Abstract
The interaction between pathogens and their multicellular hosts is initiated by activation of pathogen recognition receptors (PRRs). These receptors, that include most notably members of the toll-like receptor (TLR) family, recognize specific pathogen-associated molecular patterns (PAMPs). TLR4 is a central part of the receptor complex that is involved in the activation of the immune system by lipopolysaccharide (LPS) through the specific recognition of its endotoxic moiety (Lipid A). This is a critical event that is essential for the immune response to Gram-negative bacteria as well as the etiology of endotoxic shock. Interestingly, compared to mammals, fish are resistant to endotoxic shock. This in vivo resistance concurs with in vitro studies demonstrating significantly lowered sensitivity of fish leukocytes to LPS activation. Further, our in vitro analyses demonstrate that in trout mononuclear phagocytes, LPS fails to induce antiviral genes, an event that occurs down-stream of TLR4 and is required for the development of endotoxic shock. Finally, an in silico approach that includes mining of different piscine genomic and EST databases, reveals the presence in fish of all of the major TLR signaling elements except for the molecules specifically involved in TLR4-mediated endotoxin recognition and signaling in mammals. Collectively, our analysis questions the existence of TLR4-mediated cellular responses to LPS in fish. We further speculate that other receptors, in particular beta-2 integrins, may play a primary role in the activation of piscine leukocytes by LPS.
Keywords: innate immunity, pathogen recognition receptors, pathogen-associated molecular patterns, lipopolysaccharide, toll-like receptors, endotoxicity
1. Introduction
Pathogen recognition is one of the most basic and important properties of the immune system. It is assumed that the need for organisms to recognize pathogens and distinguish between “self” and “non-self” arose at the time of the appearance of the first metazoans [1]. This process relies on the existence of specific, structurally conserved components that are produced by certain broad groups of potentially pathogenic microorganisms. These components, which are absent in multicellular hosts, are popularly referred to as pathogen-associated molecular patterns (PAMPs). Typical PAMPs are lipopolysaccharides (LPS) of Gram-negative bacteria, peptidoglycan and its structural component muramyl dipeptide (MDP) of Gram-positive bacteria, fungal beta-glucans, and double-stranded RNA (dsRNA).
The initial recognition and biological response to PAMPs or other exogenous or endogenous substances that signal danger or directly compromise the homeostasis of an organism are mediated by genotypically encoded pathogen recognition receptors (PRRs). Structurally and functionally similar PRRs are shared between vertebrates, invertebrates, and even plants. For example, the toll-like receptors (TLRs) in vertebrates belong to a family that also includes the toll receptor in Drosophila [2] and, according to some authors, the R proteins in plants [3,4].
TLRs have drawn a great deal of attention after the discovery in the late 1990’s that mammalian TLR4 is critically involved in the etiology of LPS-induced septic shock [5–7]. LPS is widely used to experimentally induce potent immune reactions in mammals. It is the major constituent of the external layer of the outer membrane of Gram-negative bacteria. LPS is composed of three distinct parts: a carbohydrate “O-antigen” and oligosaccharide core region, and a lipid portion termed “lipid A” that is responsible for the activation of the innate immune response in mammals and confers the endotoxic properties of LPS [8,9]. With the isolation and characterization of additional TLRs in mammals, it was determined that members of this receptor family detect the presence of diverse PAMPs including, for example, LPS, peptidoglycan, double-stranded RNA, flagellin, and tri/diacylated bacterial lipoproteins. Excitingly, it also turned out that different members of the TLR family can transmit signals that activate distinct intracellular signaling cascades that may eventually result in pathogen-specific cellular responses [10,11].
Recently, there have been several studies on fish that have identified many fish orthologs of mammalian TLRs [12–14]. In addition, it appears that some of these orthologs are functionally analogous. For example, it was shown that TLR3 isolated from zebrafish (Danio rerio) is involved in the activation of NFkB, whereas trout TLR5, like its mammalian counterpart, activates the immune system upon detection of bacterial flagellin [15]. However, there still appears to be fundamental differences in the recognition and the response to certain PAMPs across vertebrates.
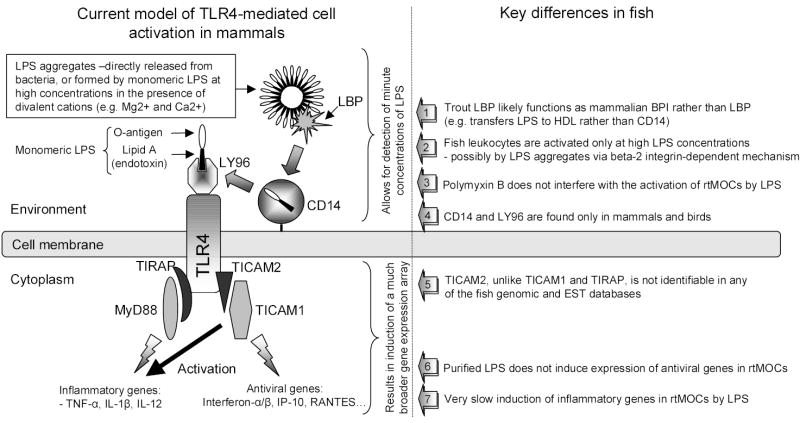
It has been known for a long time that lower vertebrates, most notably fish and amphibians, are resistant to the toxic effects of LPS [16]. It is also remarkable that in many in vitro studies on leukocytes from different fish species, extremely high (e.g., μg/ml) concentrations of LPS have been used to induce immune responses [17–21] in comparison to studies on mammals. This fact motivated us to analyze previously published data which, together with new observations, allows us to hypothesize that: The differences in the biological response to LPS between fish and mammals are likely determined by differences in their receptor-mediated recognition of LPS. Namely, it appears that the TLR4 associated molecules (CD14, LY96 (MD-2) and TICAM2 (TRAM)) that are required for the TLR4-mediated response to the endotoxic moiety of LPS in mammals, may be absent or may perform different functions in fish. Our analysis also suggests that beta-2 integrins, a group of well-conserved ancient molecules, may play a primary role in the LPS recognition by the piscine immune system.
2. TLR4-mediated endotoxin recognition in mammals
Besides being the first to be identified [22], mammalian TLR4 is by far the best functionally characterized member of the TLR family. There are two features that set TLR4 apart from other TLR family members. First, TLR4 mediates the immune response to very low (picomolar) concentrations of LPS through complex interactions with extracellular accessory molecules (discussed in further detail below) and second, compared to other TLRs, the magnitude of the TLR4-mediated cellular response is much higher and more persistent. In addition, TLR4 activation by LPS leads to activation of a much wider array of immune genes which include both proinflammatory cytokines (e.g. TNF-α, IL-1β, IL-12 etc.) and genes specifically involved in the antiviral response, including type-1 interferons and interferon-inducible genes [11,23–25].
