Abstract
1. The margin of safety for neuromuscular transmission in the tibialis and sartorius muscles of the cat has been determined by measuring the ratio by which end-plate depolarization produced by succinylcholine, decamethonium, octamethonium or iodocholine is antagonized, in the presence of neuromuscular block produced by tubocurarine, gallamine or DF-596. The estimate of the margin of safety was independent of the particular drugs chosen for the measurement.
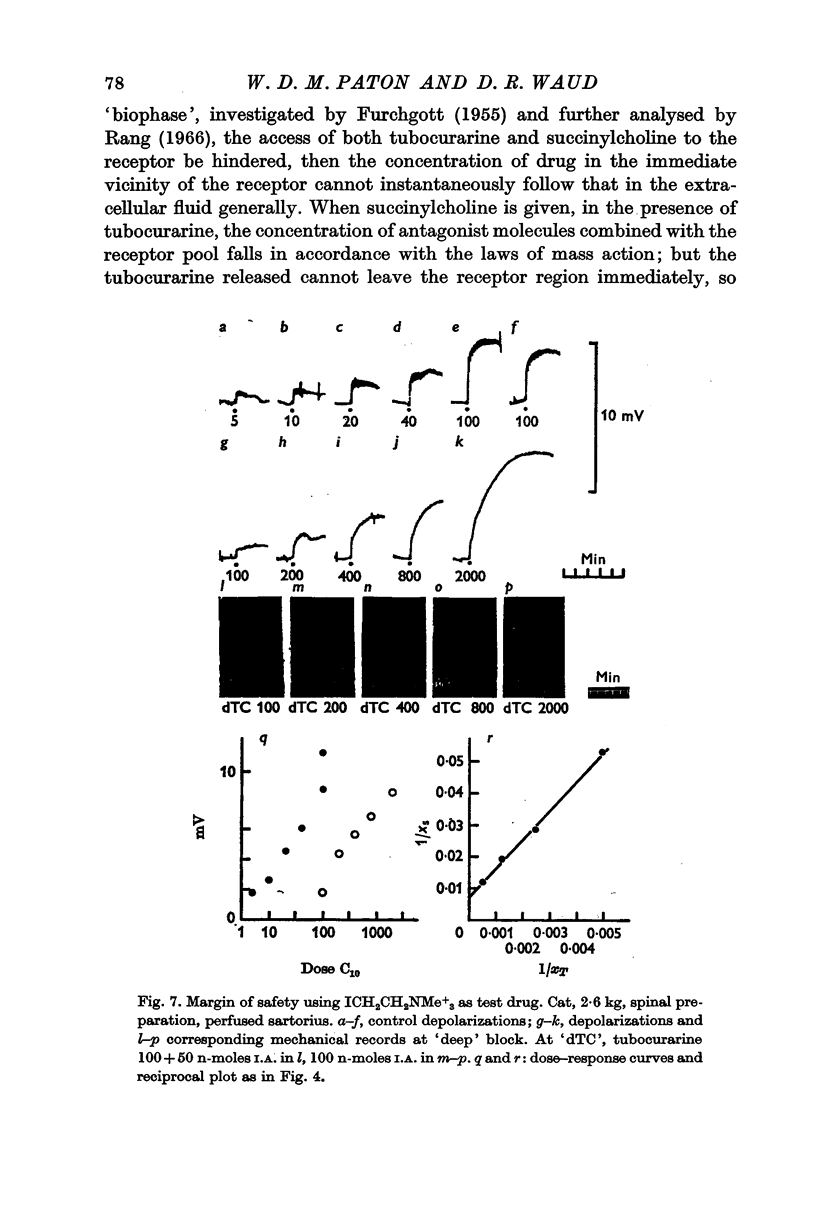
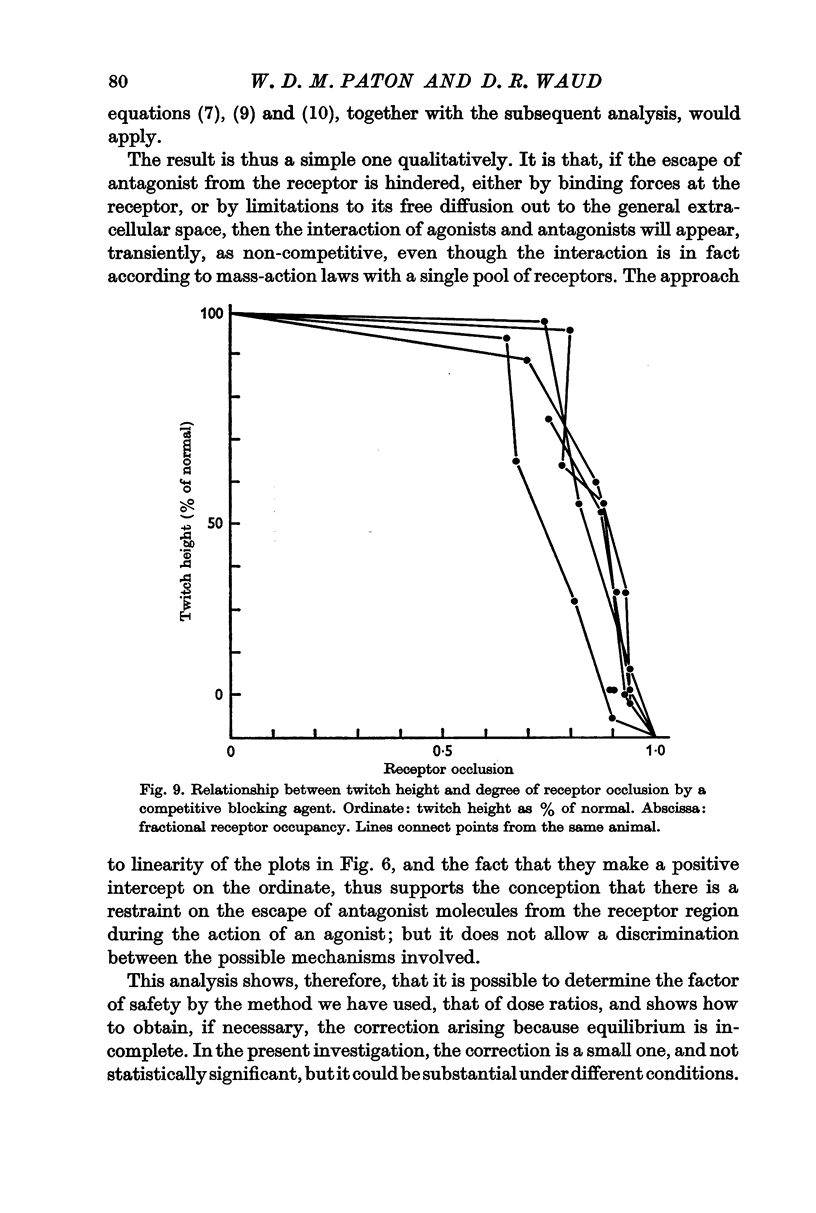
2. To produce threshold block to indirect stimulation once every 10 sec, a fractional occupancy by the antagonist of 0·76 ± 0·05 (S.D.) was required; for nearly complete block, an occupancy of 0·917 ± 0·16 (S.D.) was required. These figures