Abstract
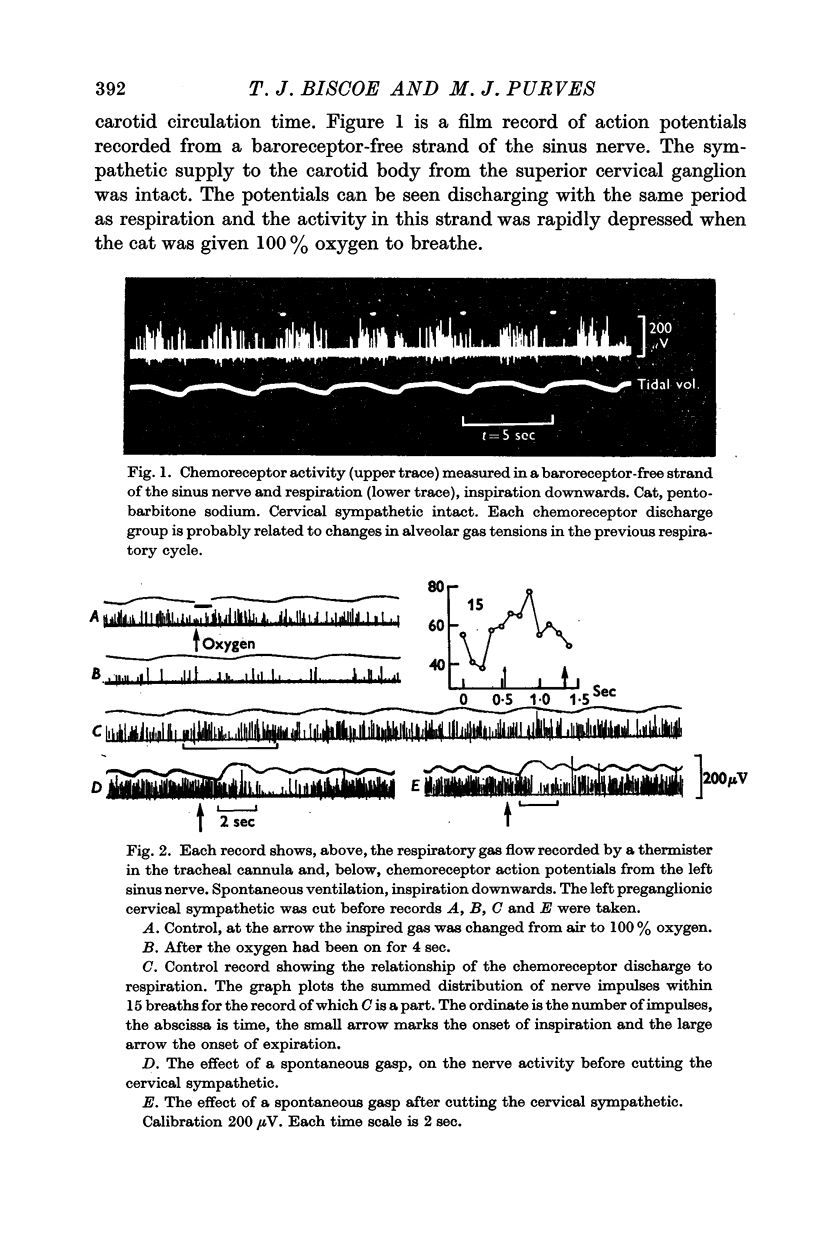
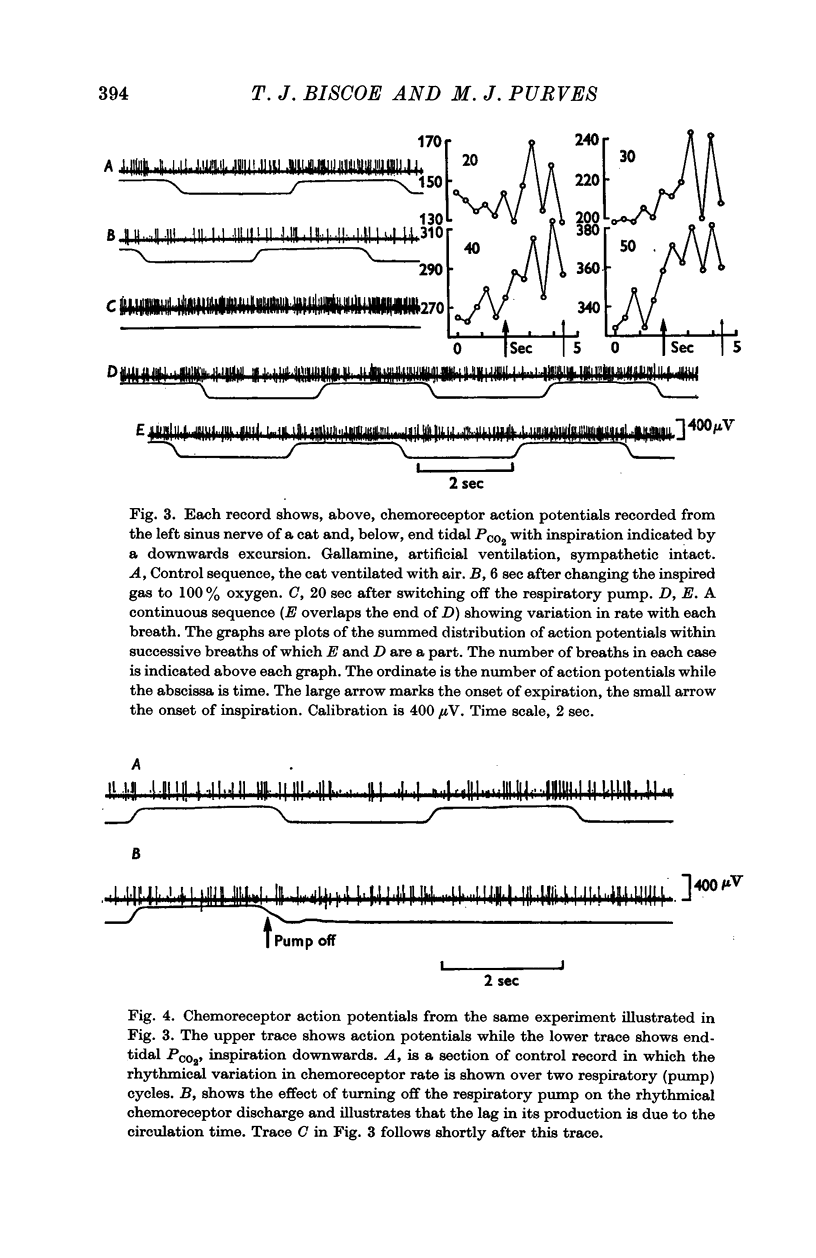