According to the current model, LPS aggregates are recognized initially by the lipopolysaccharide binding protein (LBP) which is an acute phase serum protein that is a member of the lipid transfer - LT/LBP family [26,27]. In turn, LBP facilitates the transfer of LPS to CD14 [28,29] which is followed by relocation of monomeric LPS to TLR4-associated LY96. LY96 specifically binds to the lipid A portion (the endotoxic moiety) of LPS [30] which results in homodimerisation and activation of TLR4 [31,32]. The activation of TLR4 is followed by recruitment of intracellular adapter molecules through interactions of their toll-interleukin-1 resistance (TIR) domains. Initially, it was found that TLR4 associates with MyD88, an adaptor molecule that contains both a TIR and a death domain [33] and which is implicated in the signaling of all mammalian TLRs studied so far except for TLR3 [34]. However, it was discovered later that the association between TLR4 and MyD88 is obligatorily mediated by TIRAP (Mal) which is another member of the TIR domain-containing adapter molecules [35–38]. In addition, more recent studies discovered another adapter molecule, named toll-like receptor adaptor molecule 1 (TICAM1, TRIF) which, similarly to MyD88, indirectly associates with TLR4 through the toll-like receptor adaptor molecule 2 (TICAM2). However, unlike MyD88 which mediates the early activation of NFkB, the TICAM1/2 activation is involved in both the TLR4-mediated induction of antiviral genes and the late phase of NFkB activation [38–40]. In addition to TLR4, TICAM1 is also recruited by TLR3 which is a receptor for dsRNA and the structural homolog of dsRNA, poly(I:C) [41].
MyD88 activation leads to the subsequent activation of a string of signal transduction factors most notably; IRAK4, IRAK1, TRAF6, and IKBK, IKBKB, IKBKG (IKKα/β/γ) [42–44]. Ultimately, this signaling cascade leads to the degradation of the inhibitor of NFκB (IκB) and release of NFκB p50 and p65 subunits which then migrate into the cell nucleus as heterodimers and drive the expression of proinflammatory genes [45]. In contrast to MyD88, recruitment of TICAM1 downstream of TLR4 and TLR3 is followed by activation of the NAP1/IKBKE/TBK kinase complex and subsequently IRF-3 that eventually drives the expression of type I interferons. However, TICAM1 can also activate NFkB through RIP1 and, successively, TRAF6 and its downstream targets [34].
So far, it appears that TLR4 is the only member of the TLR family that is able to recruit and activate two different pairs of TIR domain-containing adaptor molecules (TIRAP/MyD88 and TICAM1/2). This distinct ability, apparently determines its potent and often extremely adverse biological effects [23]. This was clearly demonstrated through the finding that induction of type I interferons by LPS is essential for the etiology of endotoxic shock [46].
3. Response of rainbow trout mononuclear phagocytes (rtMOCs) to LPS
In an in vitro study, part of which was published recently [47], we analyzed the dose-response of trout mononuclear phagocytes (rtMOCs) after stimulation with different PAMPs including zymosan (yeast cell wall extract), muramyl dipeptide (MDP, structural component of peptidoglycan), particulate and soluble β-glucan from yeast, poly(I:C) (dsRNA analog) and 5 different LPS preparations. Interestingly, after stimulation with zymosan, MDP, particulate β-glucan and poly(I:C), the cells responded by the up-regulation of proinflammatory cytokines with the same sensitivity as observed with mammalian cells ( Fig. 1; data showing the dose response to particulate beta glucan and poly(I:C) are not shown). On the other hand, up-regulation of TNF2 by rtMOCs required ~1000 fold higher LPS concentrations than those needed to induce a similar response in mammalian cells (Fig.1).
Figure 1. Dose-dependent TNF2 induction by different PAMPs.
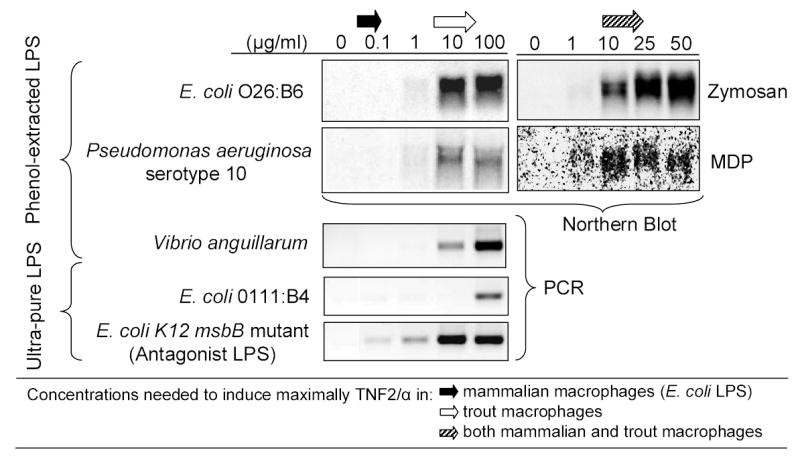
Differentiated rtMOCs, prepared as previously described [91], were stimulated for 12-hours at various PAMP concentrations in the presence of 10% fetal bovine serum (FBS). TNF2 expression in total RNA was analyzed using Northern Blot (upper 4 panels) and RT-PCR (lower 3 panels) as previously described [47]. Equal loading of RNA for Northern blotting was verified by staining ribosomal RNA with ethidium bromide prior to transfer, while the quality of the cDNA used for RT-PCR was confirmed by beta-actin amplification (data not shown). Northern data for the effects of E. coli and P. aeruginosa phenol-extracted LPS, zymosan and MDP from Iliev et al., 2005 [47]. Ultra-pure LPS from Invivogen and phenol-extracted V. anguillarum provided by Dr. J. Bogwald, University of Tromso (Tromso, Norway). The data concerning the activation of mammalian macrophages was derived from the following papers: [92–94] for LPS, [95] for zymosan and [96,97] for MDP.
Another study suggested that fish, in particular rainbow trout and cod, may lack an important component of the LPS recognition receptor complex. Namely, it was recognized that the LBP homologs that had been characterized in trout and cod [48,49] were more likely functional analogs of the mammalian bactericidal permeability increasing protein (BPI) [49]. BPI is another member of the LT/LBP family that, unlike LBP, transfers monomeric LPS to high density lipoproteins instead of CD14. This leads to the neutralization of LPS and interferes with the activation of TLR4 [50–52]. Therefore, it was speculated that the absence of a LBP analog may explain the attenuated sensitivity to LPS in fishes [49]. The hypothesis that trout lack functional LBP was further corroborated by our study that showed that the sensitivity of rtMOCs to LPS was not influenced by the presence of serum [47].