correspond to factors of safety of 4·1 and 12 for the most sensitive and the most resistant groups of fibres respectively.
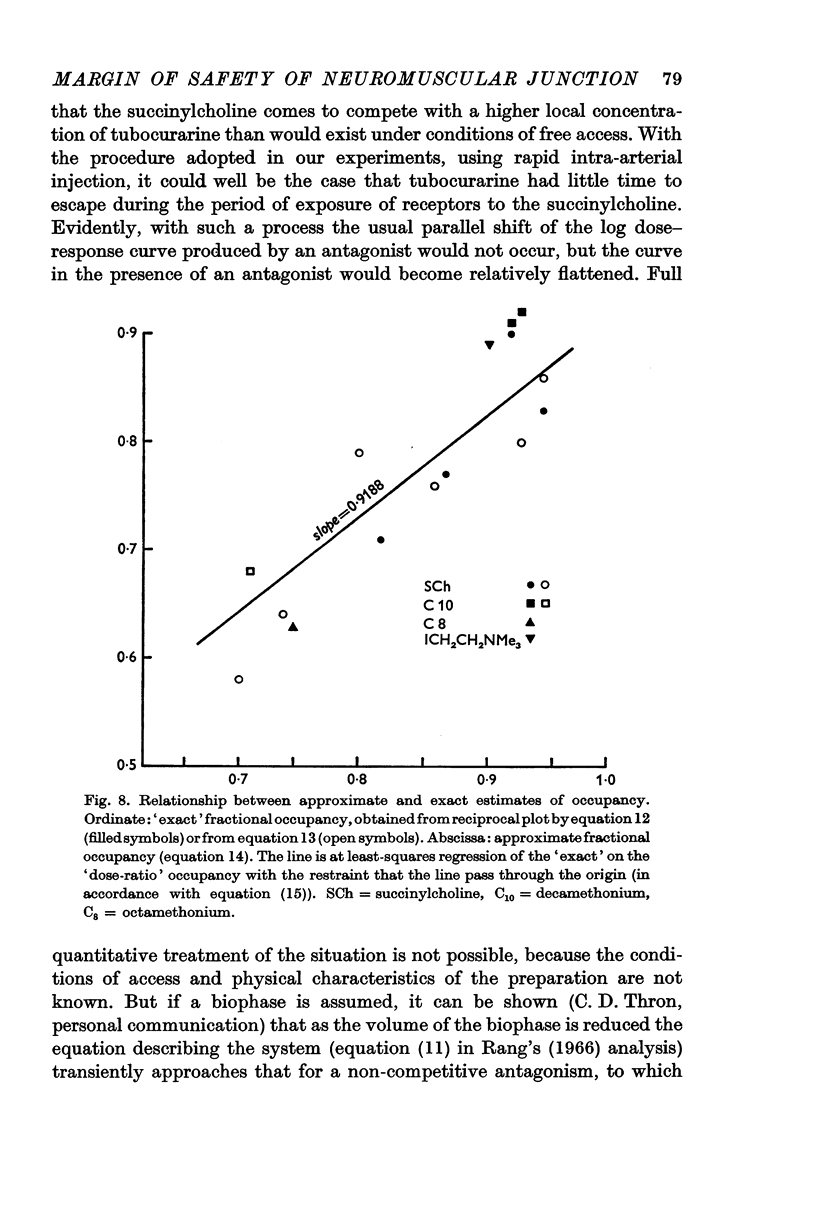
3. The interaction between the agonists and the antagonists, when tested over a wide range of dosage, did not conform with the conditions of full competitive equilibrium. It was concluded that this arose, not because of some interfering non-competitive process, but because, during the relatively brief exposure to agonist, the equilibrium between the antagonist and the receptors is not significantly disturbed. An analysis of this condition of quasi-equilibrium is given. A correction downwards of the direct estimates of the margin of safety is required, but this proves to be small, about 8%, and may not be significant.
