Abstract
1. Slices of brain cortex from rabbits were incubated in Ringer solution and in Ringer modified by the removal of calcium and sodium, and the addition of ouabain, oligomycin or extra potassium. The potassium content of the tissue, the oxygen consumption and the lactate production from glucose were measured and found to be interrelated.
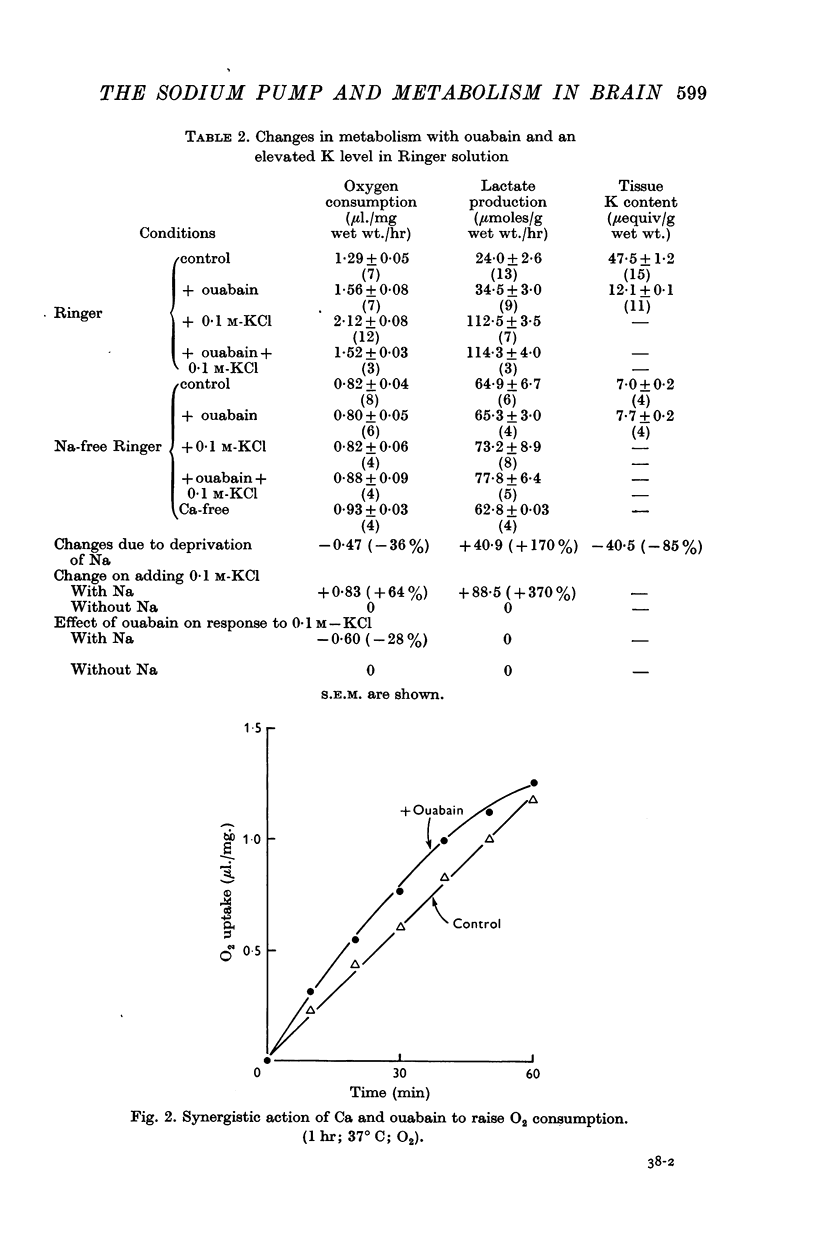
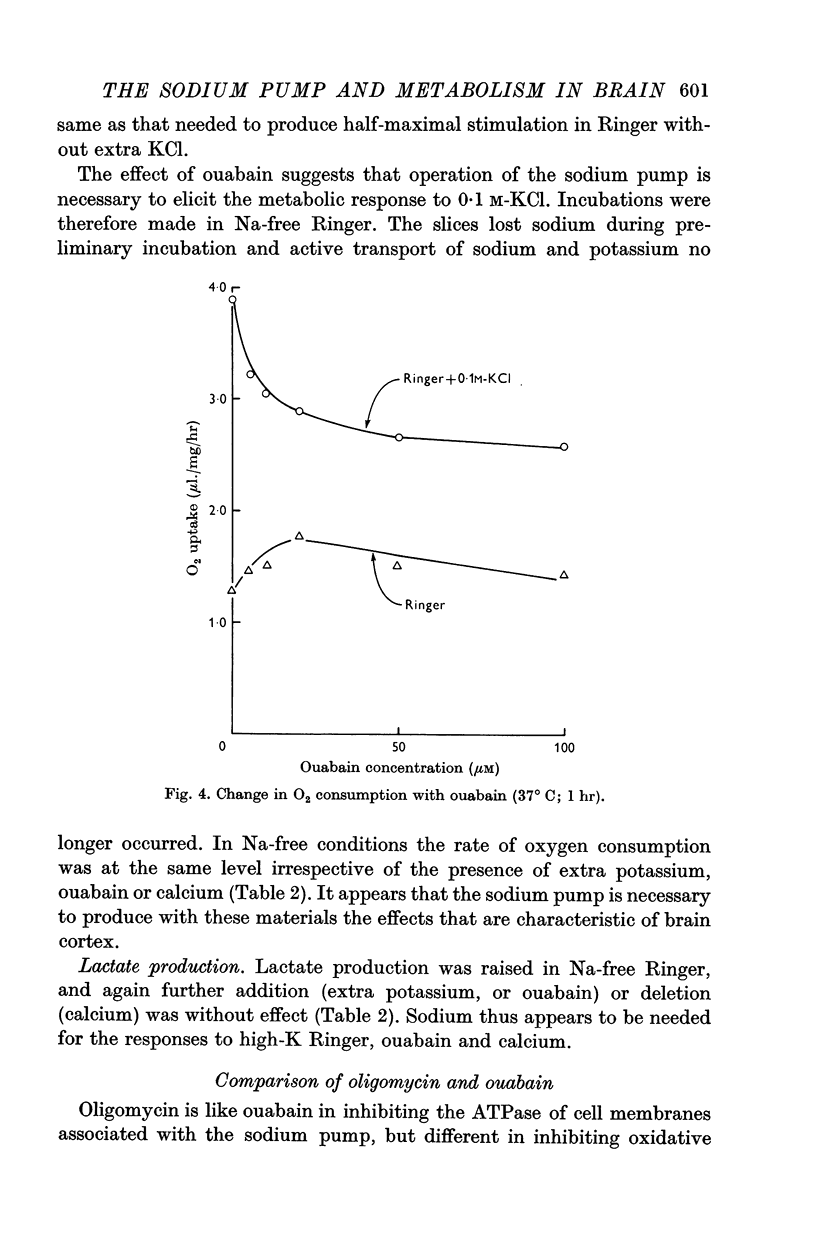
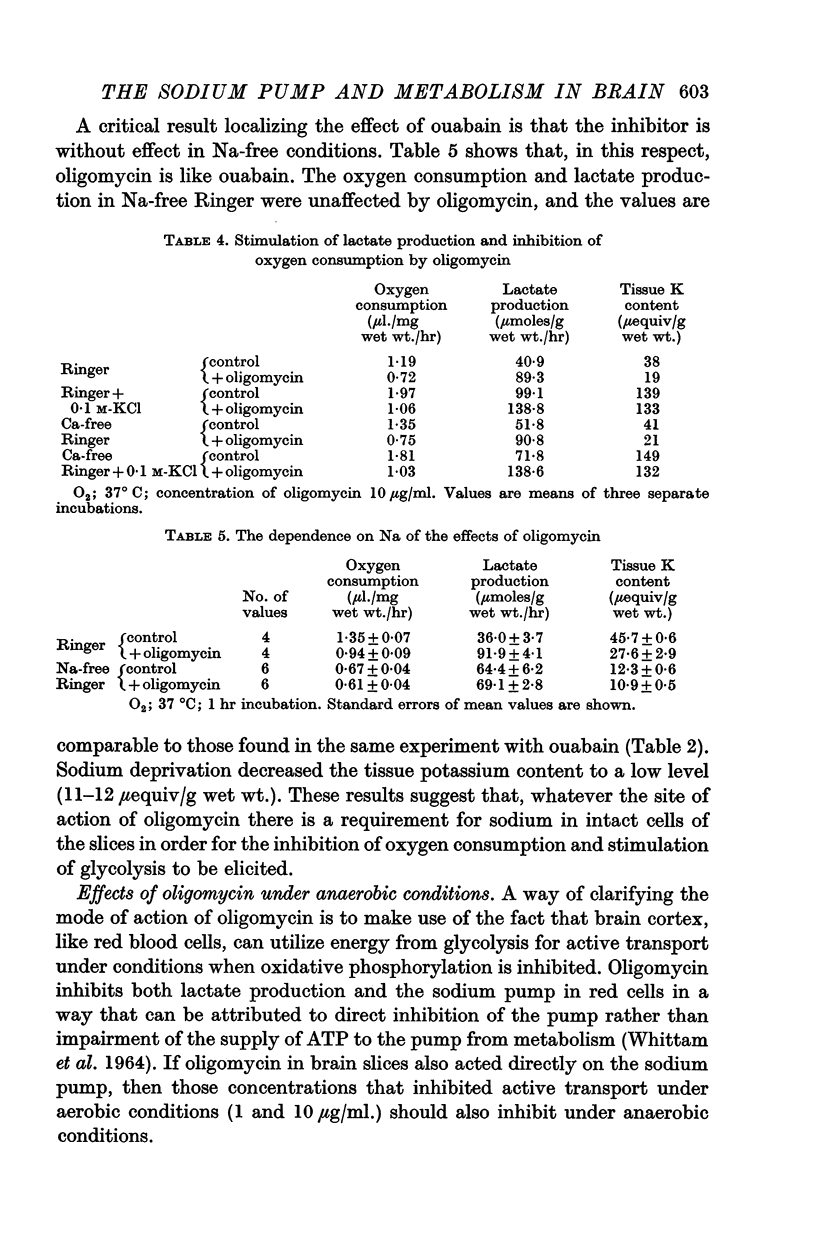
2. Incubation in high-K Ringer caused an increase in oxygen consumption that was prevented by ouabain, oligomycin and deprivation of sodium. Lactate production was also raised, but this increase was unaffected by ouabain and raised further by oligomycin.
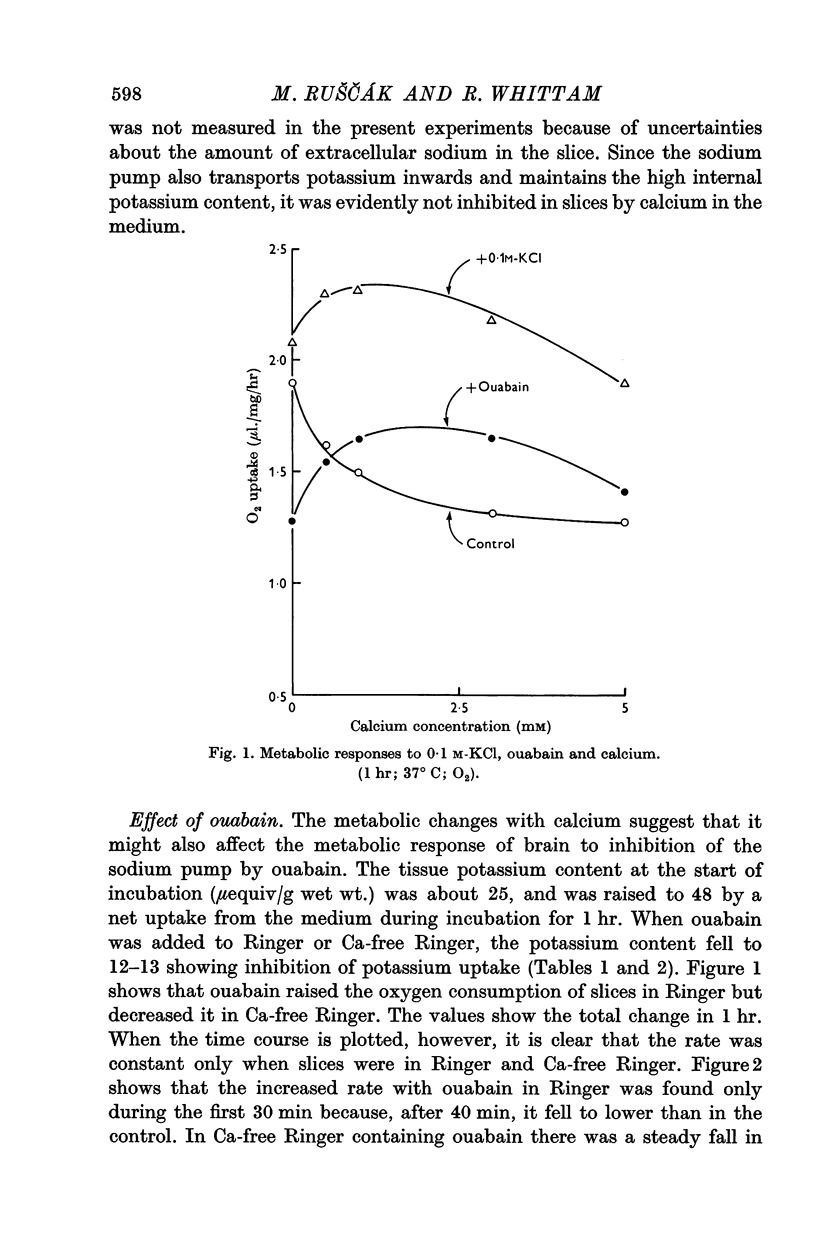
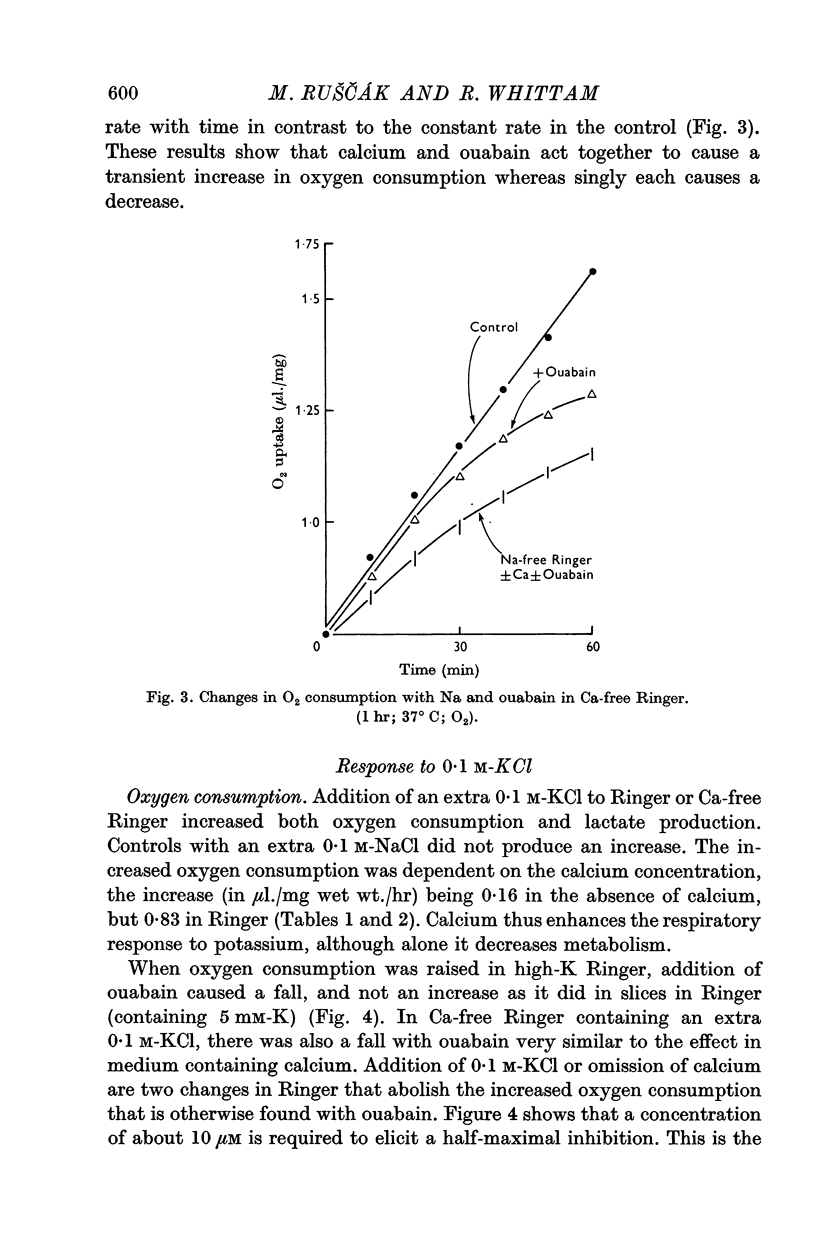
3. Calcium omission raised metabolism; the tissue K content was unaffected. Oligomycin always decreased oxygen consumption and raised lactate production further. The metabolic responses to calcium, potassium, ouabain and oligomycin depended on sodium.
4. After anaerobic incubation, the tissue potassium concentration was still 5 times higher than that in Ringer. It was unaffected by oligomycin but lowered markedly by ouabain.
