Abstract
1. Membrane properties of the muscle fibre were studied in twitch motor system of sea-water elasmobranch (Taeniura lymma, Himantura uarnak and Pastinachus sephen) and teleost fish (Periophthalmodon barbarus, Tetradon immaculata, Hemiramphus welsby, Parexocoetus brachypterus and Conger labiatus).
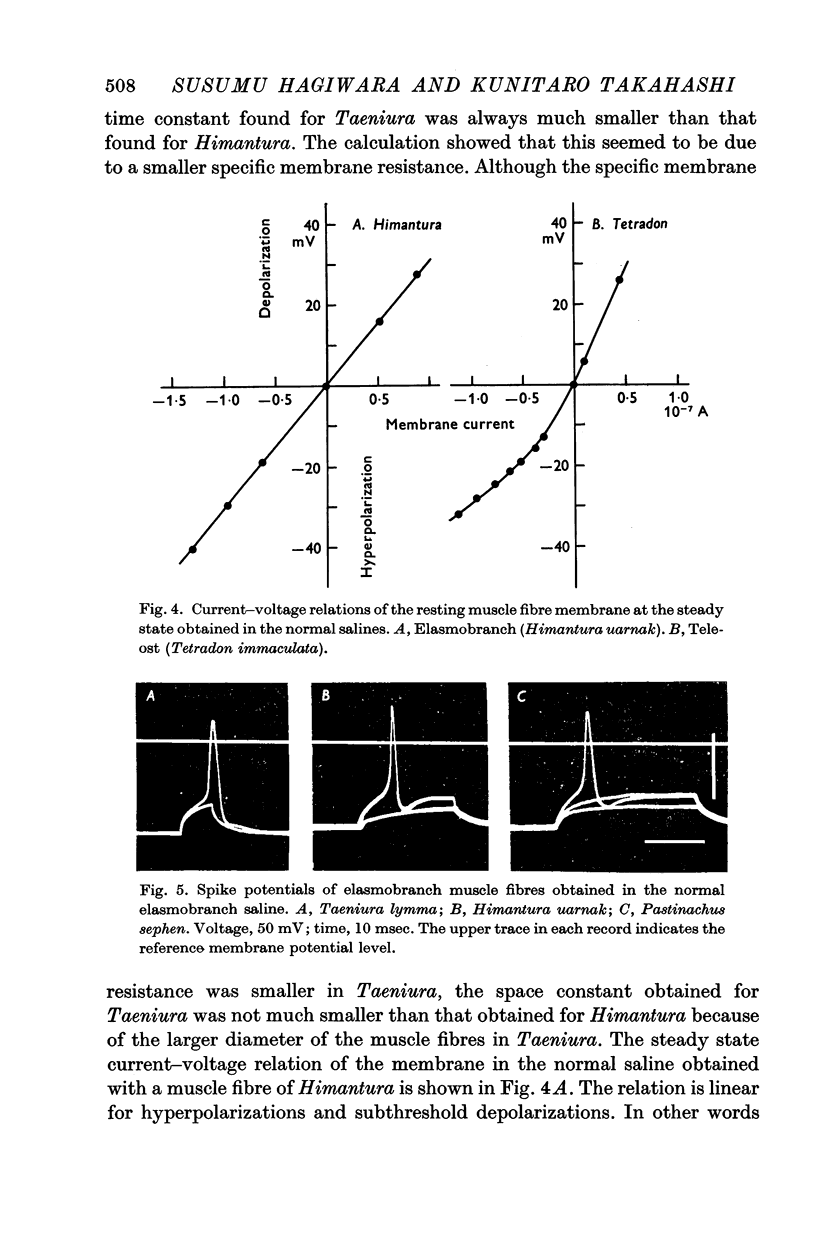
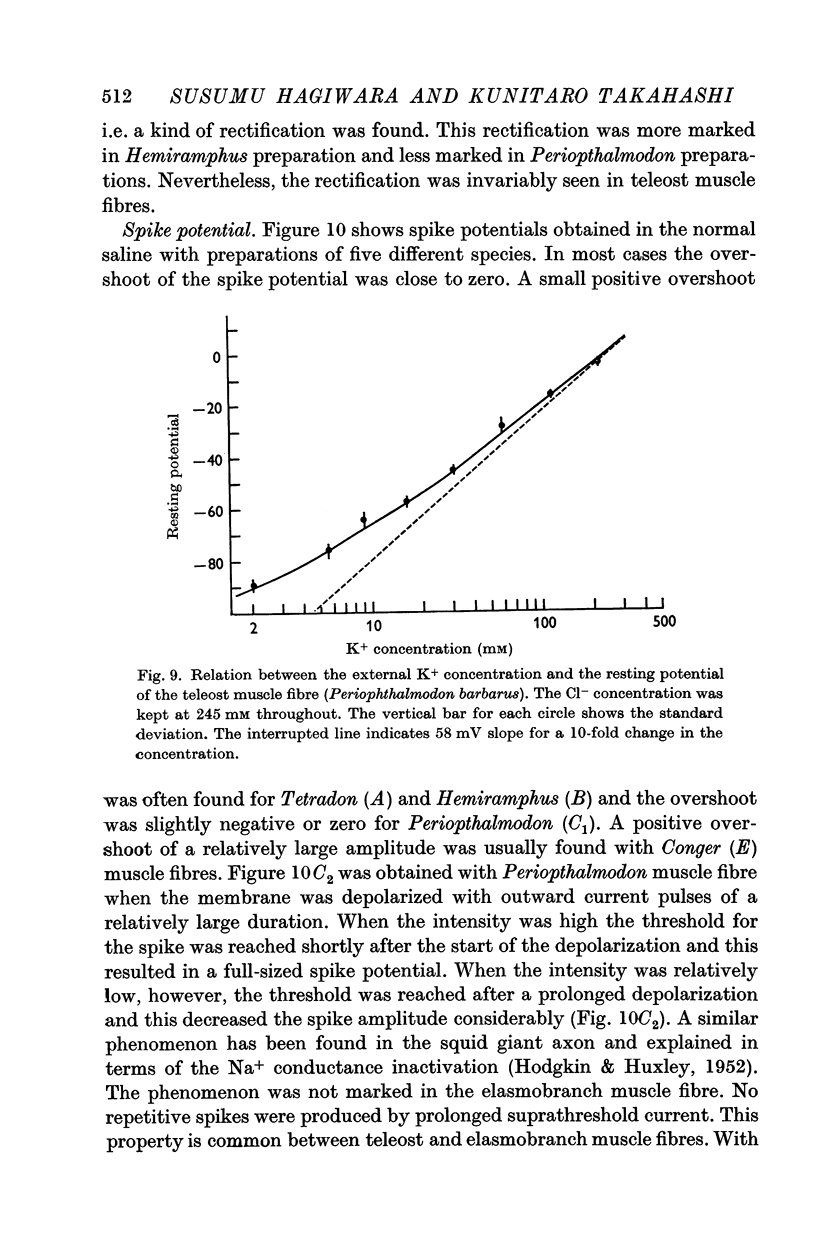
2. The resting potential of the elasmobranch fibre is mainly determined by the Cl- concentration difference between inside and outside the membrane whereas the K+ conductance is the determining factor in teleost fibres.
3. The resting membrane of the elasmobranch fibre is permeable not only to Cl- ions but also several other anions (Br-, I-, NO3-, SCN-, ClO4-, ClO3-) of large limiting conductivities in the aqueous solution.