Another interesting observation is that LPS-induced activation of rtMOCs cannot be influenced by the presence of polymyxin B (PMB) (Fig.2). PMB is a cyclic cationic polypeptide produced by Paenibacillus polymixa that binds to lipid A [53,54] and thus interferes with the association between LPS and LY96. In mammalian systems, PMB neutralizes the biological effects of LPS and is widely used to eliminate the effects of endotoxin contamination, both in vitro and in vivo. Therefore, our results with PMB and rtMOCs further suggest that there might be substantial differences in the LPS detection mechanisms between mammals and fishes.
Figure 2. Polymyxin B does not prevent LPS activation of rtMOCs.

Differentiated rtMOCs were stimulated with varying concentrations of E. coli LPS in the presence (+) or the absence (−) of polymyxin (Sigma) B. Panel A: Northern blot of TNF2 induction after stimulation for 12 hours in the presence of 10% FBS. Panel B: RT-PCR of TNF2 induction after 3 hours of stimulation in the presence of 5% homologous trout serum. Methods as described or referenced in Fig. 1.
As discussed earlier, the TLR4 mediated response to LPS involves up-regulation of antiviral genes through a TICAM2-TICAM1-IRF-3-dependent pathway [39]. These genes include type-1 interferons and interferon-inducible genes which are induced secondarily through a STAT1-dependent pathway downstream of the interferon-α/β receptor [24]. We analyzed the induction of several proinflammatory genes (TNF2 and cyclooxygenase-2, COX-2) and homologs of mammalian antiviral genes (interferon-alpha and IP-10-like protein). Crude, phenol-extracted LPS (Sigma) was much more potent in inducing the expression of proinflammatory genes compared to poly(I:C), whereas the reverse was evident for the anti-viral genes (Fig.3). In fact, ultra-pure LPS (Invivogen) did not appear to activate antiviral genes at all. This suggests that the activation of the genes involved in the antiviral response by phenol-extracted LPS may have been driven by contaminants in the preparation such as bacterial CpG DNA which can activate the expression of type-1 interferons through a TLR8-IRF5/7-dependent pathway [55]. In addition, the ultra-pure LPS preparation did not induce IFN-α and IP-10-like protein in rtMOCs when the cells were stimulated in the presence of homologous trout serum (data not shown). Further, a very recent study presented similar data confirming that in rainbow trout head kidney leukocytes, IFN-α expression was inducible by poly(I:C) and R848 (a TLR7/8 agonist) but not by LPS preparations from E. coli and Salmonella minnesota [56]. In mammals, it has been clearly shown that non-enterobacterial LPS, that signals through TLR2, does not induce the expression of type-1 interferons and fails to activate STAT1 [24,25]. This is linked to the fact that induction of antiviral genes downstream of TLR4 is TICAM2/TICAM1-dependent [39].
Figure 3. Differential gene induction by LPS from E. coli and poly(I:C).
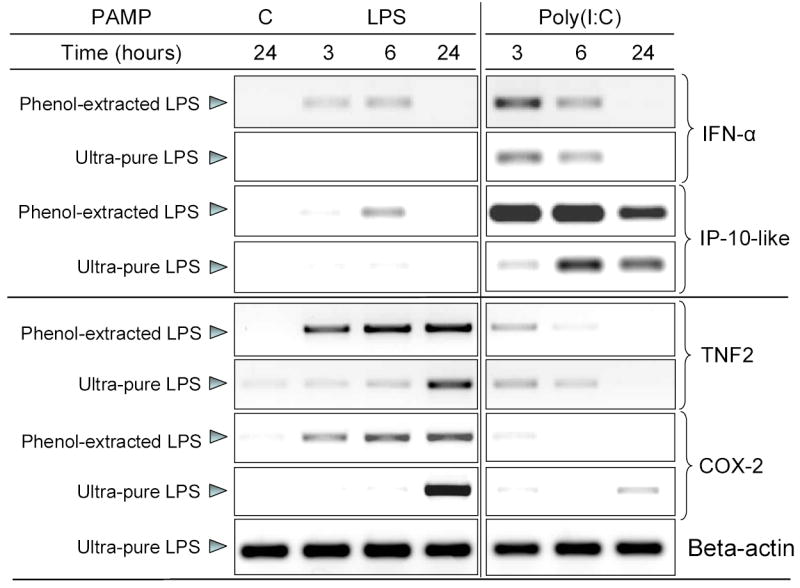
Differentiated rtMOCs were stimulated for varying times with 50 μg/ml of LPS or poly(I:C). Non-stimulated cells (“C”) were left untreated for 24 hours. Expression of IFN-α, IP-10-like, TNF2, COX-2 and beta-actin were analyzed in total RNA using RT-PCR. Results from two experiments are presented. The first experiment includes treatments with phenol-extracted LPS and poly(I:C). The second includes treatments with ultra-pure LPS and poly(I:C). The TNF2 bands across each row (i.e., under LPS and poly(I:C) stimulation) were obtained from the same experiment and were visualized on the same gel. Therefore, they can be directly compared.
Taken together, the data discussed above imply that the cellular responses to LPS in fish deviate from the model established for mammals and that the differences point to the absence of TLR4-dependent activation of piscine leukocytes by LPS stimulated under in vitro conditions.
4. TLR4 and TIR domain-containing adaptor molecules in lower vertebrates
TICAM1, TIRAP and MyD88 are very well evolutionarily conserved and easily identifiable in fish genomic and EST databases. According to our observations, TICAM2 is very well-conserved within mammals and its presence as a single gene exon greatly facilitates its identification in genomic databases. Even so, we have been unable to find a TICAM2 homolog in any of the fish genomic databases. When murine TICAM2 is used as a search query, the best match in all of the non-mammalian genomic databases is TICAM1, while the second best match is usually TIRAP. Figure 4A shows the evolutionary relations between TICAM1 and TICAM2. It is obvious that, compared to TICAM1s, TICAM2s are much more tightly clustered most likely as a result of their restricted functions and the stronger “conservative” selective pressure exerted on them. On the other hand, the evolutionary distance between TICAM1s, both within and between different vertebrate classes, is much larger. This may be a result of the diverse functions of TICAM1s (e.g., conveying signals downstream of different TLRs).
Figure 4. Phylogenetic analyses. Panel A: TRIF and TRAM are closely related; Multidimensional scaling (MDS) of the molecular distances between the TIR domains of the TLR-adapter proteins.