5. The synergistic effects of sodium with potassium, oligomycin, calcium, and calcium plus ouabain suggest that the metabolic responses of brain cortex slices to a high-K Ringer depend on the operation of the sodium pump.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford C. A., Dixon K. C. The effect of potassium on the glucolysis of brain tissue with reference to the Pasteur effect. Biochem J. 1935;29(1):157–168. doi: 10.1042/bj0290157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLINGBROKE V., MAIZELS M. Calcium ions and the permeability of human erythrocytes. J Physiol. 1959 Dec;149:563–585. doi: 10.1113/jphysiol.1959.sp006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Connelly C. M. Some properties of the external activation site of the sodium pump in crab nerve. J Physiol. 1966 Jul;185(2):270–297. doi: 10.1113/jphysiol.1966.sp007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., HERRERA F. C., FLANIGAN W. J. The effect of Ca and antidiuretic hormone on Na transport across frog skin. II. Sites and mechanisms of action. J Gen Physiol. 1963 May;46:1011–1027. doi: 10.1085/jgp.46.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON J., BONE A. WATER UPTAKE BY, AND SODIUM AND POTASSIUM CONTENT OF, BRAIN SLICES. J Neurochem. 1965 Mar;12:167–180. doi: 10.1111/j.1471-4159.1965.tb06753.x. [DOI] [PubMed] [Google Scholar]
- DIXON K. C. Action of potassium ions on brain metabolism. J Physiol. 1949 Dec 15;110(1-2):87–97. doi: 10.1113/jphysiol.1949.sp004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens F., Greville G. D. The metabolism of normal and tumour tissue: Neutral salt effects. Biochem J. 1935 Jun;29(6):1468–1483. doi: 10.1042/bj0291468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT K. A., BILODEAU F. The influence of potassium on respiration and glycolysis by brain slices. Biochem J. 1962 Aug;84:421–428. doi: 10.1042/bj0840421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSHOVE A., VAN ROSSUMG NET MOVEMENTS OF SODIUM AND POTASSIUM, AND THEIR RELATION TO RESPIRATION, IN SLICES OF RAT LIVER INCUBATED IN VITRO. J Physiol. 1963 Oct;168:531–553. doi: 10.1113/jphysiol.1963.sp007206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. TRANSPORT ADENOSINETRIPHOSPHATASE' IN ELECTRIC ORGAN. THE RELATION BETWEEN ION TRANSPORT AND OXIDATIVE PHOSPHORYLATION. J Physiol. 