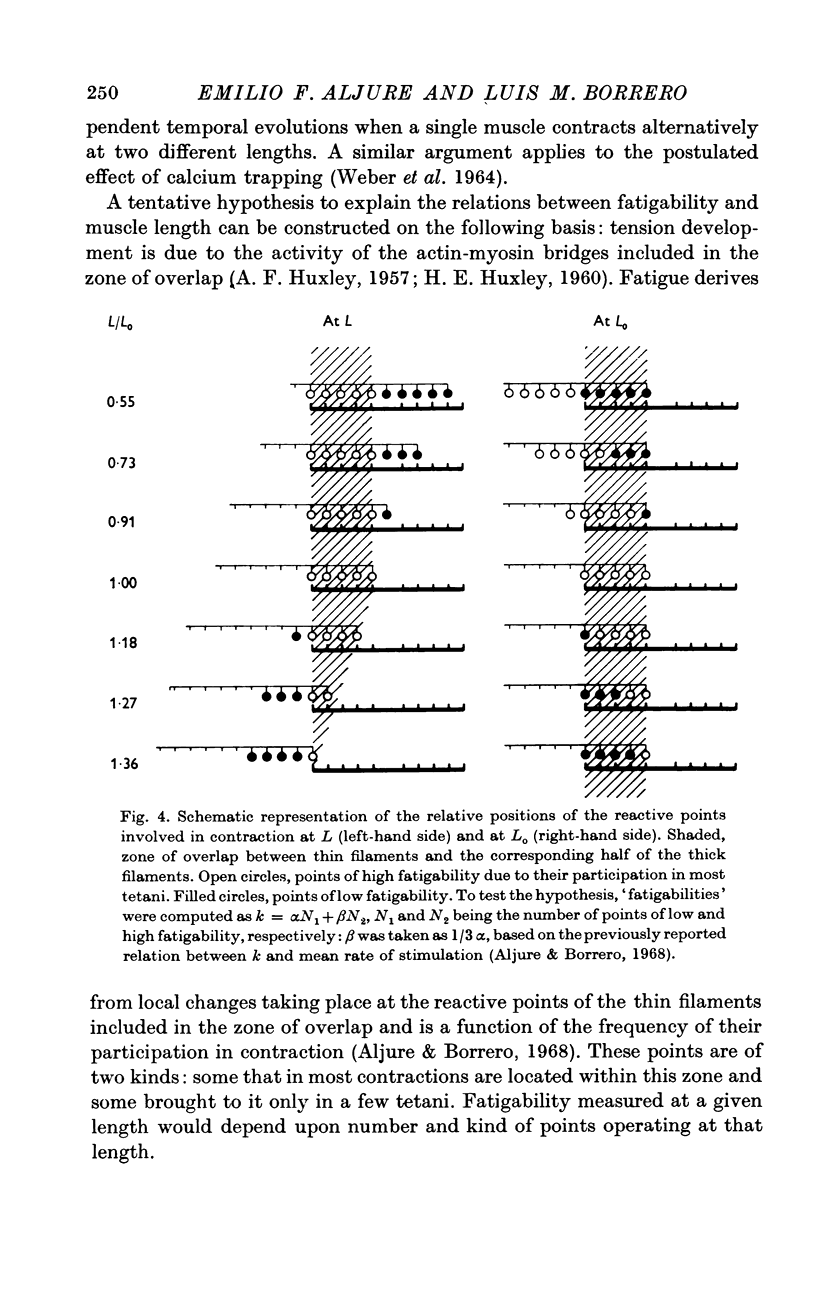
4. Points 2 and 3 of this Summary imply that fatigue is a process dependent on the actual setting of the myofilaments during contraction and not distributed uniformly within the sarcomere.
5. It is proposed that fatigue results from local changes taking place at discrete reactive points in the myofilament. The experimental results are discussed in terms of this hypothesis and of the sliding model of contraction.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C. The heat production associated with the maintenance of a prolonged contraction and the extra heat produced during large shortening. J Physiol. 1951 Feb;112(3-4):438–445. doi: 10.1113/jphysiol.1951.sp004541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUBERT X., ROQUET M. L., VAN DER ELST J. The tension-length diagram of the frog's sartorius muscle. Arch Int Physiol. 1951 Jul;59(2):239–241. doi: 10.3109/13813455109145002. [DOI] [PubMed] [Google Scholar]
- Aljure E. F., Borrero L. M. The evolution of fatigue associated with isometric contraction in toad sartorius. J Physiol. 1968 Feb;194(2):289–303. doi: 10.1113/jphysiol.1968.sp008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler A. S., Fales J. T., Zierler K. L. The active state of mammalian skeletal muscle. J Gen Physiol. 1967 Oct;50(9):2239–2253. doi: 10.1085/jgp.50.9.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INFANTE A. A., KLAUPIKS D., DAVIES R. E. LENGTH, TENSION AND METABOLISM DURING SHORT ISOMETRIC CONTRACTIONS OF FROG SARTORIUS MUSCLES. Biochim Biophys Acta. 1964 Jul 29;88:215–217. doi: 10.1016/0926-6577(64)90171-8. [DOI] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. The mechanical properties of relaxing muscle. J Physiol. 1960 Jun;152:30–47. doi: 10.1113/jphysiol.1960.sp006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- WEBER A., HERZ R., REISS I. THE REGULATION OF MYOFIBRILLAR ACTIVITY BY CALCIUM. Proc R Soc Lond B Biol Sci. 1964 Oct 27;160:489–501. doi: 10.1098/rspb.1964.0063. [DOI] [PubMed] [Google Scholar]