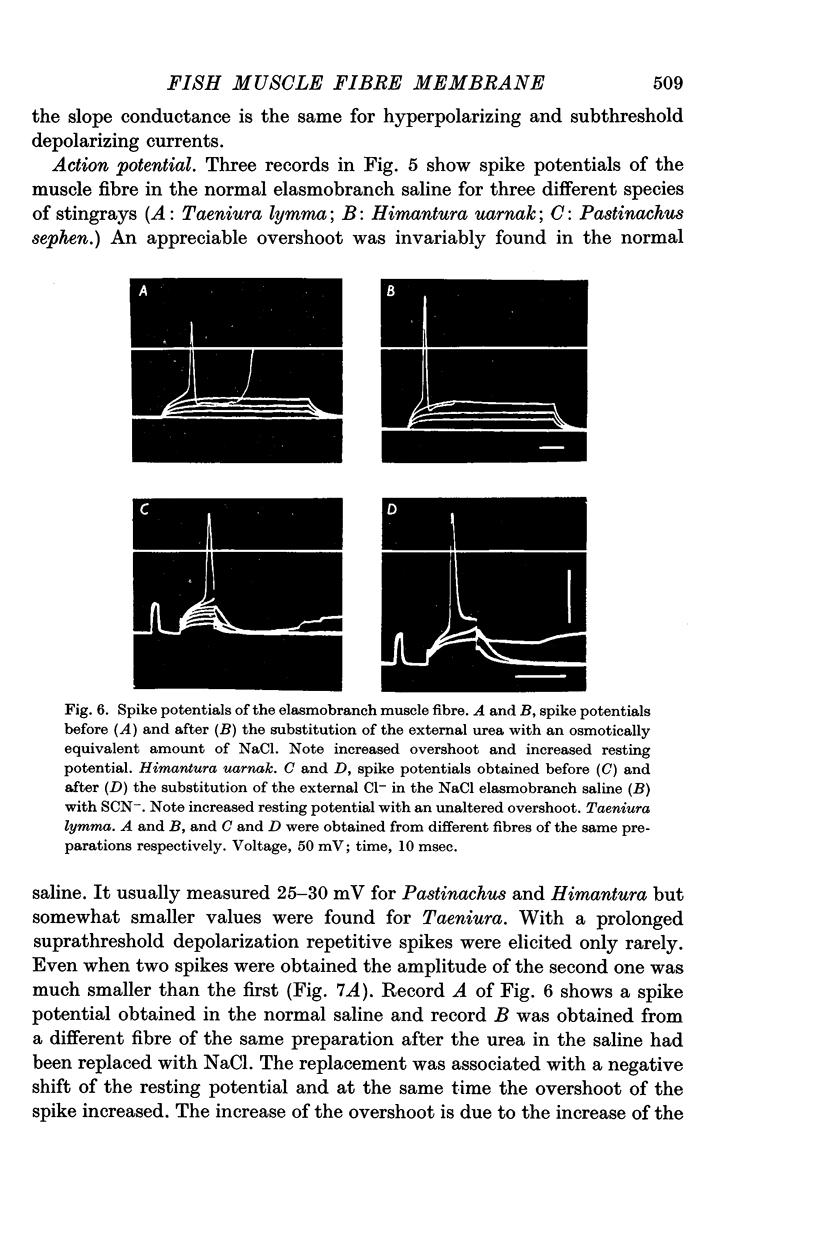
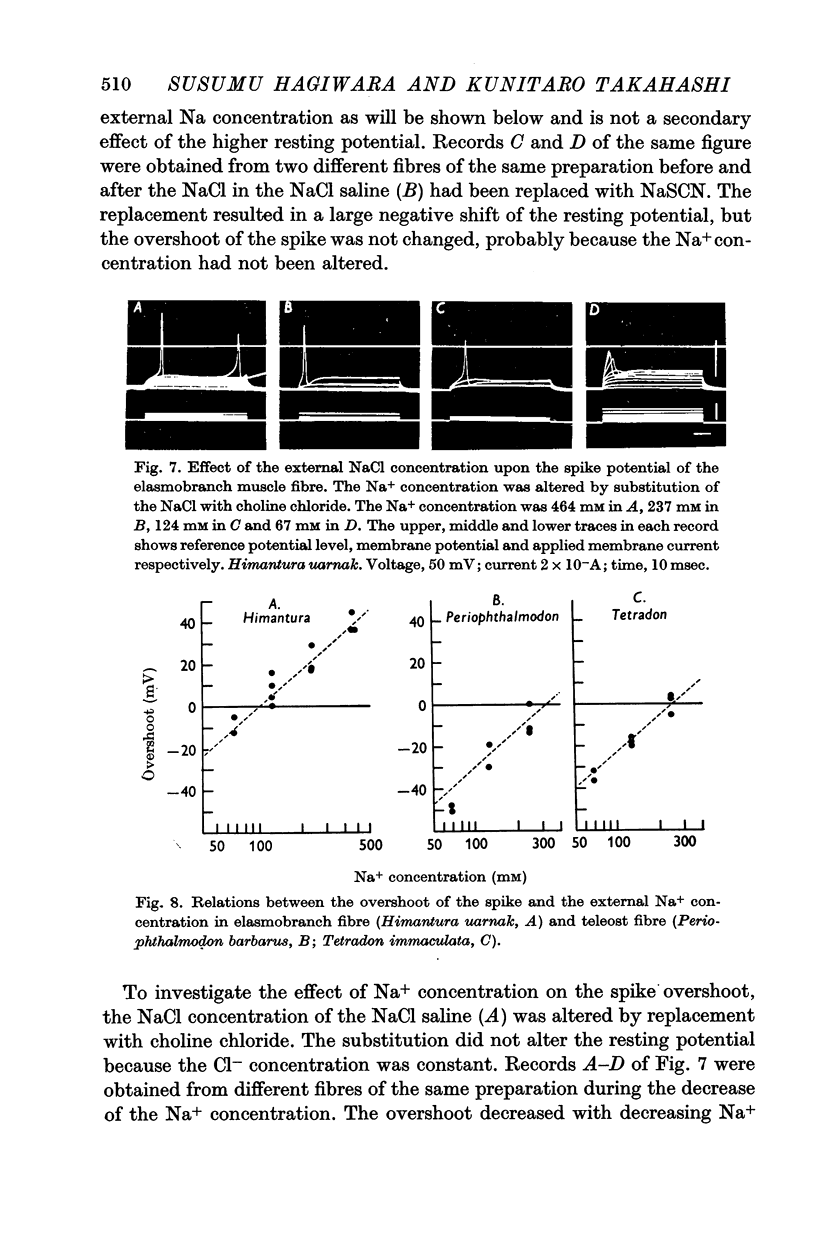
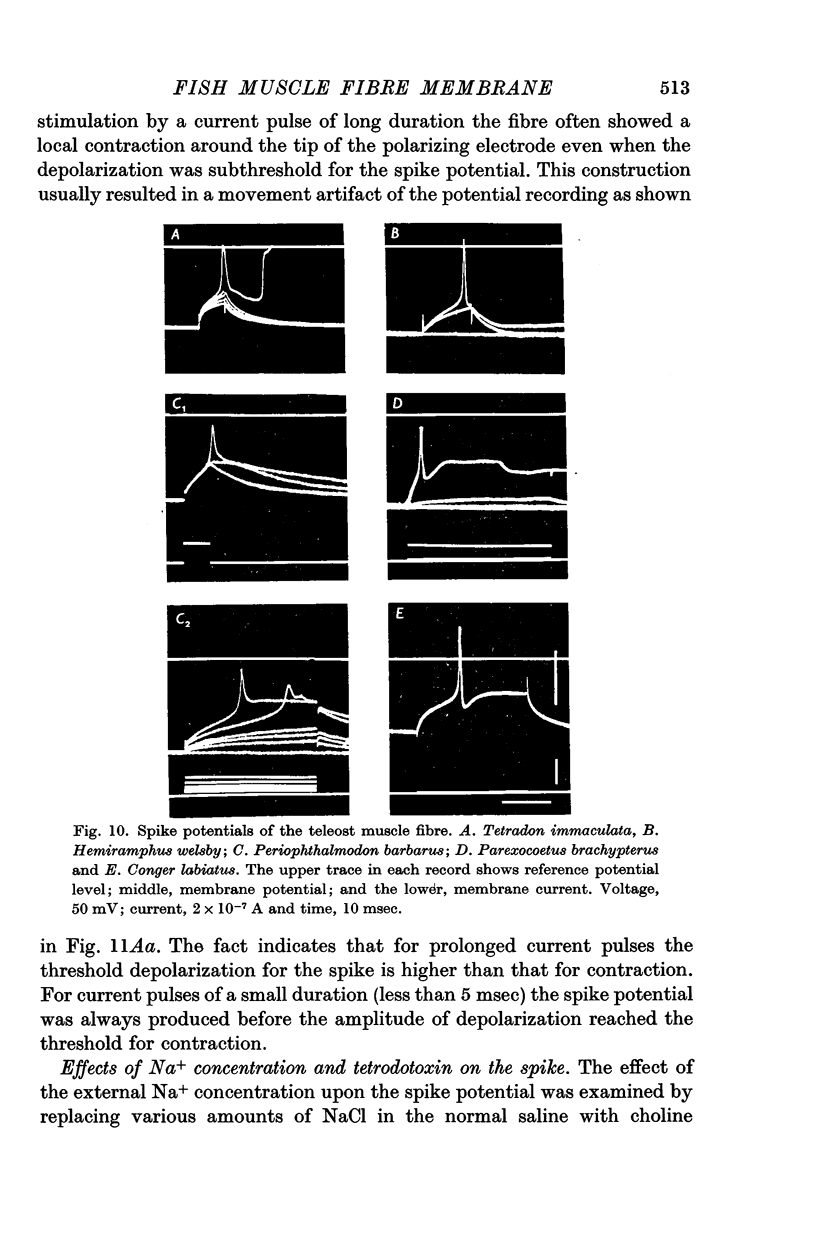
4. The spike potential of the elasmobranch fibre always shows a significant overshoot in normal saline while no significant overshoot is generally found in teleost fibres.
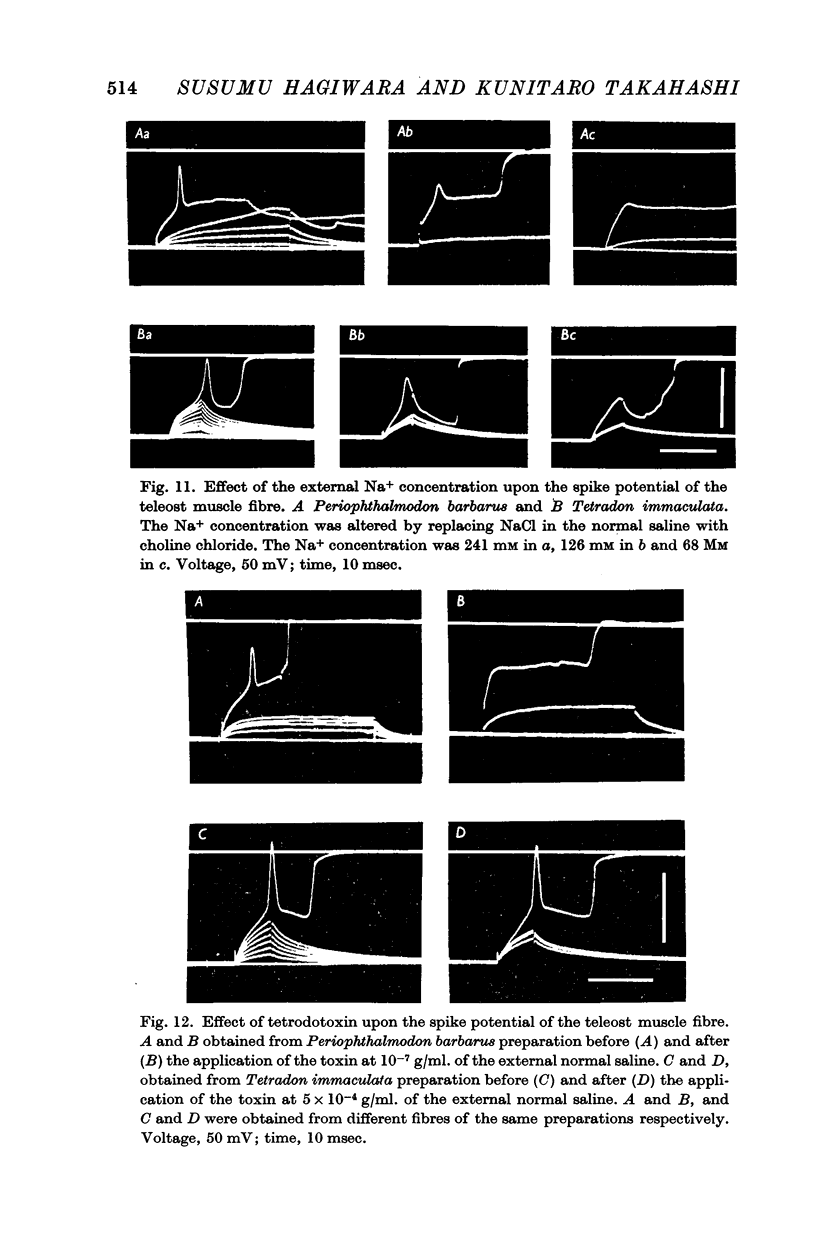
5. In both elasmobranchs and teleosts the spike is produced by the permeability increase of the membrane to Na+ ions and is effectively suppressed by tetrodotoxin at a concentration of 0·5-1·0 × 10-7 g/ml. of the external solution with one exception, i.e. the Na+ spike of Tetradon fibre is not suppressed by the toxin even when the concentration is above 5 × 10-4 g/ml.