1963 Nov;169:452–465. doi: 10.1113/jphysiol.1963.sp007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONDA O., QUASTEL J. H. Effects of ouabain on cerebral metabolism and transport mechanisms in vitro. Biochem J. 1962 Aug;84:394–406. doi: 10.1042/bj0840394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaister D., Kerly M. The oxygen consumption and carbohydrate metabolism of the retractor muscle of the foot of Mytilus edulis. J Physiol. 1936 Jun 10;87(1):56–66. doi: 10.1113/jphysiol.1936.sp003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTZ L., CLAUSEN T. EFFECTS OF POTASSIUM AND SODIUM ON RESPIRATION: THEIR SPECIFICITY TO SLICES FROM CERTAIN BRAIN REGIONS. Biochem J. 1963 Dec;89:526–533. doi: 10.1042/bj0890526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLMAN H. H., McILWAIN H. Membrane potentials in mammalian cerebral tissues in vitro: dependence on ionic environment. J Physiol. 1961 Jul;157:263–278. doi: 10.1113/jphysiol.1961.sp006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- HUIJING F., SLATER E. C. The use of oligomycin as an inhibitor of oxidative phosphorylation. J Biochem. 1961 Jun;49:493–501. doi: 10.1093/oxfordjournals.jbchem.a127334. [DOI] [PubMed] [Google Scholar]