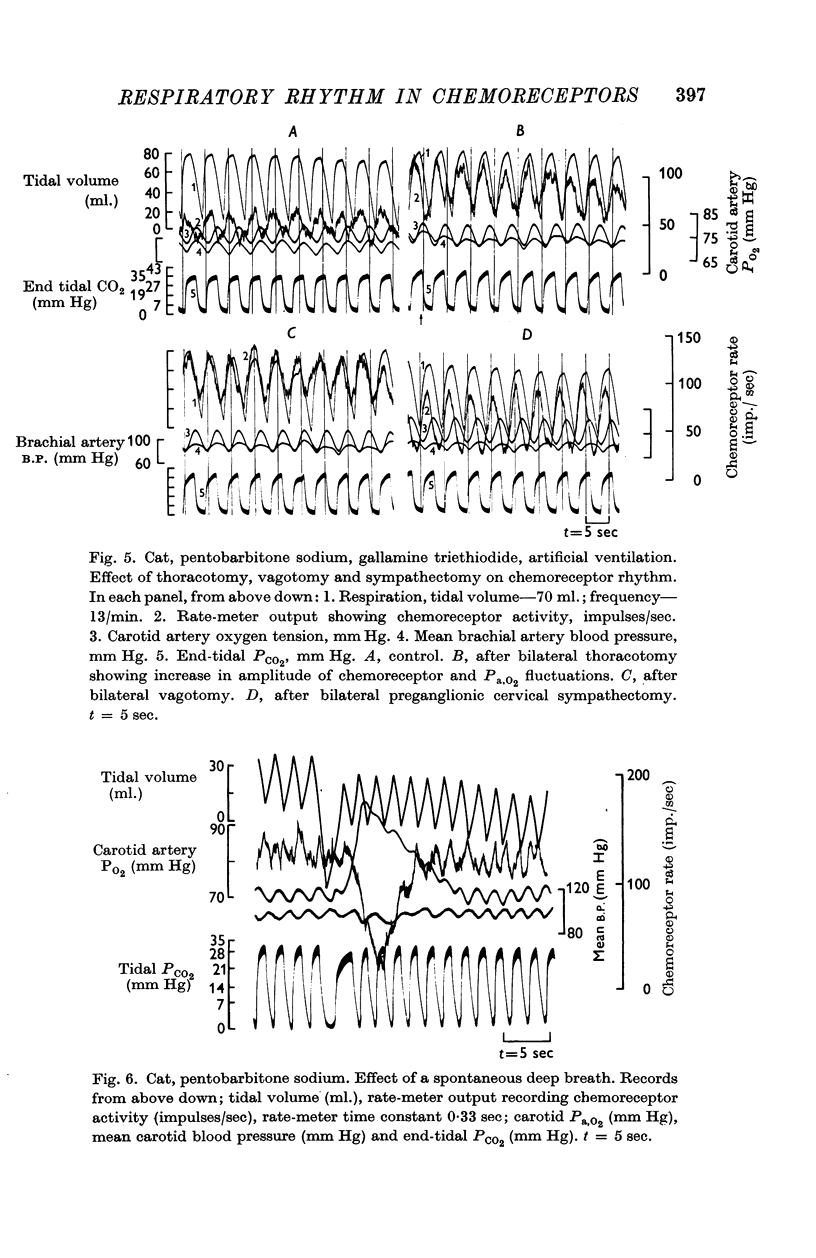
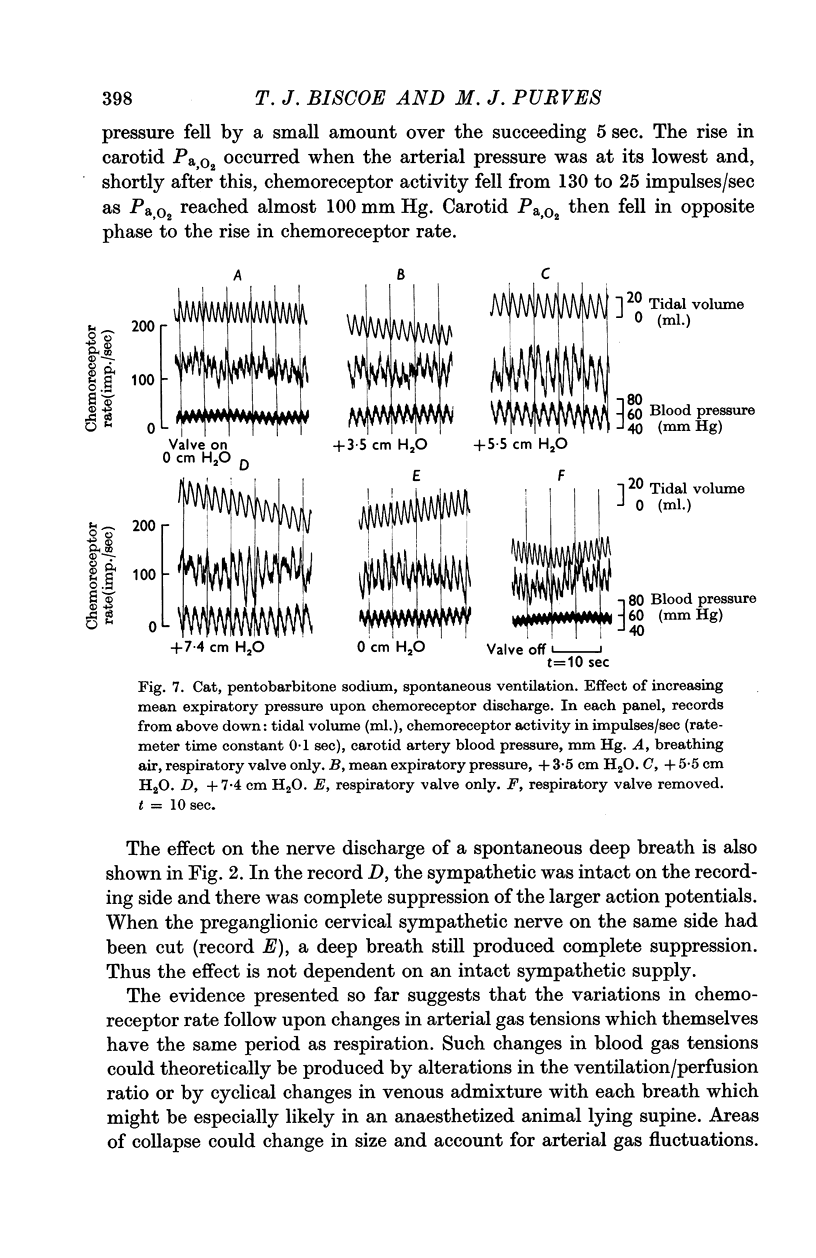
1. The activity in carotid body chemoreceptor afferent fibres in the cat has been recorded and found to have a rhythm with the same period as respiration.