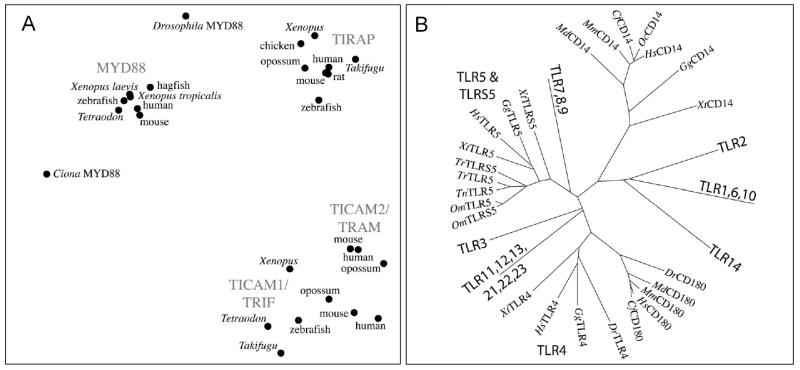
TICAM2 is only found in mammals, suggesting a recent divergence from TICAM1. The distance between the gene families compared to the distances within the gene families is so great that it is possible that portraying this information as a molecular tree could be misleading. MDS permits visualization of the proximity of TICAM1 and TICAM2 with respect to MYD88 and TIRAP. Distances are computed with PROTDIST at alignment positions with at least 80% non-gap characters (i.e., only from the TIR domain) from alignments by CLUSTALX. MDS is performed as described in [98]. Panel B: Molecular tree of the Toll-like LRR multigene family; CD180, CD14 and TLRS5 belong to the same superfamily as the vertebrate TLRs. The LRR domains of TLR4 are more closely related to CD180 than they are to any other TLR. The LRR domains of TLR5 are more closely related to TLRS5 than they are to any other TLR. This molecular tree is derived from a CLUSTALX alignment, followed by PROTDIST and FITCH from the PHYLIP package. Distance is calculated only from the 600 alignment positions with at least 80% non-gap characters (out of 855), up to the end of the CD180 alignment (i.e., not including the TIR domain of the TLRs). A single TLR is used to represent TLR clades described in [14], with additional illustrative TLRs from the TLR4 and TLR5 clades. CD180 is monophyletic, but TLRS5 is not. This difference in relationships suggests differences in mechanisms of evolution and selection pressures and thus differences in functional relationships. Mammals do not possess TLR5S. Xt: Xenopus tropicalis - frog; Gg: Gallus gallus - chicken; Hs: Homo sapiens; Dr: Danio rerio - zebrafish; Md: Monodelphis domestica - opossum; Mm: Mus musculus - mouse; Cf: Canis familiaris - dog; Om: Oncorhynchus mykiss - trout; Tr: Takifugu rubripes - Torafugu pufferfish; Tn: Tetraodon nigroviridis - Spotted green pufferfish.
In addition to TICAM2, searching for TLR4 reveals that this receptor may be absent in the genomes of pufferfish, Takifugu rubripes (fugu) and Tetraodon nigroviridis, though it is present in zebrafish [57]. Although TLR4 is present in some lower vertebrates, the data we have discussed so far raises the question of how well the function of TLR4 is conserved across vertebrates. Interestingly, the extracellular domain of TLR4 shares greater sequence similarity with CD180 (RP105) than with any of the other TLRs (Fig.4). CD180 was not included in a recently published phylogenetic analysis of the evolution of vertebrate TLRs as it did not meet the definition of a TLR used by Roach et al [14]. Our phylogenetic analysis reproduces the topology of the TLR phylogenetic tree published by these authors and reveals that the TLR4 and CD180 lineages cluster together and form a distinct evolutionary branch when compared with other TLRs. It also appears that these two genes evolved from a common ancestor shortly before the divergence between fishes and tetrapods (Fig.4B).
Like TLR4, CD180 cooperates with an accessory molecule, LY86 (MD-1), which is a close homolog to LY96 [58,59]. However, although CD180/LY86 is also implicated in the activation of the immune system by LPS, it does not directly bind endotoxin [60,61]. CD180 lacks an intracellular TIR domain and mediates cellular responses to LPS indirectly through interaction with other receptors, including TLR4 and TLR2 [62–65]. These facts raise the question of whether the common ancestor of TLR4 and CD180 was directly involved in the response to LPS or whether this function arose later, after the divergence between TLR4 and CD180. Although it is difficult to find a convincing answer to this question, a closer look at the phylogeny of LY96 seems to support the second possibility. LY96 is a small molecule that is not well conserved evolutionarily and is not present in any of the fish EST databases. In addition, the LY96 gene appears to be interrupted by several introns (our unpublished observations) which makes its in silico identification very difficult. As a result, we cannot determine from the genomic databases whether LY96 exists in fish. However, LY96 as well as CD14 and TLR4 homologs are present in chicken. Interestingly, alignment (our unpublished observations) of chicken LY96 and both LY96 and LY86 from human and mouse shows that, like mammalian LY86, the chicken LY96 homolog lacks the amino acids that are absolutely required for the LPS-binding ability of mammalian LY96 [60]. Moreover, chicken leukocytes, just like fish leukocytes, require stimulation with much higher concentrations of LPS to initiate a respiratory burst response [66]. Furthermore, although E. coli LPS is pyrogenic for chickens, excessive doses of LPS did not appear to be lethal for them, in sharp contrast to the different mammalian species studied by Berczi and colleagues [16].
Therefore, while TLR4 may be involved in the response to LPS in non-mammalian vertebrates, the function of the TLR4 receptor complex that mediates the ultra-sensitive and exceptionally robust cellular reactions to endotoxin appears to be restricted to mammals.
It may be argued that the piscine EST databases are still relatively shallow. Currently, the number of ESTs from zebrafish, trout and salmon taken together is ~10 times lower than the number of EST sequences present in the human and the murine EST databases. In addition, the sequences of the fish genomes are still not completely assembled and contain numerous gaps. Both of these caveats may obstruct the identification of mammalian orthologs in the fish databases and may account for the inability to identify elements involved in the TLR4-mediated signaling. However, so far all of the major proximal elements of the common TLR signaling pathways (including MyD88, TIRAP, TICAM1, IRAK-4 and TRAF6) have been identified in the genomes and/or ESTs of different fish species. Therefore, it is quite remarkable that, there are no identifiable piscine homologues of all the genes (namely, CD14, LY-96 and TICAM2) that are specifically involved in the TLR4-mediated immune response.
