Abstract
The membrane properties of single cells of intestinal smooth muscle of duodenum, jejunum, ileum, caecum and rectum of guinea-pig have been studied.
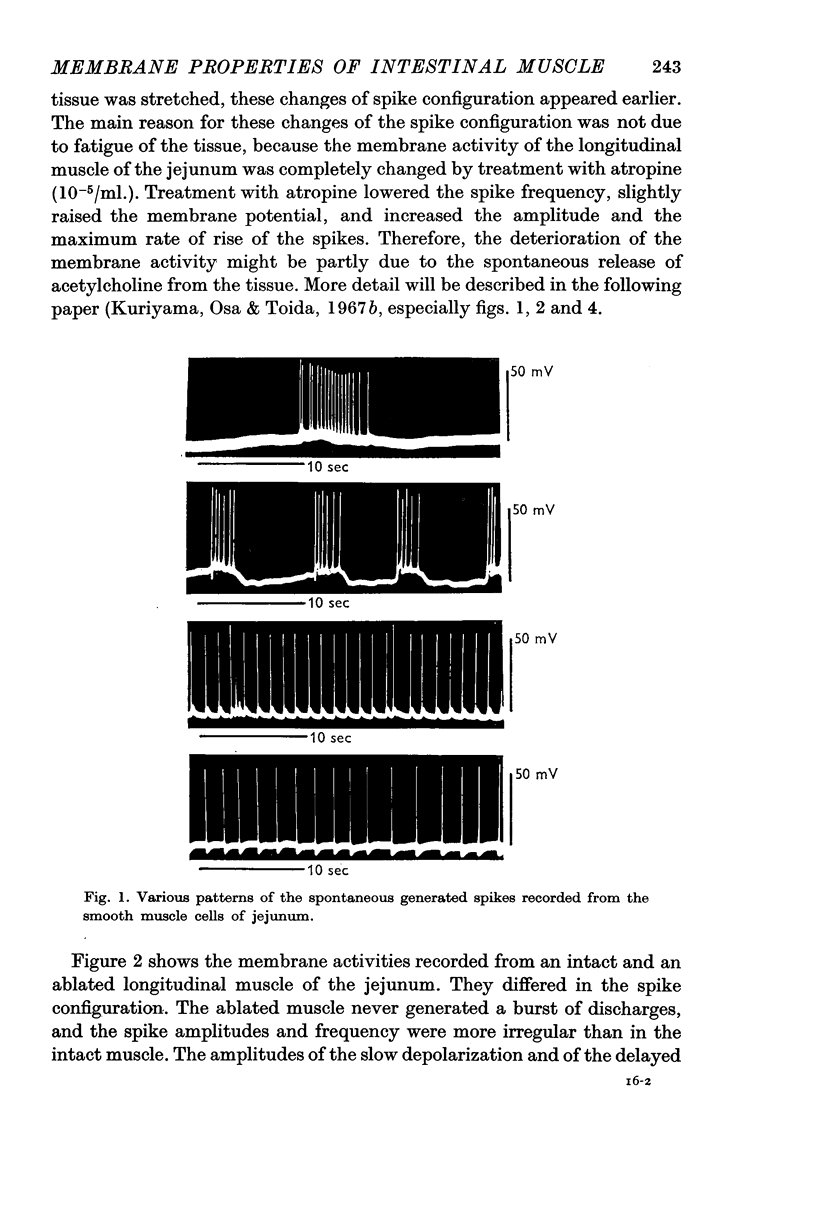
1. The membrane potentials of longitudinal muscles of the duodenum, jejunum, ileum, caecum and rectum varied from 54 to 56 mV and those of circular muscles of jejunum, ileum, caecum and rectum varied from 57 to 60 mV. The ablated longitudinal muscle had a slightly lower value (50 mV) than the intact one.