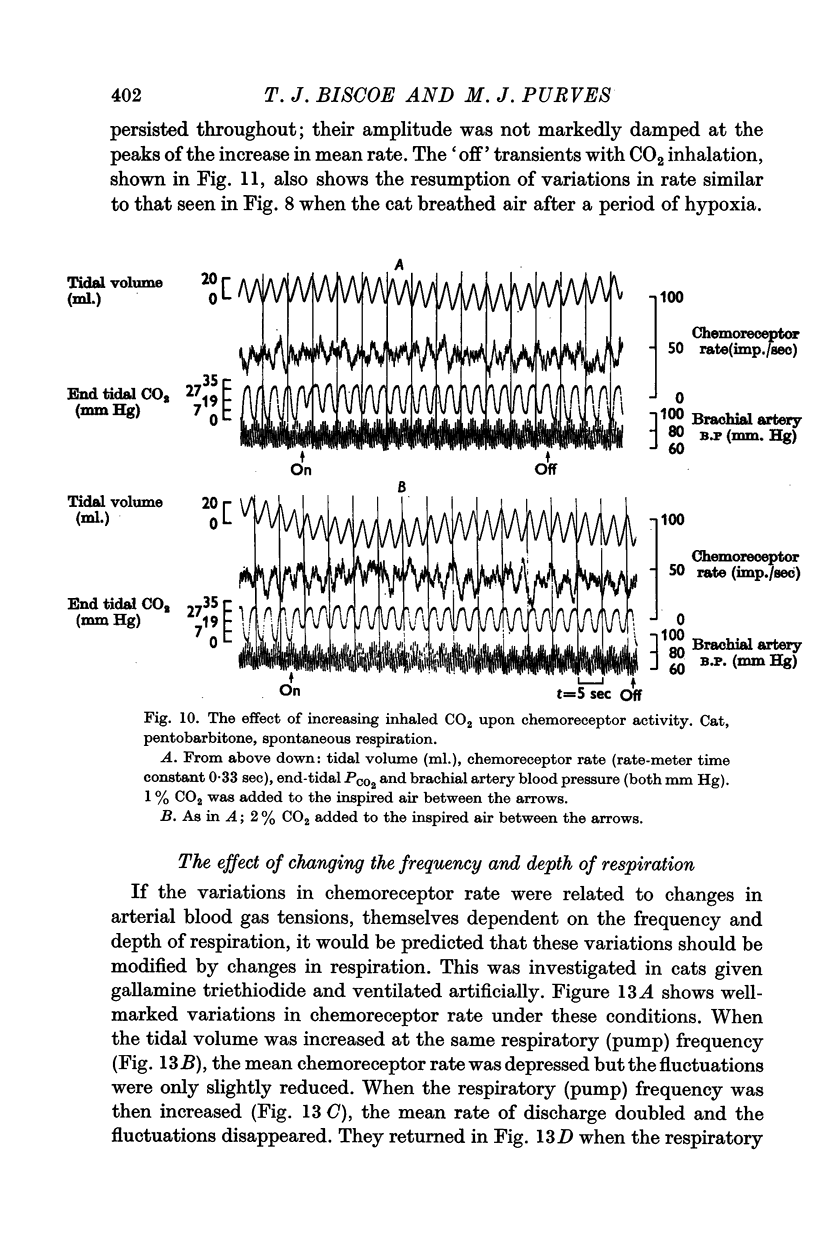
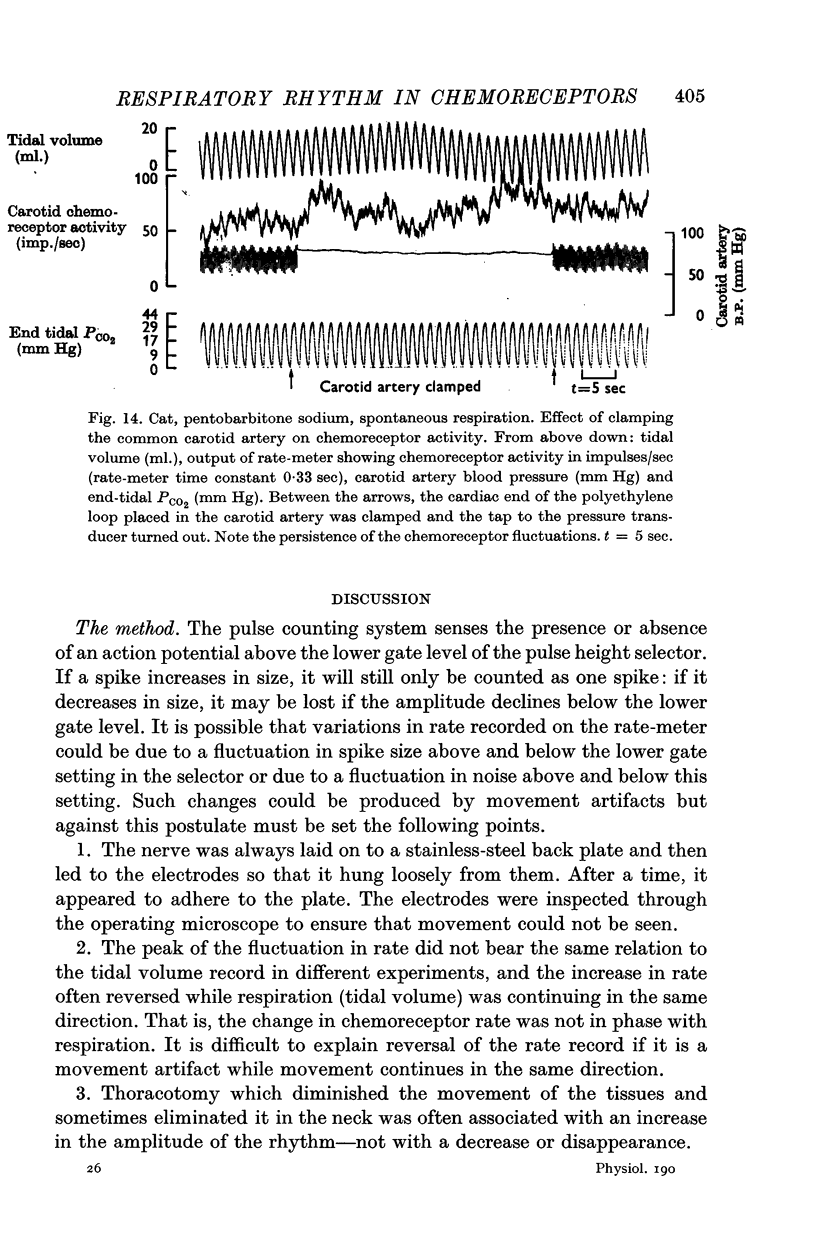
2. This rhythm is not an artifact; it is not due to arterial pressure changes with respiration nor to cyclical changes in pulmonary venous admixture. It is caused by changes in blood gas tensions during each respiratory cycle.