A second possibility that may account for the failure to detect these genes in fish may be the increased rate of their evolution. There are genes (e.g., many cytokines) that evolve rapidly resulting in the generation of highly divergent orthologs that may be difficult to identify through direct sequence comparison. However, CD14, LY96 and TICAM2 appear to be relatively well conserved within mammals. In fact, the similarity between human and murine TICAM2 is higher than the similarity between human and murine TICAM1 (Fig. 4 A). Therefore, if we assume that TICAM2, LY96 and CD14 orthologs already existed in the common predecessor of fish and tetrapods, than it would mean that the selective pressure on those genes, unlike the other proximal TLR signaling elements, differed drastically throughout vertebrate evolution. The increased rate of evolution of CD14, LY96 and TICAM2 during the evolution of tetrapods, particularly mammals, may be explained by the adoption of novel functions that may have redirected the selective pressure on those genes. Further, the “conservative” selective pressure on TICAM2, LY96 and CD14 within mammals may have been governed by the need to conserve those newly evolved functions which may have been TLR4-mediated endotoxin recognition and sensitivity to LPS. For example, CD14 which appears to originate from a TLR, possibly a member of the TLR2/14 clade, is relatively poorly conserved between the vertebrate classes as compared to TLRs (Fig.4B).
A significant part of the in vivo and the in vitro data that supports our hypothesis is derived from studies on rainbow trout for which there is no genomic data available. On the other hand, the major part of our in silico analysis is based on the genomes of fish (zebrafish, fugu and Tetraodon) for which experimental data concerning the immune response to LPS is scant. Currently, there are no published data that would suggest that zebrafish and fugu are as sensitive to LPS as mammals. In fact, papers that have reported on the in vitro stimulation of peripheral blood leukocytes from fugu and isolated kidneys from zebrafish have used fairly high concentrations of LPS (50 and 10 μg/ml, respectively) [67,68]. In addition, it is interesting that IL-12 p35, a gene whose induction in murine macrophages by LPS is strictly TLR4-dependent [69], was not activated in fugu after intraperitoneal injections with LPS though TNF and IL-1β were [70]. Furthermore, extremely high concentrations of LPS (500 μg/ml) have been used to stimulate peripheral blood leukocytes from Paralichthys olivaceus [71,72], a relative of Fugu and Tetraodon (Series Percomorpha). Finally, zebrafish belong to the family Cyprinidae and carp, another member of this family, were used in the LPS sensitivity experiments that demonstrated resistance of this species to endotoxic shock [16]. It seems unlikely that so closely related species may exhibit fundamental differences in their response to LPS.
5. Alternative receptors for LPS
In addition to the TLR4 complex, enterobacterial LPS has been shown to interact with several other membrane-bound proteins, including CD11/CD18 heterodimers (also known as beta2 integrins), heat shock protein 70 (HSP70), HSP90, the chemokine receptor CXCR4 and growth differentiation factor 5 (GDF5) [73,74]. In addition, many non-enterobacterial species produce LPS that signal through TLR2 [11,75–79]. Recently, Roach and colleagues [14] suggested that Fugu TLR23 may participate in LPS recognition which may compensate for the loss of TLR4 in this species. Also, based on the observation that LPS from E. coli and P. aeruginosa induced responses of different durations in rtMOCs, we speculated previously that different receptors might be involved in the activation of rtMOCs by E. coli and P. aeruginosa LPSs [47].
Beta2 integrins, which are one of the most abundant receptor types found on macrophages, are especially remarkable because of their ability to transmit intracellular activation signals through MAP kinases and NF-κB [80–83]. In addition, these PRRs are involved in the internalization of LPS and whole bacteria [84]. It appears that, upon encountering bacteria, beta-2 integrins recognize the hydrophilic carbohydrate moieties of LPS which are exposed to the environment [85], but not the hydrophobic endotoxic moiety that is buried in the outer bacterial membrane. When LPS is released in aqueous solutions it forms supra-molecular aggregates. The size of these structures, which can assume micellar or lamellar shapes, correlates with the LPS concentration and the availability of divalent cations (e.g. Ca2+ and Mg2+) [86–88]. The LPS aggregates apparently resemble the surface of Gram-negative bacteria and perhaps it is these aggregates, rather than the endotoxic moiety of LPS, that trigger the beta-2 integrin-mediated cellular response. In support of this, the LPS concentrations required for beta-2 integrin-mediated activation of NFkB are relatively high (e.g., μg/ml) and close to the critical micelle concentration (concentration above which LPS micelles can be detected) that has been reported by different authors [88,89].
Interestingly, we found that the LPS concentrations required to activate rtMOCs were similarly high (Fig.1). In addition, we observed that the immunostimulatory potency of a given LPS preparation was inversely correlated with its ability to disperse in water. For example, a highly dispersible, ultra-pure LPS preparation (E. coli 0111:B4) weakly induced TNF2 expression in rtMOCs even when administered at high concentrations (Fig.1). On the other hand, an ultra-pure LPS preparation (E. Coli K12 msbB mutant), that was difficult to disperse in water and formed a hazy stock solution (most likely because of the presence of large LPS aggregates), activated rtMOCs at much lower concentrations. We were also able to observe this correlation with other LPS preparations that we tested on rtMOCs. These observations suggest that the activation of rtMOCs by LPS depends on the presence of supra-molecular structures. Finally, the kinetics of activation of rtMOCs by LPS are relatively delayed, taking hours to reach maximal expression of inflammatory genes [47]. These features parallel the characteristics of the beta2 integrin-mediated activation of mammalian cells which require stimulation with high concentrations of LPS to slowly activate NFkB [80]. Therefore, our results suggest that beta-2 integrins may be key elements in the recognition of LPS by trout macrophages. Nevertheless, this does not exclude the possibility that other receptors, including TLRs may be involved in the activation of rtMOCs by LPS through collateral interactions with beta-2 integrins and, subsequently, transmission of intracellular activation signals. A similar process has been observed in mammals [90].
In summary, our analysis questions the existence of CD14/LY96/TLR4-mediated cellular responses to LPS in fish. While TLR4 is present in non-mammalian vertebrates and might still be involved in the immune response to LPS, its function appears to differ significantly between mammals and other vertebrates. It is likely that the ability of TLR4 to initiate a robust immune response upon exposure to minute amounts of endotoxin arose relatively late in the evolution of vertebrates, and may be pertinent only to higher vertebrates. This seems to have depended on the evolution of accessory (CD-14 and LY96) and adapter molecules (TICAM2) which, so far, have not been found in fish. Our analysis also suggests that alternative signaling receptors such as beta-2 integrins may play a primary role in the activation of piscine leukocytes by LPS. The overall supporting facts of our hypothesis are summarized in figure 5.
Figure 5. A schematic representation of the model of the CD14/LY96/TLR4-dependent response to LPS.