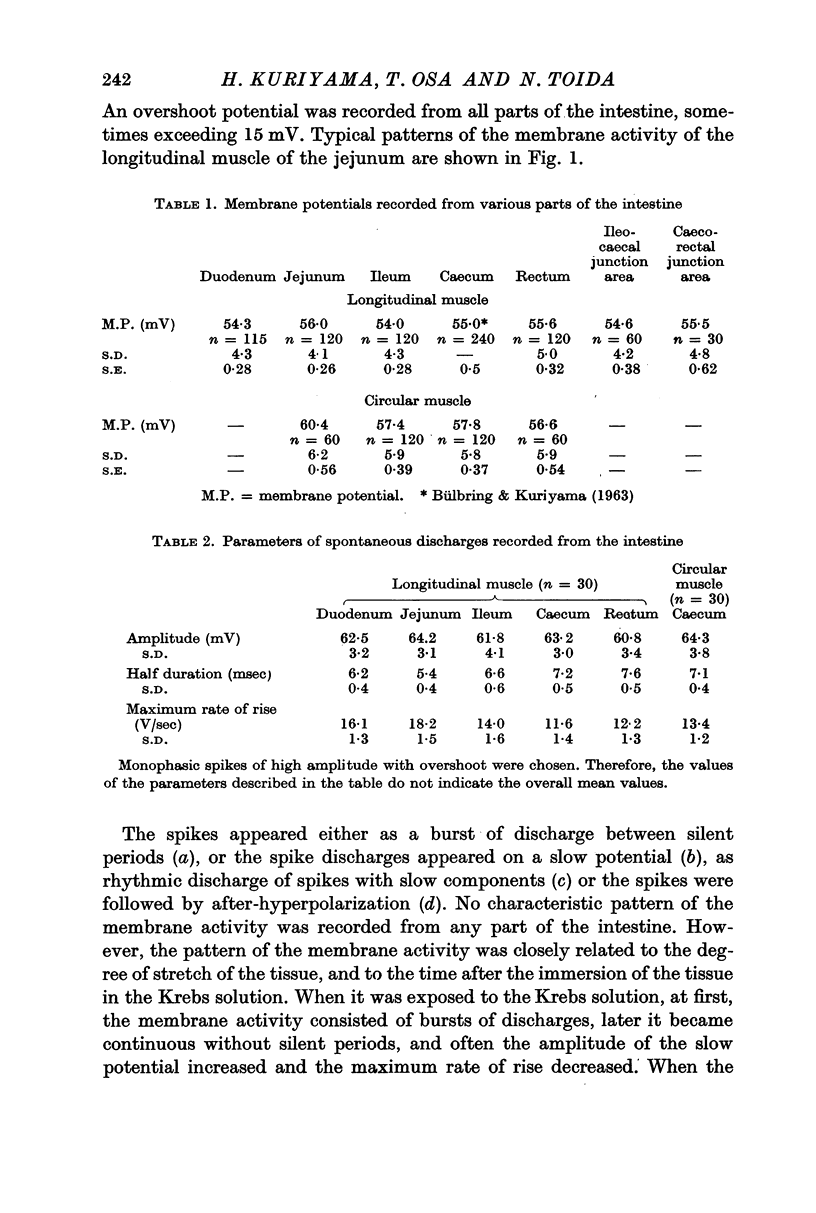
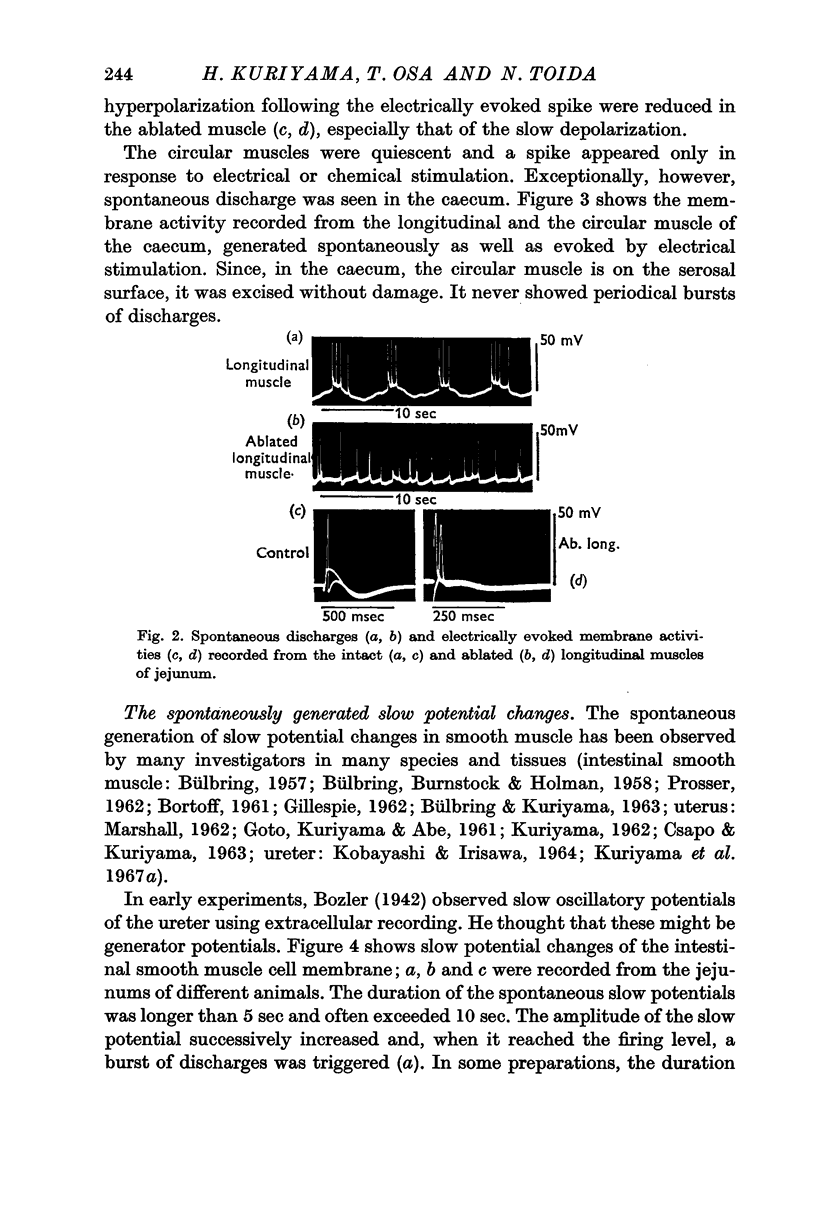
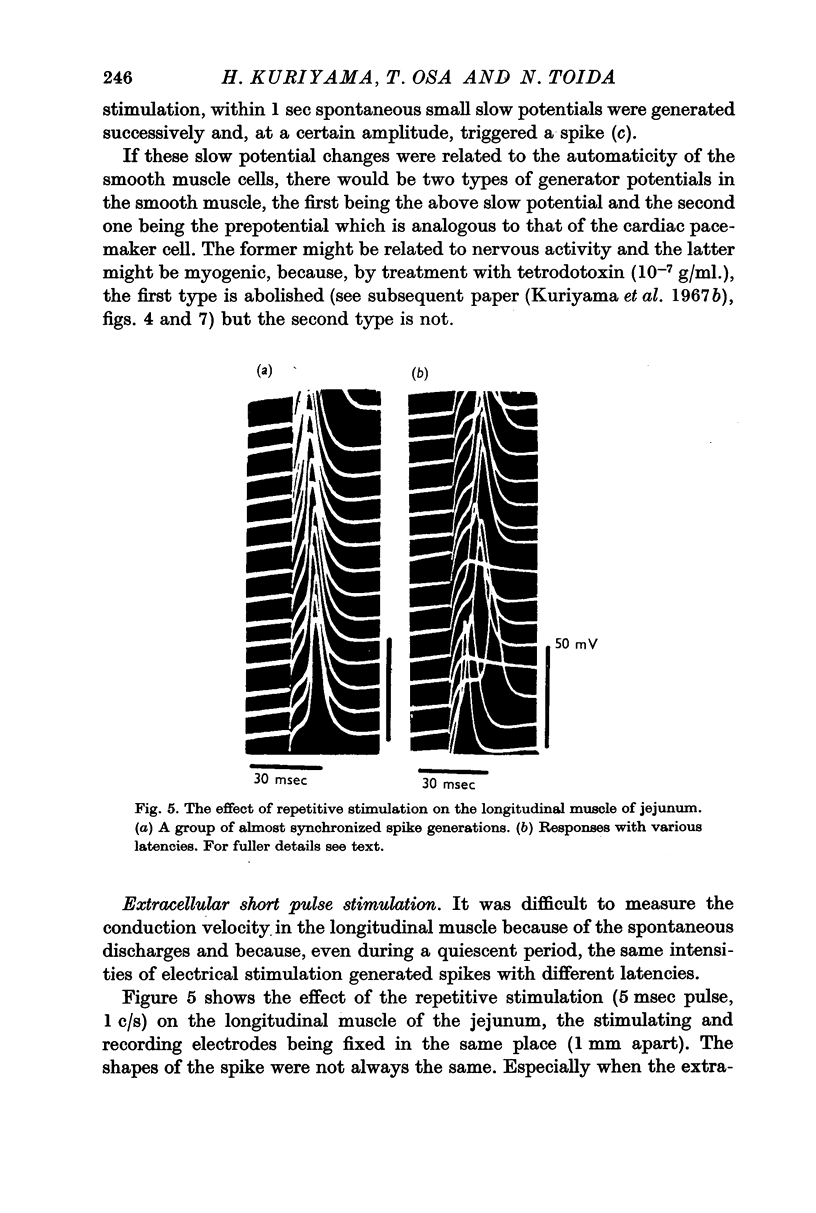
2. The longitudinal muscle generated spontaneous discharges but these were rare in the circular muscles of the intestine except for the caecum. Overshoot potentials could be observed in all regions of the intestine. The maximum rate of rise of the spontaneously discharging longitudinal muscles varied from 11 to 18 V/sec.