Full text
PDF

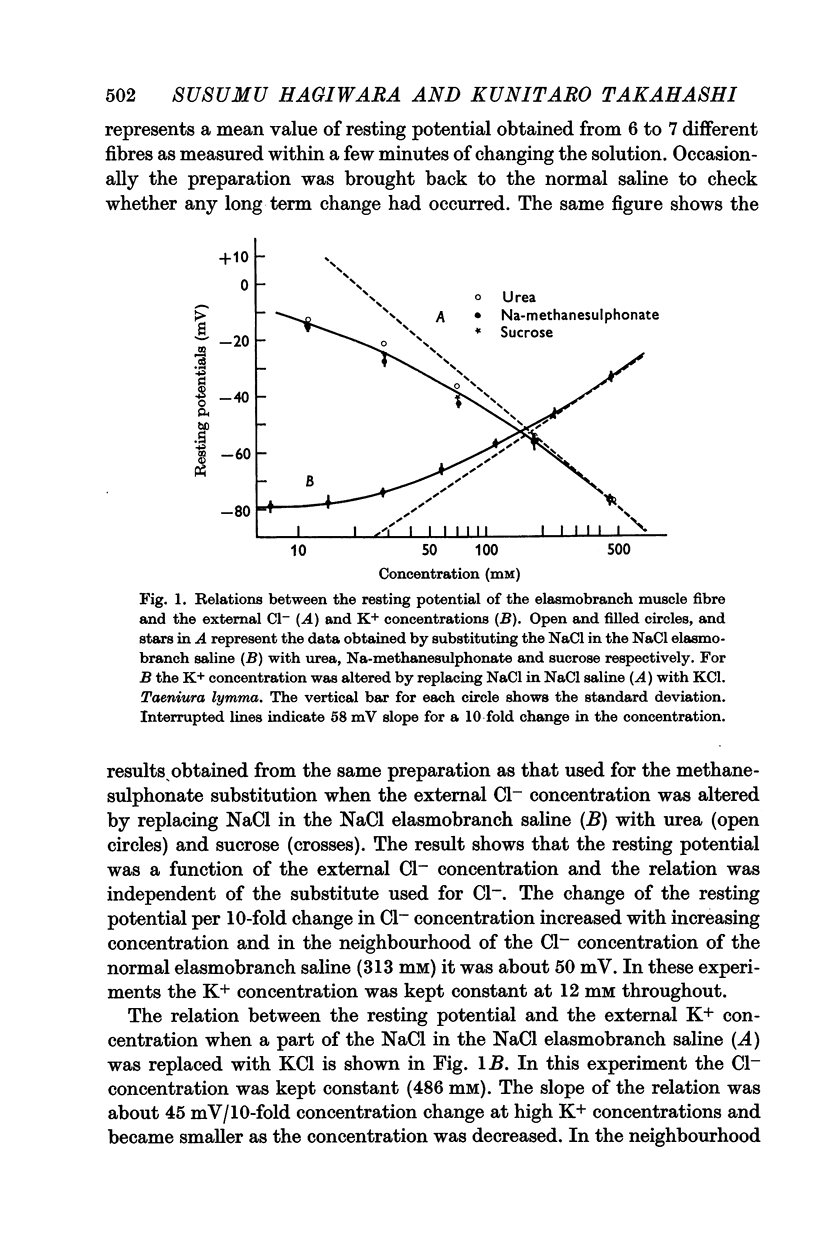








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSEN P., JANSEN J. K., LOYNING Y. Slow and fast muscle fibres in the Atlantic hagfish (Myxine glutinosa). Acta Physiol Scand. 1963 Jan-Feb;57:167–179. doi: 10.1111/j.1748-1716.1963.tb02583.x. [DOI] [PubMed] [Google Scholar]
- ARAKI T., ITO M., OSCARSSON O. Anion permeability of the synaptic and non-synaptic motoneurone membrane. J Physiol. 1961 Dec;159:410–435. doi: 10.1113/jphysiol.1961.sp006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARETS A. Caractéristiques morphologiques des deux types d'innervation motrice du muscle latéral