The right panel summarizes on the key observations that question the presence of this model in fish. The TLR4 homodimerization is not depicted for schematic clarity. See text for details.
5. Concluding remarks
Elimination of pathogens by the immune systems of their multicellular hosts may occur directly through phagocytosis which is mediated by receptors, such as integrins and scavenger receptors. Alternatively, potentially pathogenic microorganisms or their PAMPs may activate signaling receptors such as TLRs that can consecutively initiate cellular and humoral immune responses leading to indirect clearance of pathogens. Unlike direct pathogen elimination, the TLR-mediated immune reaction does not require the presence of the whole pathogen and can potentially initiate the immune reaction without an immediate rendezvous between the pathogen and the host leukocytes. This “reconnaissance” mechanism can add more flexibility to the immune reaction and can also provide the host immunity with additional tactics to cope with an infection. However, in mammals, erroneous reconnaissance input, such as the uncontrolled activation of the TLR4 complex by excessive amounts of LPS, may lead to an endotoxic reaction that is devastating for the host. The intimate mechanisms of this process are relatively well understood; nevertheless, it is still unknown whether the endotoxicity is merely a side-effect that accompanies the immune reaction to Gram-negative bacteria or whether it provides any advantage for the species that exhibit it. Therefore, more detailed studies on non-mammalian vertebrates that do not manifest this phenomenon can be of critical importance for its adequate understanding.
Acknowledgments
Research leading to this paper was supported by National Research Initiative Competitive Grant nos. 2004-35024-14232 and -14709 from the USDA Cooperative State Research, Education, and Extension Service to FWG and by a grant to JCR from NIAID (5K08AI056092).
References
- 1.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–59. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 3.Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–66. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 4.Nurnberger T, Brunner F. Innate immunity in plants and animals: emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr Opin Plant Biol. 2002;5:318–24. doi: 10.1016/s1369-5266(02)00265-0. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 7.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop RE. Fundamentals of endotoxin structure and function. Contrib Microbiol. 2005;12:1–27. doi: 10.1159/000081687. [DOI] [PubMed] [Google Scholar]
- 9.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 11.Hirschfeld M, et al. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–82. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jault C, Pichon L, Chluba J. Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol Immunol. 2004;40:759–71. doi: 10.1016/j.molimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Oshiumi H, Tsujita T, Shida K, Matsumoto M, Ikeo K, Seya T. Prediction of the prototype of the human Toll-like receptor gene family from the pufferfish, Fugu rubripes, genome. Immunogenetics. 2003;54:791–800. doi: 10.1007/s00251-002-0519-8. [DOI] [PubMed] [Google Scholar]
- 14.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–82. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsujita T, Tsukada H, Nakao M, Oshiumi H, Matsumoto M, Seya T. Sensing bacterial flagellin by membrane and soluble orthologs of Toll-like receptor 5 in rainbow trout (Onchorhynchus mikiss) J Biol Chem. 2004;279:48588–97. doi: 10.1074/jbc.M407634200. [DOI] [PubMed] [Google Scholar]
- 16.Berczi I, Bertok L, Bereznai T. Comparative studies on the toxicity of Escherichia coli lipopolysaccharide endotoxin in various animal species. Can J Microbiol. 1966;12:1070–1. doi: 10.1139/m66-143. [DOI] [PubMed] [Google Scholar]
- 17.Hirono I, Takami M, Miyata M, Miyazaki T, Han HJ, Takano T, Endo M, Aoki T. Characterization of gene structure and expression of two toll-like receptors from Japanese flounder, Paralichthys olivaceus. Immunogenetics. 2004;56:38–46. doi: 10.1007/s00251-004-0657-2. [DOI] [PubMed] [Google Scholar]
- 18.MacKenzie S, Planas JV, Goetz FW. LPS-stimulated expression of a tumor necrosis factor-alpha mRNA in primary trout monocytes and in vitro differentiated macrophages. Dev Comp Immunol. 2003;27:393–400. doi: 10.1016/s0145-305x(02)00135-0. [DOI] [PubMed] [Google Scholar]
- 19.Pelegrin P, Garcia-Castillo J, Mulero V, Meseguer J. Interleukin-1beta isolated from a marine fish reveals up-regulated expression in macrophages following activation with lipopolysaccharide and lymphokines. Cytokine. 2001;16:67–72. doi: 10.1006/cyto.2001.0949. [DOI] [PubMed] [Google Scholar]
- 20.Stafford JL, Ellestad KK, Magor KE, Belosevic M, Magor BG. A toll-like receptor (TLR) gene that is up-regulated in activated goldfish macrophages. Dev Comp Immunol. 2003;27:685–98. doi: 10.1016/s0145-305x(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 21.Zou J, Secombes CJ, Long S, Miller N, Clem LW, Chinchar VG. Molecular identification and expression analysis of tumor necrosis factor in channel catfish (Ictalurus punctatus) Dev Comp Immunol. 2003;27:845–58. doi: 10.1016/s0145-305x(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 23.Vogel SN, Fitzgerald KA, Fenton MJ. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Interv. 2003;3:466–77. doi: 10.1124/mi.3.8.466. [DOI] [PubMed] [Google Scholar]
- 24.Toshchakov V, et al. TLR2 and TLR4 agonists stimulate unique repertoires of host resistance genes in murine macrophages: interferon-beta-dependent signaling in TLR4-mediated responses. J Endotoxin Res. 2003;9:169–75. doi: 10.1179/096805103125001577. [DOI] [PubMed] [Google Scholar]
- 25.Toshchakov V, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–8. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 26.Mathison JC, Tobias PS, Wolfson E, Ulevitch RJ. Plasma lipopolysaccharide (LPS)-binding protein. A key component in macrophage recognition of gram-negative LPS. J Immunol. 1992;149:200–6. [PubMed] [Google Scholar]
- 27.Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–31. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 28.Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–8. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 29.Hailman E, et al. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–77. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akashi S, et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med. 2003;198:1035–42. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gioannini TL, Teghanemt A, Zhang D, Levis EN, Weiss JP. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res. 2005;11:117–23. doi: 10.1179/096805105X35198. [DOI] [PubMed] [Google Scholar]