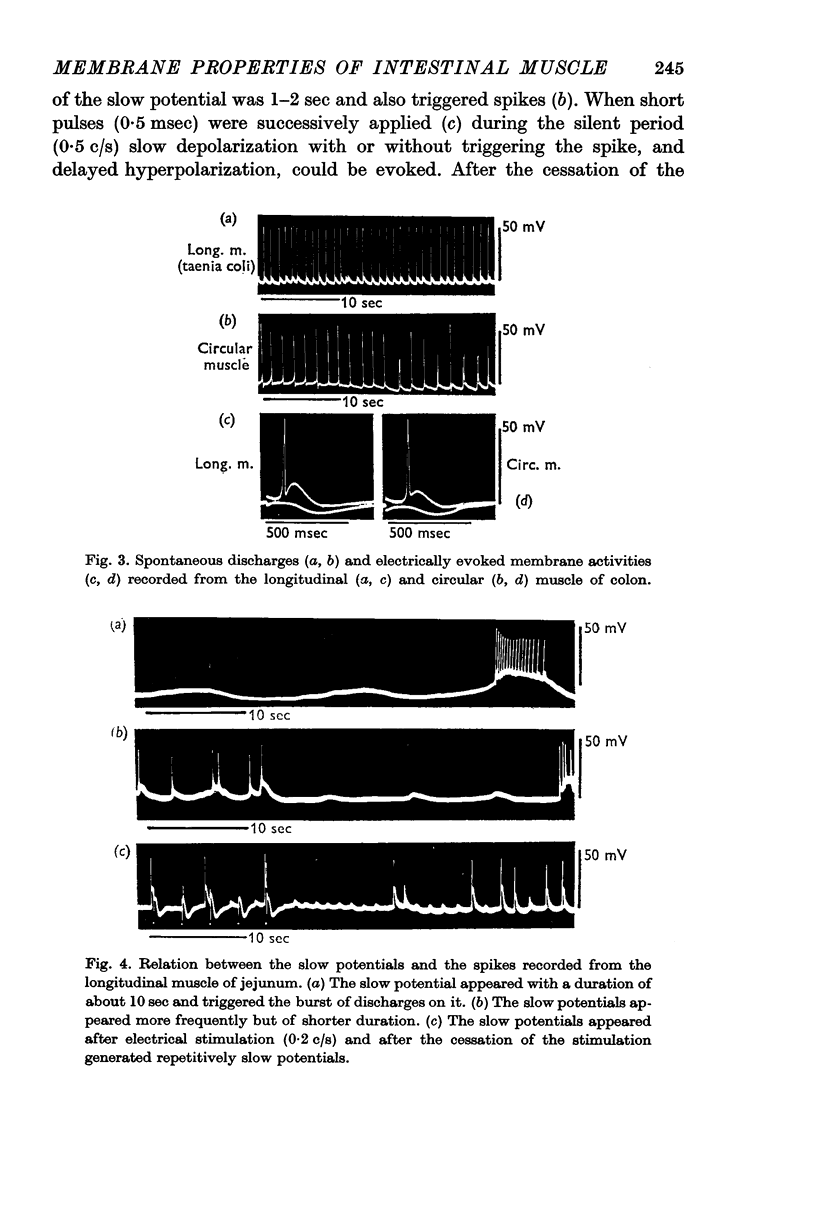
3. Not all of the slow potential changes (but at least some) were generated by the nervous elements distributed between the muscle layers and in them.