- Hempling H. G. Sources of energy for the transport of potassium and sodium across the membrane of the Ehrlich mouse ascites tumor cell. Bibl Laeger. 1966 Mar 14;112(3):503–518. doi: 10.1016/0926-6585(66)90253-6. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- LOLLEY R. N. THE CALCIUM CONTENT OF ISOLATED CEREBRAL TISSUES AND THEIR STEADY-STATE EXCHANGE OF CALCIUM. J Neurochem. 1963 Sep;10:665–676. doi: 10.1111/j.1471-4159.1963.tb08938.x. [DOI] [PubMed] [Google Scholar]
- ROSE S. P. EFFECTS OF OUABAIN, GLUTAMATE AND CATIONS ON PHOSPHATE INCORPORATION IN BRAIN SLICES. Biochem Pharmacol. 1965 Apr;14:589–601. doi: 10.1016/0006-2952(65)90231-5. [DOI] [PubMed] [Google Scholar]
- RUMMEL W., SEIFEN E., BALDAUF J. Influence of calcium and ouabain upon the potassium influx in human erythrocytes. Biochem Pharmacol. 1963 Jun;12:557–563. doi: 10.1016/0006-2952(63)90131-x. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ A. The effect of ouabain on potassium content, phosphoprotein metabolism and oxygen consumption of guinea pig cerebral tissue. Biochem Pharmacol. 1962 Apr-May;11:389–391. doi: 10.1016/0006-2952(62)90061-8. [DOI] [PubMed] [Google Scholar]
- SOLOMON A. K. Red cell membrane structure and ion transport. J Gen Physiol. 1960 May;43:1–15. doi: 10.1085/jgp.43.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin R. B., Slater E. C. The effect of oligomycin on the respiration of tissue slices. Biochim Biophys Acta. 1965 Aug 24;105(2):214–220. doi: 10.1016/s0926-6593(65)80146-1. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R. The dependence of the respiration of brain cortex on active cation transport. Biochem J. 1962 Jan;82:205–212. doi: 10.1042/bj0820205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R., WHEELER K. P., BLAKE A. OLIGOMYCIN AND ACTIVE TRANSPORT REACTIONS IN CELL MEMBRANES. Nature. 1964 Aug 15;203:720–724. doi: 10.1038/203720a0. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WILLIS J. S. ION MOVEMENTS AND OXYGEN CONSUMPTION IN KIDNEY CORTEX SLICES. J Physiol. 1963 Aug;168:158–177. doi: 10.1113/jphysiol.1963.sp007184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Blond D. M. Respiratory control by an adenosine triphosphatase involved in active transport in brain cortex. Biochem J. 1964 Jul;92(1):147–158. doi: 10.1042/bj0920147. [DOI] [PMC free article] [PubMed] [Google Scholar]