- 32.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004;101:4186–91. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns K, et al. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–9. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 34.Dunne A, O'Neill L. Adaptor usage and Toll-like receptor signaling specificity. FEBS Lett. 2005 doi: 10.1016/j.febslet.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–7. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 36.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–41. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 37.Fitzgerald KA, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 38.Horng T, Barton GM, Flavell RA, Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–33. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–50. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto M, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–9. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J Biol Chem. 2003;278:16713–9. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki N, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–6. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 43.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–6. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 44.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–62. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 46.Karaghiosoff M, et al. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol. 2003;4:471–7. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- 47.Iliev DB, Liarte CQ, MacKenzie S, Goetz FW. Activation of rainbow trout (Oncorhynchus mykiss) mononuclear phagocytes by different pathogen associated molecular pattern (PAMP) bearing agents. Mol Immunol. 2005;42:1215–23. doi: 10.1016/j.molimm.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Inagawa H, Honda T, Kohchi C, Nishizawa T, Yoshiura Y, Nakanishi T, Yokomizo Y, Soma G. Cloning and characterization of the homolog of mammalian lipopolysaccharide-binding protein and bactericidal permeability-increasing protein in rainbow trout Oncorhynchus mykiss. J Immunol. 2002;168:5638–44. doi: 10.4049/jimmunol.168.11.5638. [DOI] [PubMed] [Google Scholar]
- 49.Stenvik J, Solstad T, Strand C, Leiros I, Jorgensen TT. Cloning and analyses of a BPI/LBP cDNA of the Atlantic cod (Gadus morhua L.) Dev Comp Immunol. 2004;28:307–23. doi: 10.1016/j.dci.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Wiese A, Brandenburg K, Lindner B, Schromm AB, Carroll SF, Rietschel ET, Seydel U. Mechanisms of action of the bactericidal/permeability-increasing protein BPI on endotoxin and phospholipid monolayers and aggregates. Biochemistry. 1997;36:10301–10. doi: 10.1021/bi970176m. [DOI] [PubMed] [Google Scholar]
- 51.Elsbach P, Weiss J. Bactericidal/permeability increasing protein and host defense against gram-negative bacteria and endotoxin. Curr Opin Immunol. 1993;5:103–7. doi: 10.1016/0952-7915(93)90088-a. [DOI] [PubMed] [Google Scholar]
- 52.Dentener MA, Von Asmuth EJ, Francot GJ, Marra MN, Buurman WA. Antagonistic effects of lipopolysaccharide binding protein and bactericidal/permeability-increasing protein on lipopolysaccharide-induced cytokine release by mononuclear phagocytes. Competition for binding to lipopolysaccharide. J Immunol. 1993;151:4258–65. [PubMed] [Google Scholar]
- 53.Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–8. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 54.Srimal S, Surolia N, Balasubramanian S, Surolia A. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochem J. 1996;315(Pt 2):679–86. doi: 10.1042/bj3150679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai T, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–8. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 56.Purcell M.K., Smith K.D., Hood L., Winton J.R., and Roach J.C. (2005). Conservation of Toll-Like Receptor Signaling Pathways in Teleost Fish. Comparative Biochemistry and Physiology - Part D: Genomics and Proteomics. In Press. [DOI] [PMC free article] [PubMed]
- 57.Meijer AH, Gabby Krens SF, Medina Rodriguez IA, He S, Bitter W, Ewa Snaar-Jagalska B, Spaink HP. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol Immunol. 2004;40:773–83. doi: 10.1016/j.molimm.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Miyake K, et al. Mouse MD-1, a molecule that is physically associated with RP105 and positively regulates its expression. J Immunol. 1998;161:1348–53. [PubMed] [Google Scholar]
- 59.Nagai Y, et al. Requirement for MD-1 in cell surface expression of RP105/CD180 and B-cell responsiveness to lipopolysaccharide. Blood. 2002;99:1699–705. doi: 10.1182/blood.v99.5.1699. [DOI] [PubMed] [Google Scholar]
- 60.Tsuneyoshi N, Fukudome K, Kohara J, Tomimasu R, Gauchat JF, Nakatake H, Kimoto M. The functional and structural properties of MD-2 required for lipopolysaccharide binding are absent in MD-1. J Immunol. 2005;174:340–4. doi: 10.4049/jimmunol.174.1.340. [DOI] [PubMed] [Google Scholar]
- 61.Visintin A, Latz E, Monks BG, Espevik T, Golenbock DT. Lysines 128 and 132 enable lipopolysaccharide binding to MD-2, leading to Toll-like receptor-4 aggregation and signal transduction. J Biol Chem. 2003;278:48313–20. doi: 10.1074/jbc.M306802200. [DOI] [PubMed] [Google Scholar]
- 62.Miyake K, Yamashita Y, Ogata M, Sudo T, Kimoto M. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J Immunol. 1995;154:3333–40. [PubMed] [Google Scholar]
- 63.Ogata H, et al. The toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23–9. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Divanovic S, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6:571–8. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagai Y, et al. The radioprotective 105/MD-1 complex links TLR2 and TLR4/MD-2 in antibody response to microbial membranes. J Immunol. 2005;174:7043–9. doi: 10.4049/jimmunol.174.11.7043. [DOI] [PubMed] [Google Scholar]
- 66.Farnell MB, Crippen TL, He H, Swaggerty CL, Kogut MH. Oxidative burst mediated by toll like receptors (TLR) and CD14 on avian heterophils stimulated with bacterial toll agonists. Dev Comp Immunol. 2003;27:423–9. doi: 10.1016/s0145-305x(02)00115-5. [DOI] [PubMed] [Google Scholar]
- 67.Igawa D, Sakai M, Savan R. An unexpected discovery of two interferon gamma-like genes along with interleukin (IL)-22 and -26 from teleost: IL-22 and -26 genes have been described for the first time outside mammals. Molecular Immunology. 2005 doi: 10.1016/j.molimm.2005.05.009. In press, Corrected proof. [DOI] [PubMed] [Google Scholar]
- 68.Bei JX, Suetake H, Araki K, Kikuchi K, Yoshiura Y, Lin HR, Suzuki Y. Two interleukin (IL)-15 homologues in fish from two distinct origins. Molecular Immunology. 2005 doi: 10.1016/j.molimm.2005.06.040. In press, corrected proof. [DOI] [PubMed] [Google Scholar]