4. The safety factor diminished when the motor nerve had been cut more than 5 hr; it is suggested that this represents an early sign of nerve degeneration.
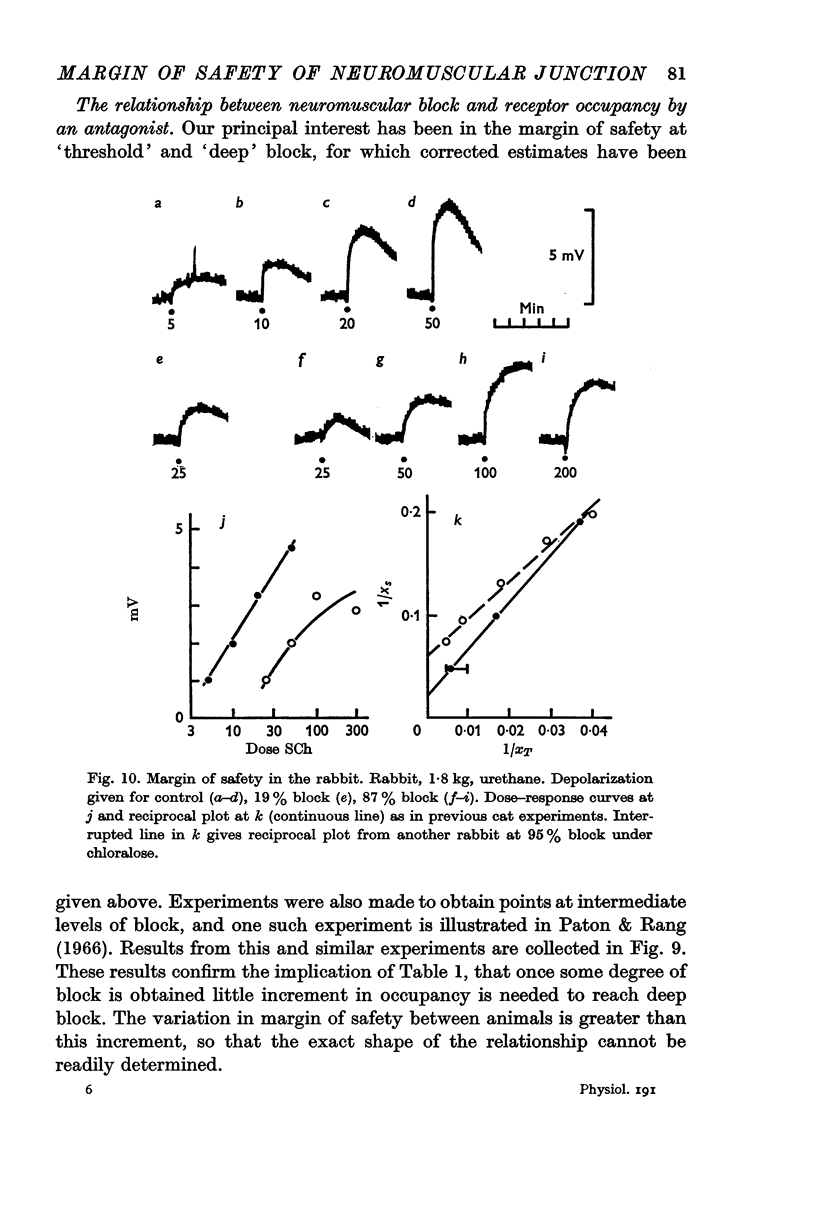
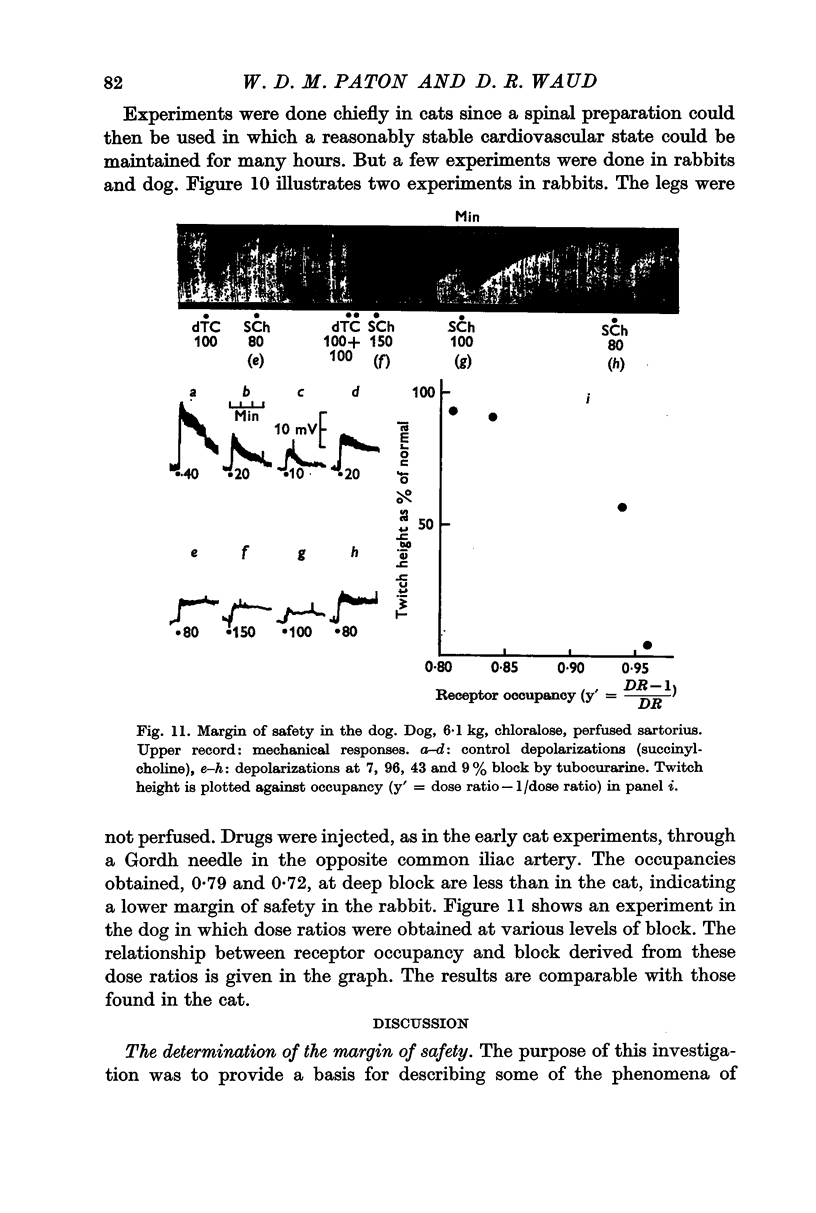
5. With dog sartorius muscle, results similar to those in the cat were obtained. But for deep block in the rabbit, the safety factor was only about 4.
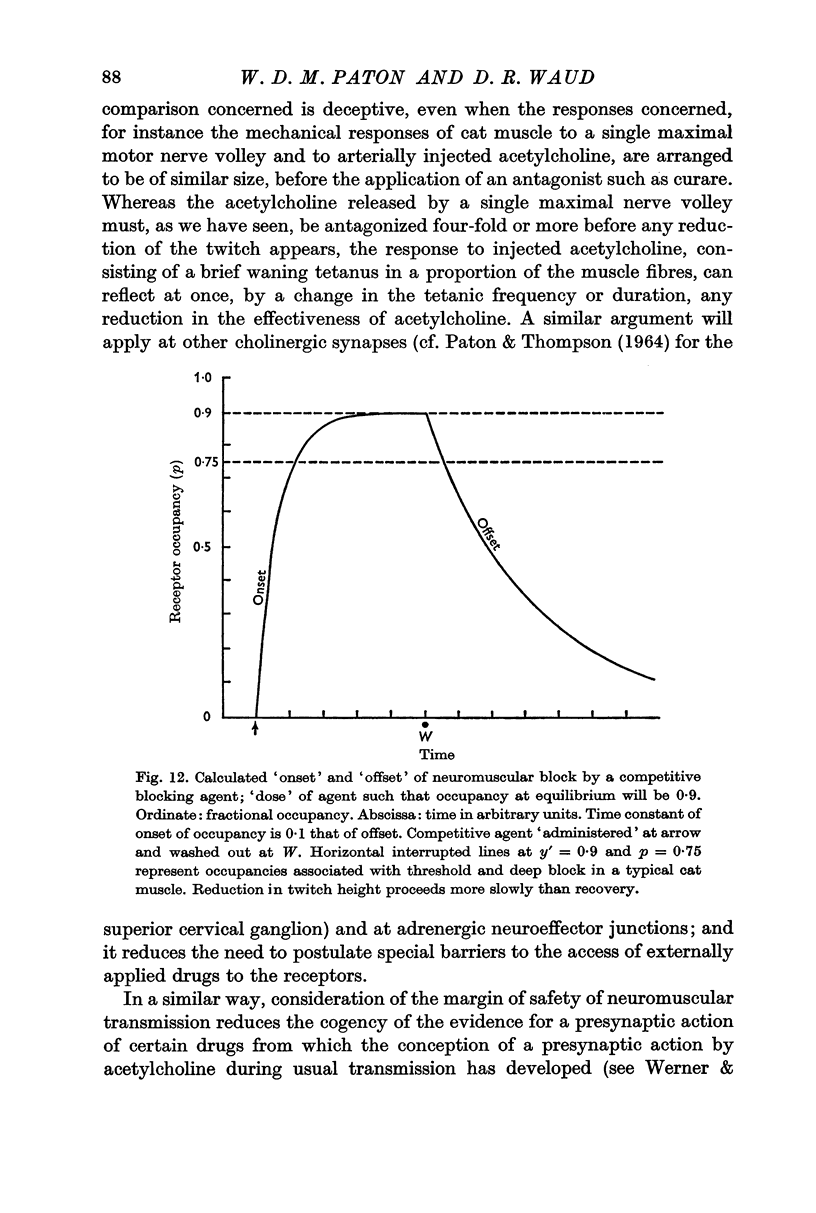
6. The existence of a substantial margin of safety influences considerably the interpretation of the time course of action of blocking drugs, and of comparisons between responses to nervous excitation and drug injection.
Full text
PDF



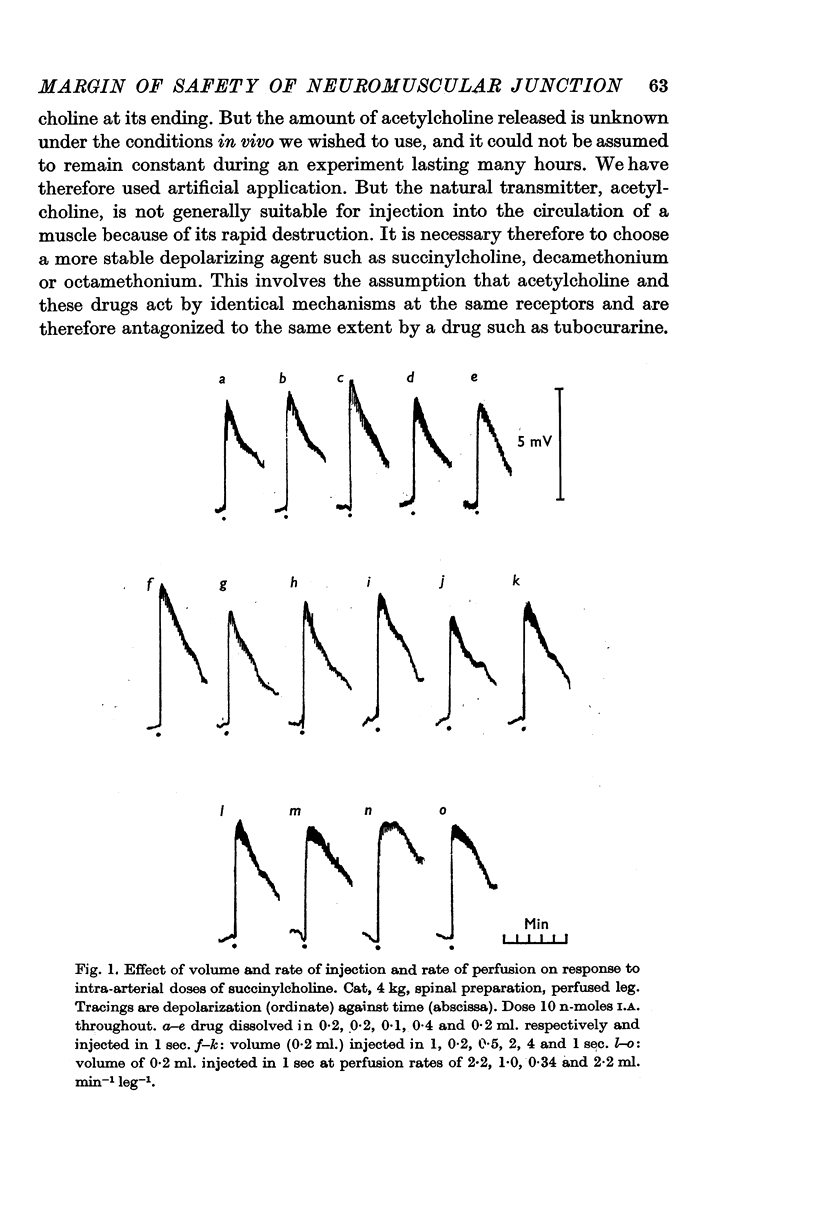
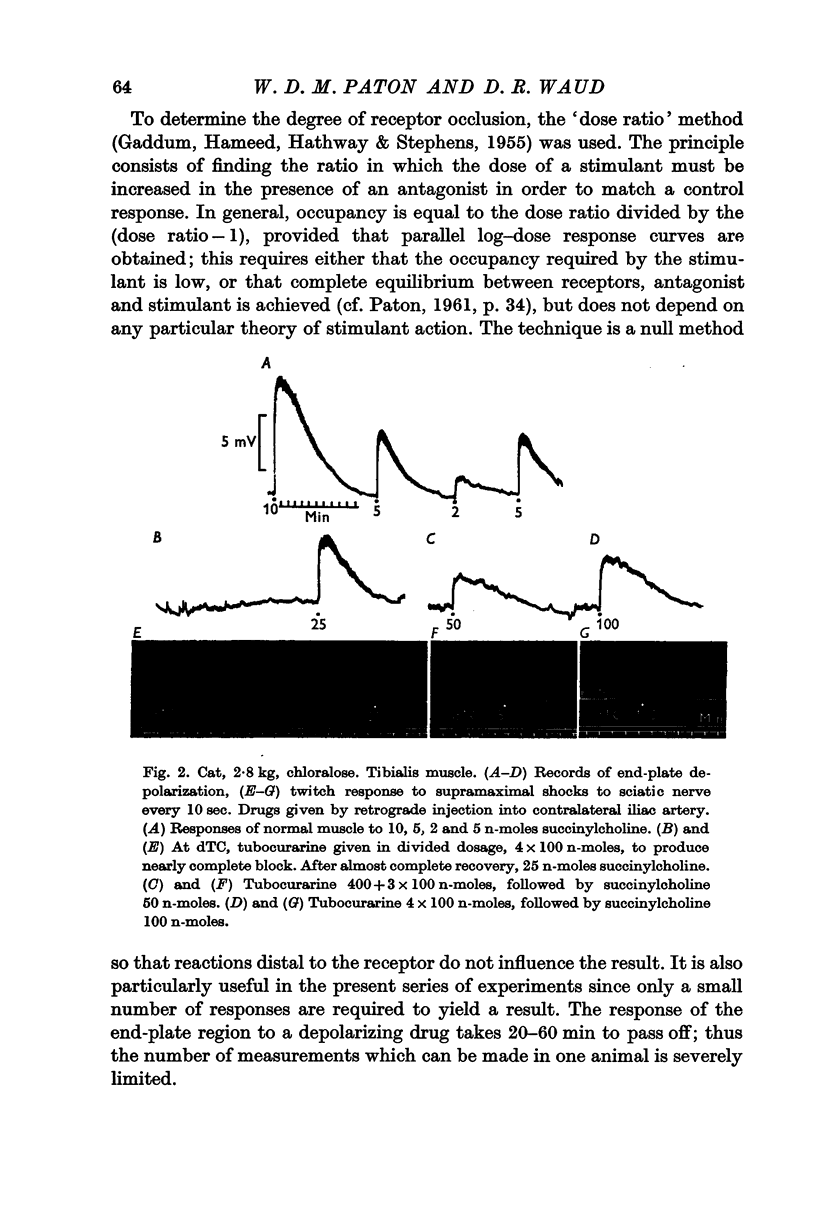

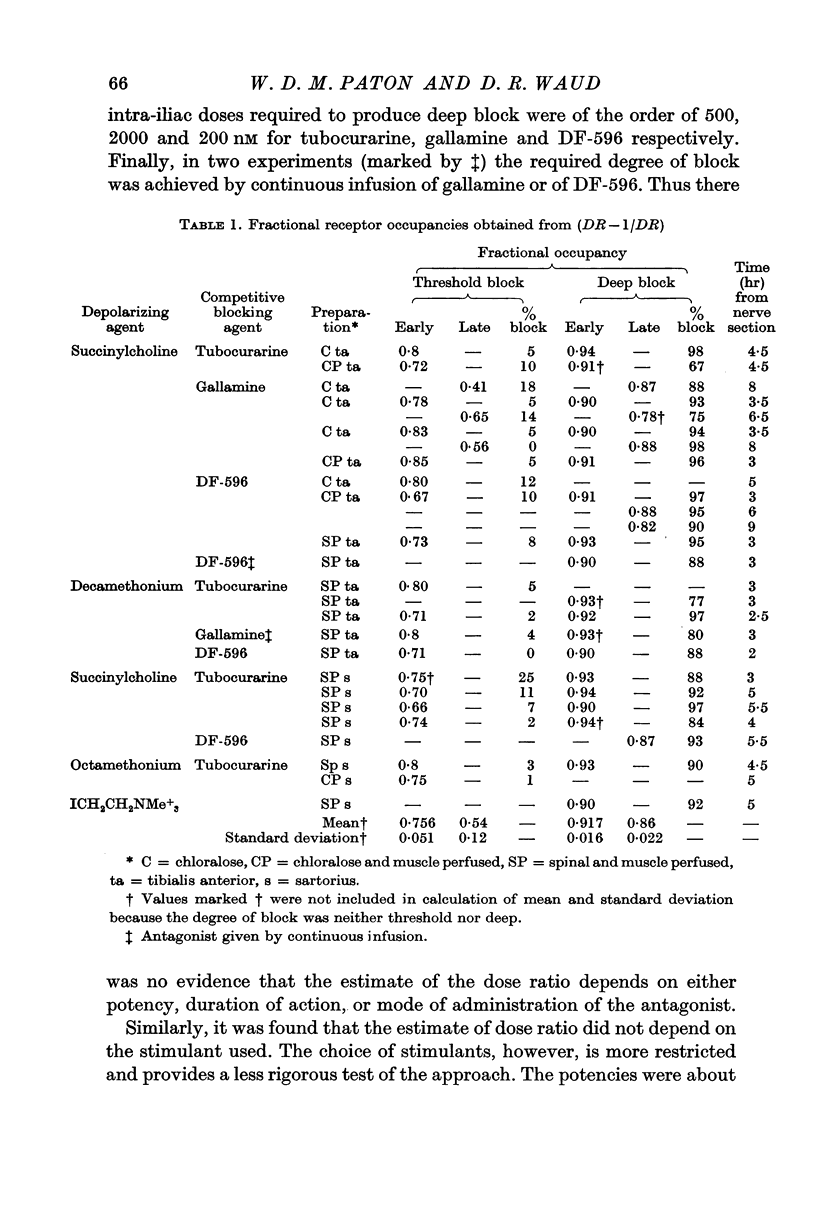
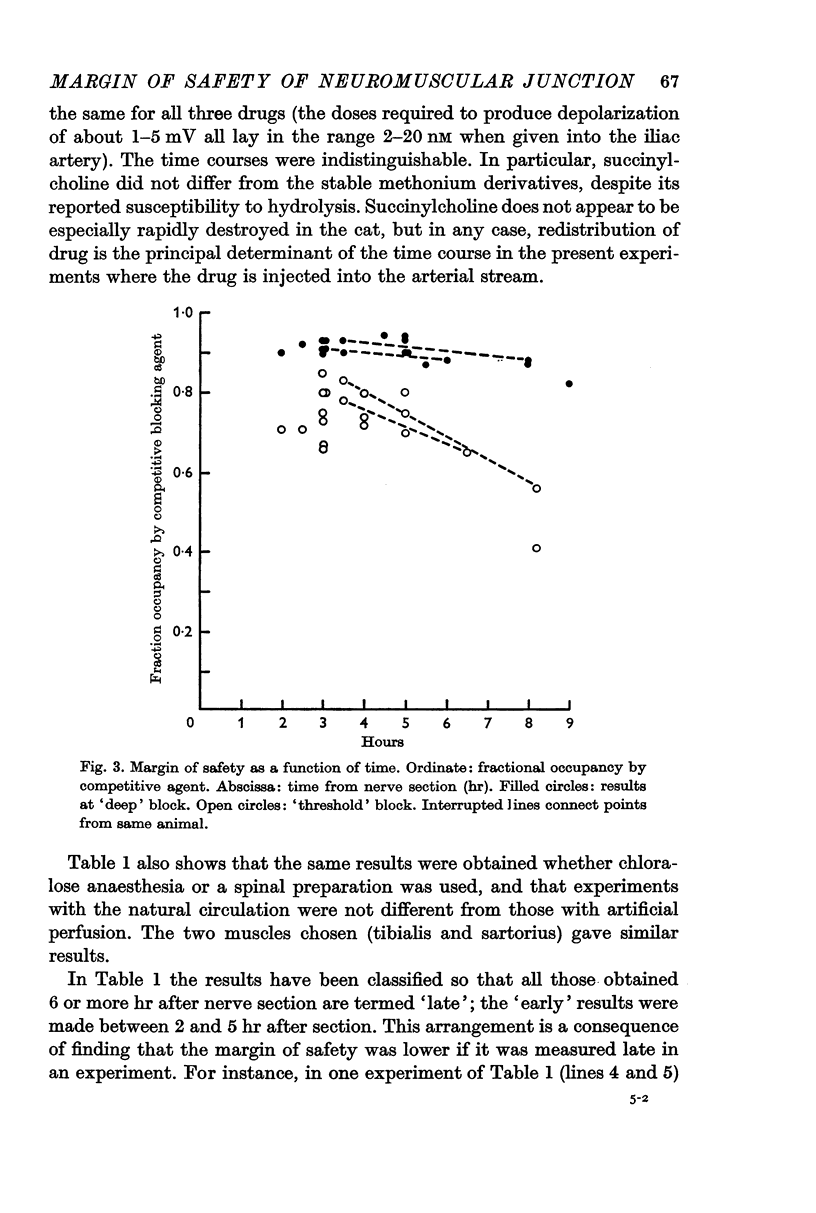