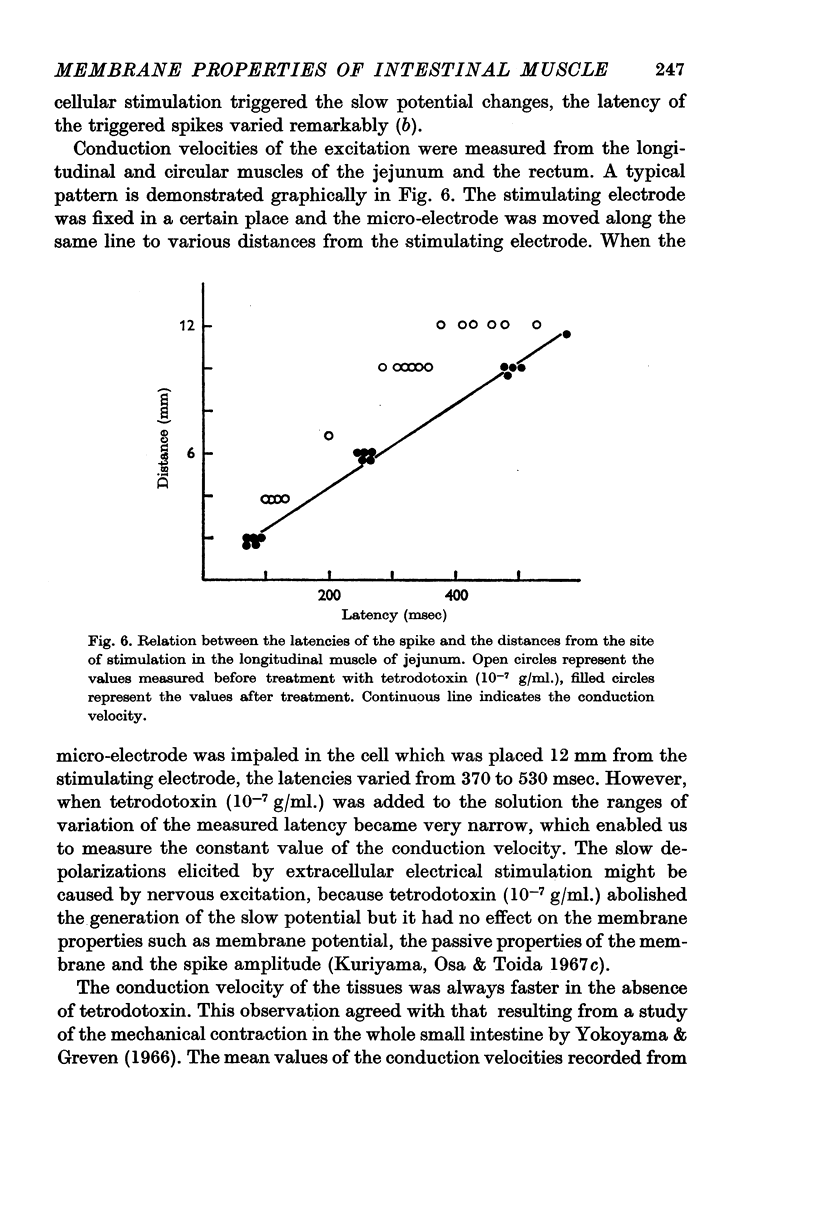
4. The conduction velocities measured from the longitudinal muscles of jejunum and rectum in the presence of tetrodotoxin were 2·1 cm/sec and 4·0 cm/sec respectively.
5. Chronaxies of the longitudinal muscles of jejunum and rectum were 2-5 msec and 5-18 msec respectively.
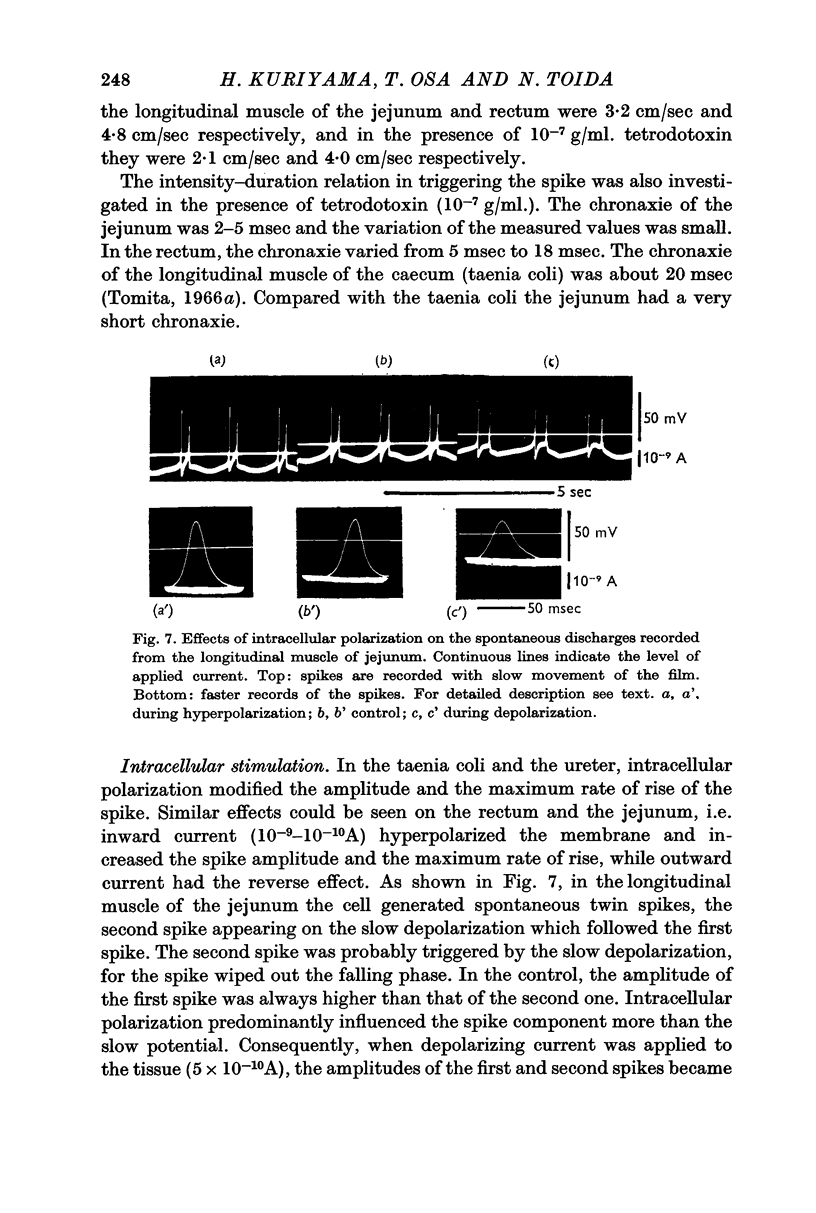
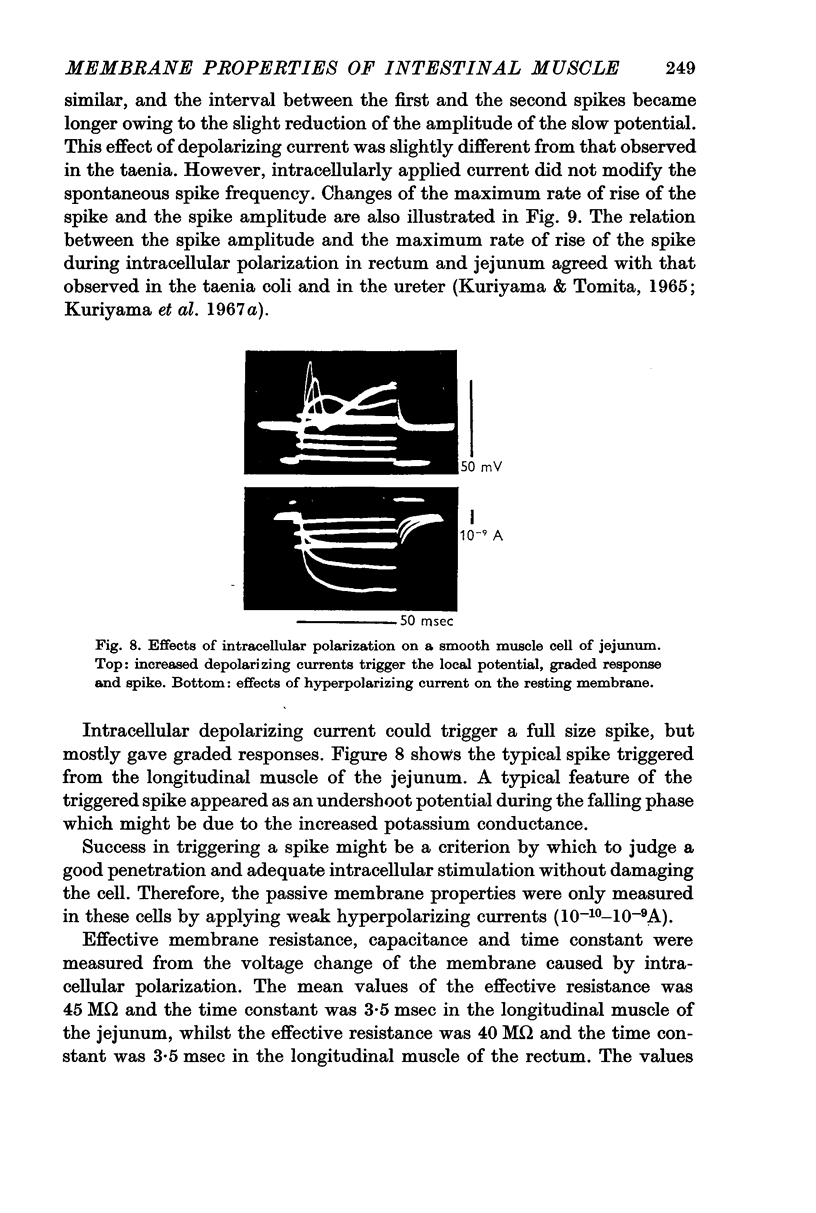
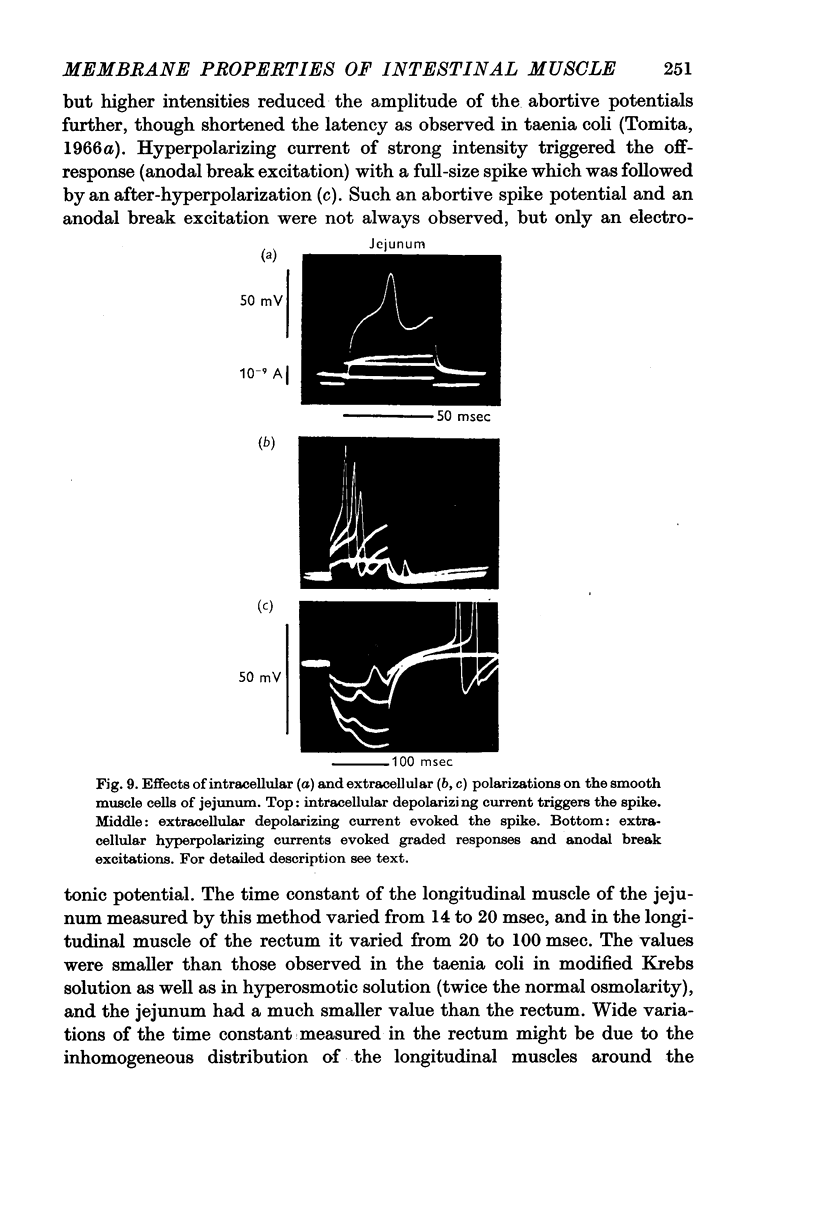
6. Intracellular stimulation of the single cells of the duodenum and caecum could trigger a spike, similar to that observed in the taenia coli. The spikes were mostly graded ones; a spike of full size was rarely elicited. When the spikes were triggered, the after-hyperpolarization appeared consistently presumably caused by the increased potassium conductance.
7. The effective membrane resistance and the time constant were measured for the longitudinal muscles of the jejunum and rectum. When spikes were generated by intracellular stimulation the observed values were 40-50 MΩ and 3-5 msec in both tissues. These values were the same as those observed in the taenia coli.
8. When the time constant of the membrane was measured by the extracellular polarizing method, the longitudinal muscles of the jejunum especially and the rectum had smaller time constants than the taenia coli.