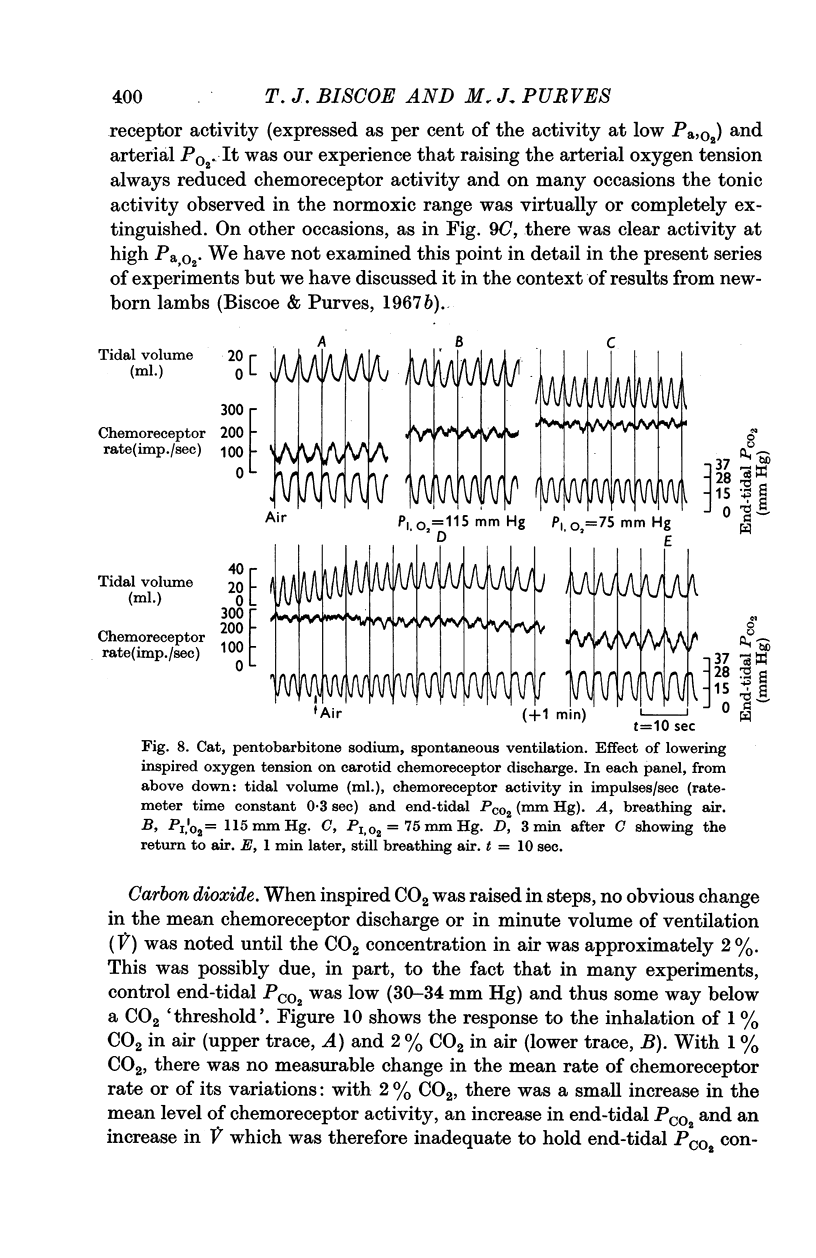
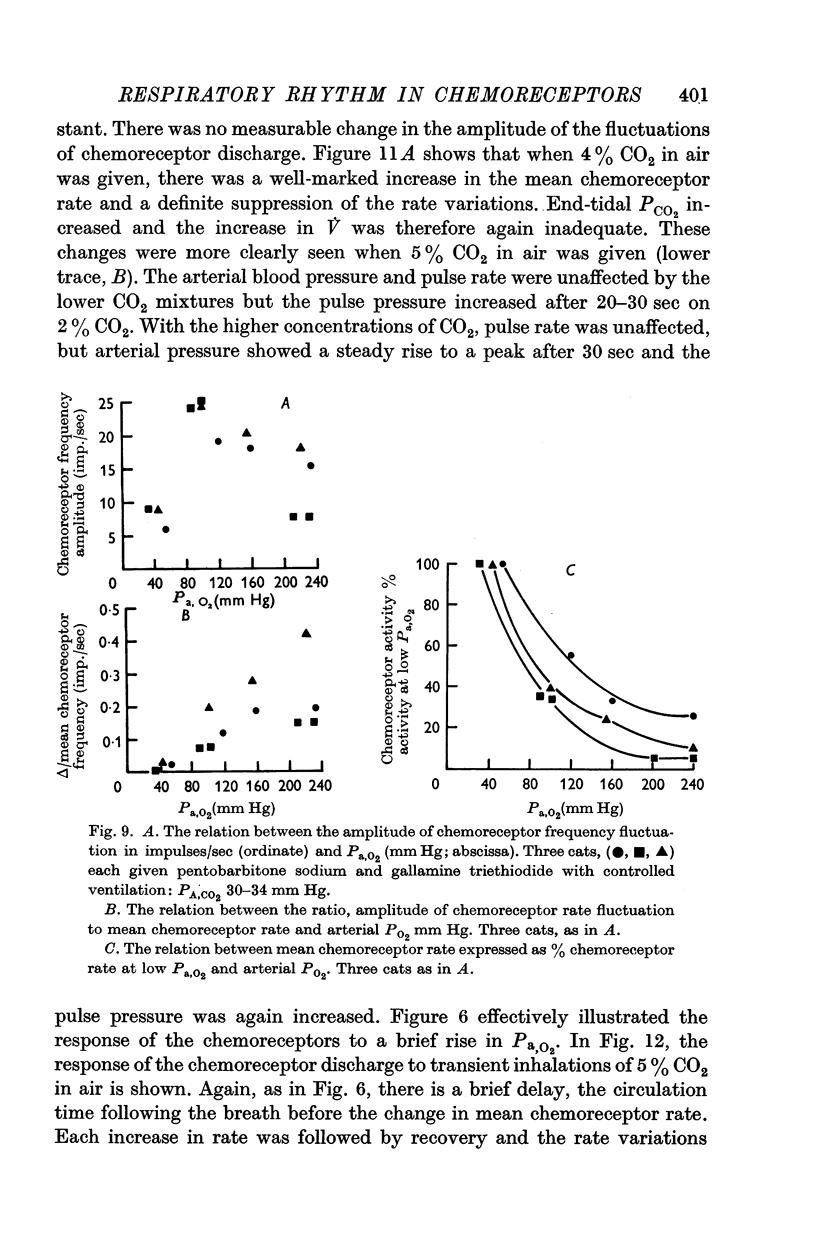
3. The amplitude of the rhythm is modified by transient and long-term changes in inspired oxygen or CO2 so that a rise or fall in O2 or CO2 tensions of arterial blood (Pa,O2, Pa,CO2) from the physiological range reduces it. The ratio of the rhythm amplitude to the mean rate of chemoreceptor discharge increases with Pa,O2 over the range 40-240 mm Hg.