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIENS E. J., VAN ROSSUM J. M., SIMONIS A. M. Affinity, intrinsic activity and drug interactions. Pharmacol Rev. 1957 Jun;9(2):218–236. [PubMed] [Google Scholar]
- BANISTER J., SCRASE M. Acetylcholine synthesis in normal and denervated sympathetic ganglia of the cat. J Physiol. 1950 Oct 16;111(3-4):437–444. doi: 10.1113/jphysiol.1950.sp004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B., THIES R. E. Reduction of quantum content during neuromuscular transmission. J Physiol. 1962 Jul;162:298–310. doi: 10.1113/jphysiol.1962.sp006934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D., PATON W. D. M. Depolarization of the motor end-plate by decamethonium and acetylcholine. J Physiol. 1951 Sep;115(1):41–73. doi: 10.1113/jphysiol.1951.sp004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The identity of intrinsic and extrinsic acetylcholine receptors in the motor end-plate. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):357–361. doi: 10.1098/rspb.1957.0016. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO L., KATZ B. A study of curare action with an electrical micromethod. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):339–356. doi: 10.1098/rspb.1957.0015. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., PATON W. D. The mechanisms of motor end-plate depolarization due to a cholinesterase-inhibiting drug. J Physiol. 1954 May 28;124(2):325–344. doi: 10.1113/jphysiol.1954.sp005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELIN N. Submaxillary and sublingual secretion in cats during degeneration of post-ganglionic parasympathetic fibres. J Physiol. 