9. The differences of conduction velocity and chronaxie of the different regions of the intestine are discussed in relation to the cable properties of the tissues which are directly influenced by the morphological arrangements of the tissues.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARR L. Transmembrane resistance of smooth muscle cells. Am J Physiol. 1961 Jun;200:1251–1255. doi: 10.1152/ajplegacy.1961.200.6.1251. [DOI] [PubMed] [Google Scholar]
- BULBRING E., BURNSTOCK G., HOLMAN M. E. Excitation and conduction in the smooth muscle of the isolated taenia coli of the guinea-pig. J Physiol. 1958 Aug 6;142(3):420–437. doi: 10.1113/jphysiol.1958.sp006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Changes in configuration of spontaneously discharged spike potentials from smooth muscle of the guinea-pig's taenia coli; the effect of electrotonic currents and of adrenaline, acetylcholine and histamine. J Physiol. 1957 Feb 15;135(2):412–425. doi: 10.1113/jphysiol.1957.sp005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., PROSSER C. L. Delayed repolarization in smooth muscles. Proc Soc Exp Biol Med. 1960 Feb;103:269–270. doi: 10.3181/00379727-103-25484. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., STRAUB R. W. A method for studying the effects of ions and drugs on the resting and action potentials in smooth muscle with external electrodes. J Physiol. 1958 Jan 23;140(1):156–167. doi: 10.1113/jphysiol.1958.sp005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., KURIYAMA H. MEMBRANE POTENTIAL AND IONIC CONTENT IN PREGNANT AND NON-PREGNANT RAT MYOMETRIUM. J Physiol. 1965 Mar;177:263–287. doi: 10.1113/jphysiol.1965.sp007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO I. A., KURIYAMA H. A. Effects of ions and drugs on cell membrane activity and tension in the postpartum rat myometrium. J Physiol. 1963 Mar;165:575–592. doi: 10.1113/jphysiol.1963.sp007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWEY M. M., BARR L. A STUDY OF THE STRUCTURE AND DISTRIBUTION OF THE NEXUS. J Cell Biol. 1964 Dec;23:553–585. doi: 10.1083/jcb.23.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. S. Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic-nerve stimulation. J Physiol. 1962 Jun;162:54–75. doi: 10.1113/jphysiol.1962.sp006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTO M., KURIYAMA H., ABE Y. Refractory period and conduction of excitation in the uterine muscle cells of the mouse. Jpn J Physiol. 1961 Aug 15;11:369–377. doi: 10.2170/jjphysiol.11.369. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Holman M. E., Tille J. Electrical properties of the smooth muscle membrane of the guinea-pig vas deferens. J Physiol. 1966 Sep;186(1):27–41. doi: 10.1113/jphysiol.1966.sp008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI M., IRISAWA H. EFFECT OF SODIUM DEFICIENCY ON THE ACTION POTENTIAL OF THE SMOOTH MUSCLE OF URETER. Am J Physiol. 1964 Jan;206:205–210. doi: 10.1152/ajplegacy.1964.206.1.205. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H., TOMITA T. THE RESPONSES OF SINGLE SMOOTH MUSCLE CELLS OF GUINEA-PIG TAENIA COLI TO INTRACELLULARLY APPLIED CURRENTS, AND THEIR EFFECT ON THE SPONTANEOUS ELECTRICAL ACTIVITY. J Physiol. 1965 May;178:270–289. doi: 10.1113/jphysiol.1965.sp007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Membrane properties of the smooth muscle of guinea-pig ureter. J Physiol. 1967 Jul;191(2):225–238. doi: 10.1113/jphysiol.1967.sp008247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Nervous factors influencing the membrane activity of intestinal smooth muscle. J Physiol. 1967 Jul;191(2):257–270. doi: 10.1113/jphysiol.1967.sp008249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAI T., PROSSER C. L. Electrical parameters of smooth muscle cells. Am J Physiol. 1963 May;204:915–924. doi: 10.1152/ajplegacy.1963.204.5.915. [DOI] [PubMed] [Google Scholar]
- NAGAI T., PROSSER C. L. Patterns of conduction in smooth muscle. Am J Physiol. 1963 May;204:910–914. doi: 10.1152/ajplegacy.1963.204.5.910. [DOI] [PubMed] [Google Scholar]
- PROSSER C. L., BURNSTOCK G., KAHN J. Conduction in smooth muscle: comparative structural properties. Am J Physiol. 1960 Sep;199:545–552. doi: 10.1152/ajplegacy.1960.199.3.545. [DOI] [PubMed] [Google Scholar]
- PROSSER C. L. Conduction in nonstriated muscles. Physiol Rev Suppl. 1962 Jul;5:193–212. [PubMed] [Google Scholar]
- PROSSER C. L., SPERELAKIS N. Transmission in ganglion-free circular muscle from the cat intestine. Am J Physiol. 1956 Dec;187(3):536–545. doi: 10.1152/ajplegacy.1956.187.3.536. [DOI] [PubMed] [Google Scholar]
- SPERELAKIS N., TARR M. WEAK ELECTRONIC INTERACTION BETWEEN NEIGHBORING VISCERAL SMOOTH MUSCLE CELLS. Am J Physiol. 1965 Apr;208:737–747. doi: 10.1152/ajplegacy.1965.208.4.737. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Membrane capacity and resistance of mammalian smooth muscle. J Theor Biol. 1966 Nov;12(2):216–227. doi: 10.1016/0022-5193(66)90114-7. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Greven K. Weitere Studien über die Erregungsleitung in der Dünndarmlängsmuskulatur nach Untersuchungen mit extracellulär abgeleiteten Aktionspotentialen. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(2):147–162. [PubMed] [Google Scholar]