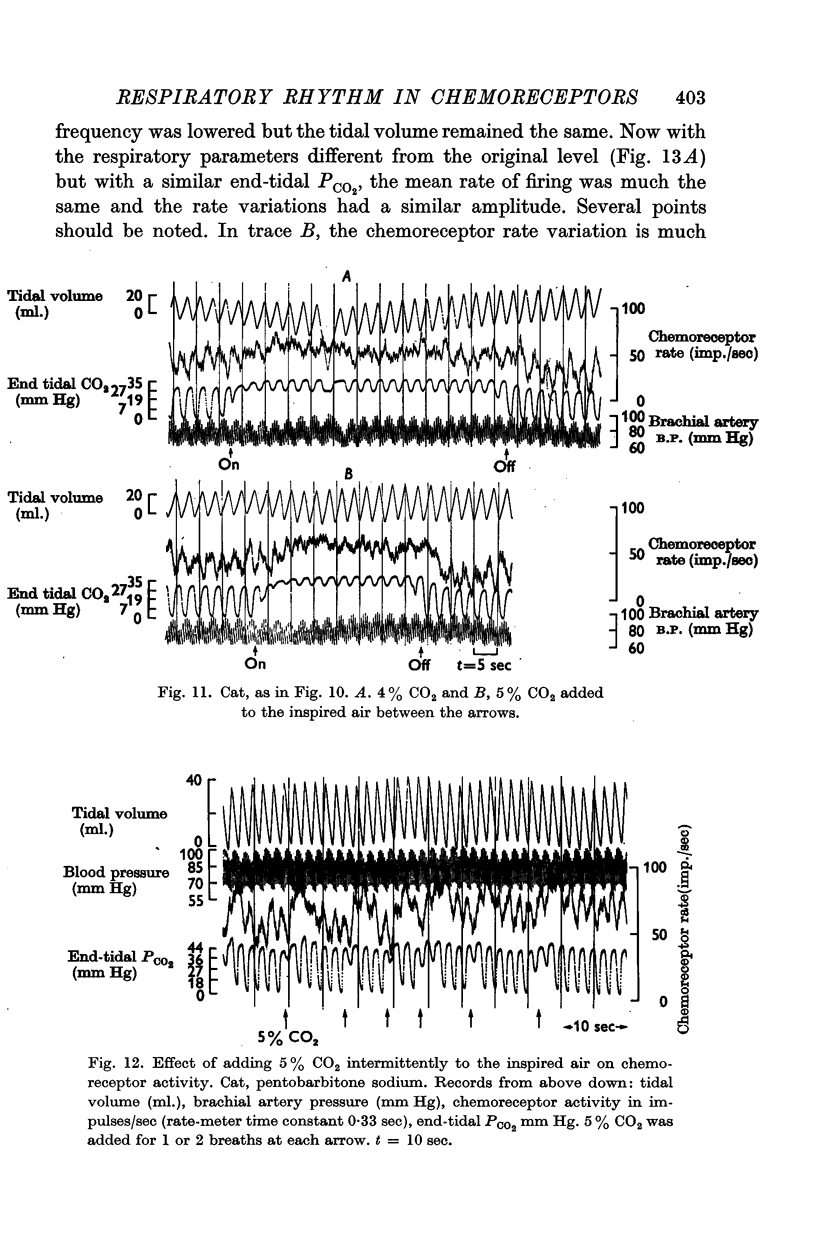
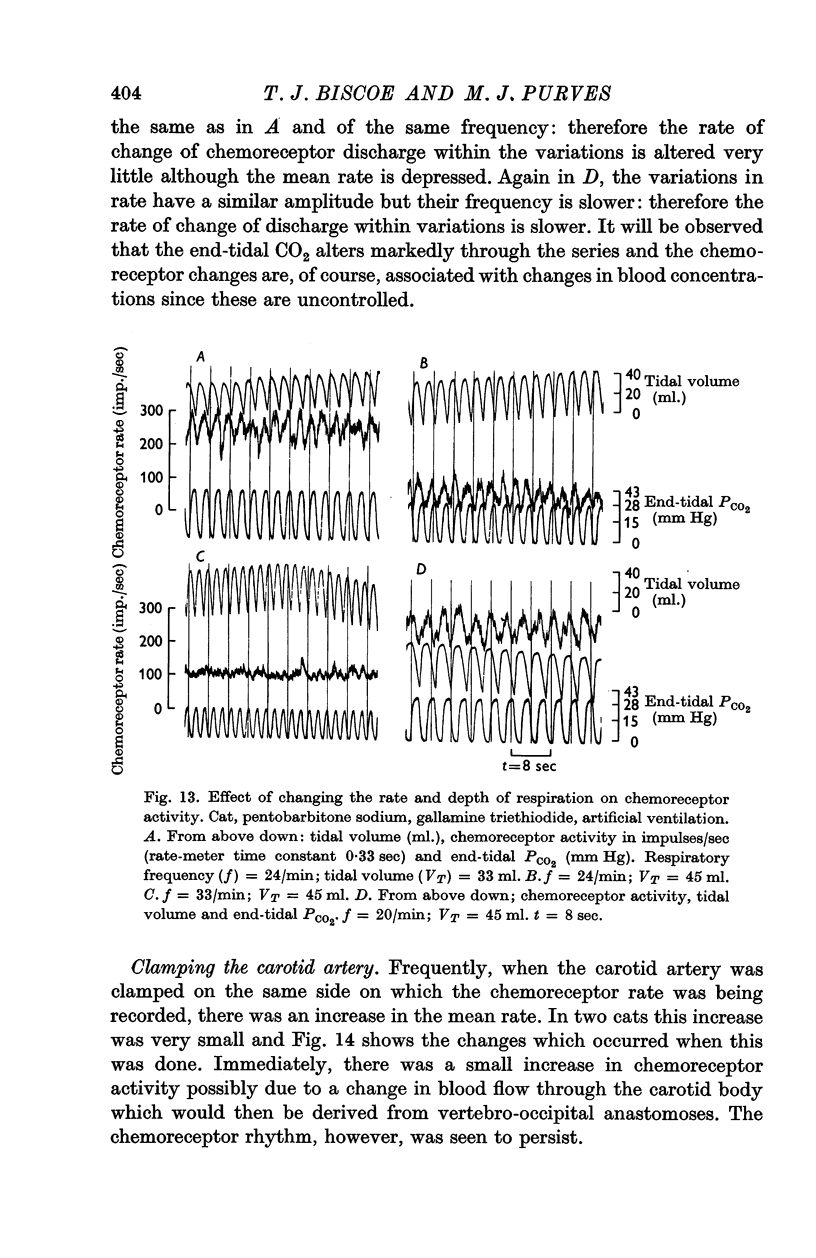
4. The rhythm is modified by changes in respiratory frequency and volume.
5. The fluctuations of arterial oxygen tension which have the same period as respiration are shown to be conducted up the vertebral artery at least as far as the vertebro-occipital anastomosis.