- 69.Schuetze N, Schoeneberger S, Mueller U, Freudenberg MA, Alber G, Straubinger RK. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella Enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int Immunol. 2005;17:649–59. doi: 10.1093/intimm/dxh247. [DOI] [PubMed] [Google Scholar]
- 70.Yoshiura Y, Kiryu I, Fujiwara A, Suetake H, Suzuki Y, Nakanishi T, Ototake M. Identification and characterization of Fugu orthologues of mammalian interleukin-12 subunits. Immunogenetics. 2003;55:296–306. doi: 10.1007/s00251-003-0582-9. [DOI] [PubMed] [Google Scholar]
- 71.Park CI, Kurobe T, Hirono I, Aoki T. Cloning and characterization of cDNAs for two distinct tumor necrosis factor receptor superfamily genes from Japanese flounder Paralichthys olivaceus. Dev Comp Immunol. 2003;27:365–75. doi: 10.1016/s0145-305x(02)00118-0. [DOI] [PubMed] [Google Scholar]
- 72.Hirono I, Nam BH, Kurobe T, Aoki T. Molecular cloning, characterization, and expression of TNF cDNA and gene from Japanese flounder Paralychthys olivaceus. J Immunol. 2000;165:4423–7. doi: 10.4049/jimmunol.165.8.4423. [DOI] [PubMed] [Google Scholar]
- 73.Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–45. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 74.Triantafilou M, Triantafilou K. The dynamics of LPS recognition: complex orchestration of multiple receptors. J Endotoxin Res. 2005;11:5–11. doi: 10.1179/096805105225006641. [DOI] [PubMed] [Google Scholar]
- 75.Neumeister B, Faigle M, Sommer M, Zahringer U, Stelter F, Menzel R, Schutt C, Northoff H. Low endotoxic potential of Legionella pneumophila lipopolysaccharide due to failure of interaction with the monocyte lipopolysaccharide receptor CD14. Infect Immun. 1998;66:4151–7. doi: 10.1128/iai.66.9.4151-4157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Girard R, Pedron T, Uematsu S, Balloy V, Chignard M, Akira S, Chaby R. Lipopolysaccharides from Legionella and Rhizobium stimulate mouse bone marrow granulocytes via Toll-like receptor 2. J Cell Sci. 2003;116:293–302. doi: 10.1242/jcs.00212. [DOI] [PubMed] [Google Scholar]
- 77.Werts C, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat Immunol. 2001;2:346–52. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 78.Netea MG, van der Graaf C, Van der Meer JW, Kullberg BJ. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol. 2004;75:749–55. doi: 10.1189/jlb.1103543. [DOI] [PubMed] [Google Scholar]
- 79.Wyllie DH, Kiss-Toth E, Visintin A, Smith SC, Boussouf S, Segal DM, Duff GW, Dower SK. Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J Immunol. 2000;165:7125–32. doi: 10.4049/jimmunol.165.12.7125. [DOI] [PubMed] [Google Scholar]
- 80.Flaherty SF, Golenbock DT, Milham FH, Ingalls RR. CD11/CD18 leukocyte integrins: new signaling receptors for bacterial endotoxin. J Surg Res. 1997;73:85–9. doi: 10.1006/jsre.1997.5195. [DOI] [PubMed] [Google Scholar]
- 81.Ingalls RR, Golenbock DT. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–9. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ingalls RR, Arnaout MA, Delude RL, Flaherty S, Savedra R, Jr, Golenbock DT. The CD11/CD18 integrins: characterization of three novel LPS signaling receptors. Prog Clin Biol Res. 1998;397:107–17. [PubMed] [Google Scholar]
- 83.Medvedev AE, Flo T, Ingalls RR, Golenbock DT, Teti G, Vogel SN, Espevik T. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–42. [PubMed] [Google Scholar]
- 84.Wright SD, Jong MT. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876–88. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chateau MT, Caravano R. The oxidative burst triggered by Salmonella typhimurium in differentiated U937 cells requires complement and a complete bacterial lipopolysaccharide. FEMS Immunol Med Microbiol. 1997;17:57–66. doi: 10.1111/j.1574-695X.1997.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 86.Olins AL, Warner RC. Physicochemical studies on a lipopolysaccharide from the cell wall of Azotobacter vinelandii. J Biol Chem. 1967;242:4994–5001. [PubMed] [Google Scholar]
- 87.Galanos C. Physical state and biological activity of lipopolysaccharides. Toxicity and immunogenicity of the lipid A component. Z Immunitatsforsch Exp Klin Immunol. 1975;149:214–29. [PubMed] [Google Scholar]
- 88.Santos NC, Silva AC, Castanho MA, Martins-Silva J, Saldanha C. Evaluation of lipopolysaccharide aggregation by light scattering spectroscopy. Chembiochem. 2003;4:96–100. doi: 10.1002/cbic.200390020. [DOI] [PubMed] [Google Scholar]
- 89.Aurell CA, Wistrom AO. Critical aggregation concentrations of gram-negative bacterial lipopolysaccharides (LPS) Biochem Biophys Res Commun. 1998;253:119–23. doi: 10.1006/bbrc.1998.9773. [DOI] [PubMed] [Google Scholar]
- 90.Ingalls RR, Arnaout MA, Golenbock DT. Outside-in signaling by lipopolysaccharide through a tailless integrin. J Immunol. 1997;159:433–8. [PubMed] [Google Scholar]
- 91.Goetz, F.W., Iliev, D.B., McCauley, L.A.R., Liarte, C.Q., Tort, L.B., Planas, J.V. and McKenzie, S. (2004). Analysis of genes isolated from lipopolysaccharide-stimulated rainbow trout (Oncorhynchus mykiss) macrophages. Mol Immunol (in press). [DOI] [PubMed]
- 92.Tsan MF, Clark RN, Goyert SM, White JE. Induction of TNF-alpha and MnSOD by endotoxin: role of membrane CD14 and Toll-like receptor-4. Am J Physiol Cell Physiol. 2001;280:C1422–30. doi: 10.1152/ajpcell.2001.280.6.C1422. [DOI] [PubMed] [Google Scholar]
- 93.Ueno M, Sonoda Y, Funakoshi M, Mukaida N, Nose K, Kasahara T. Differential induction of JE/MCP-1 in subclones from a murine macrophage cell line, RAW 264.7: role of kappaB-3 binding protein. Cytokine. 2000;12:207–19. doi: 10.1006/cyto.1999.0544. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto A, Ochiai M, Kataoka M, Toyoizumi H, Horiuchi Y. Development of a highly sensitive in vitro assay method for biological activity of endotoxin contamination in biological products. Biologicals. 2002;30:85–92. doi: 10.1006/biol.2002.0323. [DOI] [PubMed] [Google Scholar]
- 95.Sato M, et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–25. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 96.Wolfert MA, Murray TF, Boons GJ, Moore JN. The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J Biol Chem. 2002;277:39179–86. doi: 10.1074/jbc.M204885200. [DOI] [PubMed] [Google Scholar]
- 97.Le Contel C, Temime N, Charron DJ, Parant MA. Modulation of lipopolysaccharide-induced cytokine gene expression in mouse bone marrow-derived macrophages by muramyl dipeptide. J Immunol. 1993;150:4541–9. [PubMed] [Google Scholar]
- 98.Roach JC, Wang K, Gan L, Hood L. The molecular evolution of the vertebrate trypsinogens. J Mol Evol. 1997;45:640–52. doi: 10.1007/pl00006268. [DOI] [PubMed] [Google Scholar]