1962 Jul;162:270–281. doi: 10.1113/jphysiol.1962.sp006931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F. The pharmacology of vascular smooth muscle. Pharmacol Rev. 1955 Jun;7(2):183–265. [PubMed] [Google Scholar]
- GADDUM J. H., HAMEED K. A., HATHWAY D. E., STEPHENS F. F. Quantitative studies of antagonists for 5-hydroxytryptamine. Q J Exp Physiol Cogn Med Sci. 1955 Jan;40(1):49–74. doi: 10.1113/expphysiol.1955.sp001097. [DOI] [PubMed] [Google Scholar]
- Gill E. W., Rang H. P. An alkylating derivative of benzilylcholine with specific and long-lasting parasympatholytic activity. Mol Pharmacol. 1966 Jul;2(4):284–297. [PubMed] [Google Scholar]
- HAINING C. G., JOHNSTON R. G., SMITH J. M. The neuromuscular blocking properties of a series of bis-quaternary tropeines. Br J Pharmacol Chemother. 1960 Mar;15:71–81. doi: 10.1111/j.1476-5381.1960.tb01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBB C. O., KRNJEVIC K., SILVER A. ACETYLCHOLINE AND CHOLINE ACETYLTRANSFERASE IN THE DIAPHRAGM OF THE RAT. J Physiol. 1964 Jun;171:504–513. doi: 10.1113/jphysiol.1964.sp007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES P. E. B., JENDEN D. J., TAYLOR D. B. The analysis of the mode of action of curare on neuromuscular transmission; the effect of temperature changes. J Pharmacol Exp Ther. 1951 Dec;103(4):382–402. [PubMed] [Google Scholar]
- Hebb C. O. Acetylcholine content of the rabbit plantaris muscle after denervation. J Physiol. 1962 Sep;163(2):294–306. doi: 10.1113/jphysiol.1962.sp006975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENDEN D. J., KAMIJO K., TAYLOR D. B. The action of decamethonium on the isolated rabbit lumbrical muscle. J Pharmacol Exp Ther. 1954 Jun;111(2):229–240. [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUPP H., KRAUPP O., HONETZ N., KOBINGER W., LOUDON M. Uber die Freistzung von Kalium aus der Muskulatur unter der Einwirkung einiger Muskelrelaxantien. Arch Int Pharmacodyn Ther. 1954 Jul 1;98(3):340–354. [PubMed] [Google Scholar]
- PATON W. D., THOMPSON J. W. THE GANGLION BLOCKING ACTION OF PROCAINAMIDE. Br J Pharmacol Chemother. 1964 Feb;22:143–153. doi: 10.1111/j.1476-5381.1964.tb01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. The kinetics of action of acetylcholine antagonists in smooth muscle. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):488–510. doi: 10.1098/rspb.1966.0045. [DOI] [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