6. It is proposed that the chemoreceptor rhythm reflects the moment to moment changes in blood gas tensions.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVAREZ-BUYLLA R. Estudio oscilográfico de la actividad quimiorreceptora del glomus carotideo en gatos descerebrados. Arch Inst Cardiol Mex. 1951 Dec;21(5-6):724–739. [PubMed] [Google Scholar]
- ANDERSEN P., CURTIS D. R. THE EXCITATION OF THALAMIC NEURONES BY ACETYLCHOLINE. Acta Physiol Scand. 1964 May-Jun;61:85–99. doi: 10.1111/j.1748-1716.1964.tb02945.x. [DOI] [PubMed] [Google Scholar]
- BERGMAN N. A. Cyclic variations in blood oxygenation with the respiratory cycle. Anesthesiology. 1961 Nov-Dec;22:900–908. doi: 10.1097/00000542-196111000-00006. [DOI] [PubMed] [Google Scholar]
- BISCOE T. J., TAYLOR A. THE DISCHARGE PATTERN RECORDED IN CHEMORECEPTOR AFFERENT FIBRES FROM THE CAT CAROTID BODY WITH NORMAL CIRCULATION AND DURING PERFUSION. J Physiol. 1963 Sep;168:332–344. doi: 10.1113/jphysiol.1963.sp007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSS J., GREEN J. H. The histology of the common carotid baroceptor areas of the cat. Circ Res. 1956 Jan;4(1):12–17. doi: 10.1161/01.res.4.1.12. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Carotid body chemoreceptor activity in the new-born lamb. J Physiol. 1967 Jun;190(3):443–454. doi: 10.1113/jphysiol.1967.sp008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on carotid body chemoreceptor activity and cervical sympathetic discharge in the cat. J Physiol. 1967 Jun;190(3):413–424. doi: 10.1113/jphysiol.1967.sp008218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS A. B., BRITT A. G., FENN W. O. Alveolar CO2 during the respiratory cycle. J Appl Physiol. 1952 Jan;4(7):535–548. doi: 10.1152/jappl.1952.4.7.535. [DOI] [PubMed] [Google Scholar]
- DUTTON R. E., CHERNICK V., MOSES H., BROMBERGER-BARNEA B., PERMUTT S., RILEY R. L. VENTILATORY RESPONSE TO INTERMITTENT INSPIRED CARBON DIOXIDE. J Appl Physiol. 1964 Sep;19:931–936. doi: 10.1152/jappl.1964.19.5.931. [DOI] [PubMed] [Google Scholar]
- FENN W. O., CRAIG A. B., Jr EFFECT OF CO2 ON RESPIRATION USING A NEW METHOD OF ADMINISTERING CO2. J Appl Physiol. 1963 Sep;18:1023–1024. doi: 10.1152/jappl.1963.18.5.1023. [DOI] [PubMed] [Google Scholar]
- GREEN J. H. Further baroceptor areas associated with the right common carotid artery in the cat. J Physiol. 1954 Feb 26;123(2):41–2P. [PubMed] [Google Scholar]
- HORNBEIN T. F., GRIFFO Z. J., ROOS A. Quantitation of chemoreceptor activity: interrelation of hypoxia and hypercapnia. J Neurophysiol. 1961 Nov;24:561–568. doi: 10.1152/jn.1961.24.6.561. [DOI] [PubMed] [Google Scholar]
- LEE K. D., MAYOU R. A., TORRANCE R. W. THE EFFECT OF BLOOD PRESSURE UPON CHEMORECEPTOR DISCHARGE TO HYPOXIA, AND THE MODIFICATION OF THIS EFFECT BY THE SYMPATHETIC-ADRENAL SYSTEM. Q J Exp Physiol Cogn Med Sci. 1964 Apr;49:171–183. doi: 10.1113/expphysiol.1964.sp001717. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO W. S. Mathematical analysis of the time course of alveolar carbon dioxide. J Appl Physiol. 1960 Mar;15:215–219. doi: 10.1152/jappl.1960.15.2.215. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO W. S. Transmission of information by the arterial blood stream with particular reference to carbon dioxide. Biophys J. 1962 Mar;2:143–159. doi: 10.1016/s0006-3495(62)86846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