des Téléostéens. C R Seances Soc Biol Fil. 1955 Jul;149(13-14):1420–1422. [PubMed] [Google Scholar]
- BARETS A., FESSARD A., LE TOUZE S. Etude électrophysiologique d'un type particulier de jonction neuromusculaire: le système moteur rapide des Téléostéens. J Physiol (Paris) 1956 May-Jun;48(3):381–383. [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., SASAOKA T., HOSOYA Y. Effects of tetrodotoxin on the neuromuscular junction. Jpn J Physiol. 1959 Jun 25;9(2):143–152. doi: 10.2170/jjphysiol.9.143. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., PADSHA S. M. Effect of nitrate and other anions on the membrane resistance of frog skeletal muscle. J Physiol. 1959 Apr 23;146(1):117–132. doi: 10.1113/jphysiol.1959.sp006182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LING G., GERARD R. W. The normal membrane potential of frog sartorius fibers. J Cell Physiol. 1949 Dec;34(3):383–396. doi: 10.1002/jcp.1030340304. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA S., IWASAKI S., OBATA K. Delayed rectification and anomalous rectification in frog's skeletal muscle membrane. J Gen Physiol. 1962 Sep;46:97–115. doi: 10.1085/jgp.46.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., DEGUCHI T., URAKAWA N., OHKUBO Y. Stabilization and rectification of muscle fiber membrane by tetrodotoxin. Am J Physiol. 1960 May;198:934–938. doi: 10.1152/ajplegacy.1960.198.5.934. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Nakajima S., Grundfest H. The action of tetrodotoxin on electrogenic components of squid giant axons. J Gen Physiol. 1965 Jul;48(6):975–996. [PubMed] [Google Scholar]
- TAKEUCHI A. Neuromuscular transmission of fish skeletal muscles investigated with intracellular microelectrode. J Cell Comp Physiol. 1959 Dec;54:211–220. doi: 10.1002/jcp.1030540302. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Slow and fast groups of pyramidal tract cells and their respective membrane properties. J Neurophysiol. 1965 Sep;28(5):908–924. doi: 10.1152/jn.1965.28.5.908. [DOI] [PubMed] [Google Scholar]


