Abstract
The effects of various chemical agents on the spontaneous membrane activities and those electrically elicited in the smooth muscles of small intestine were investigated.
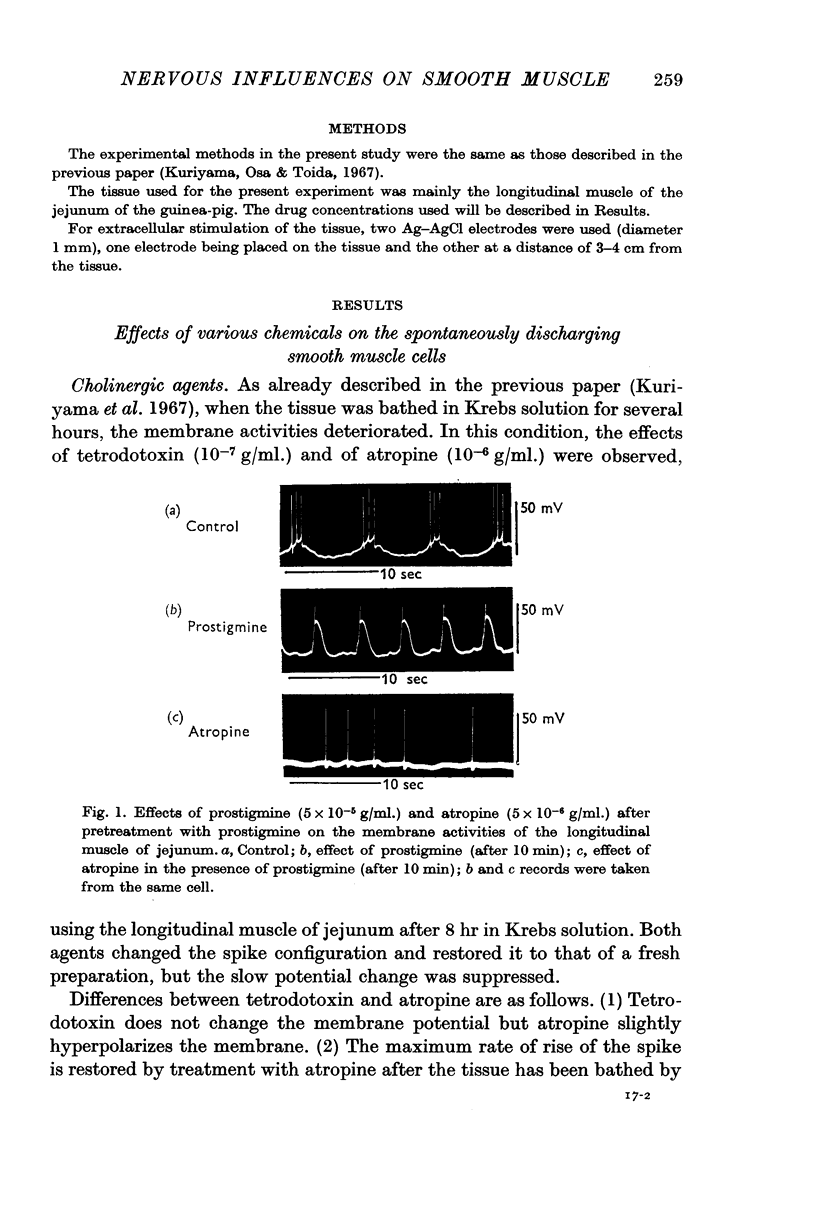
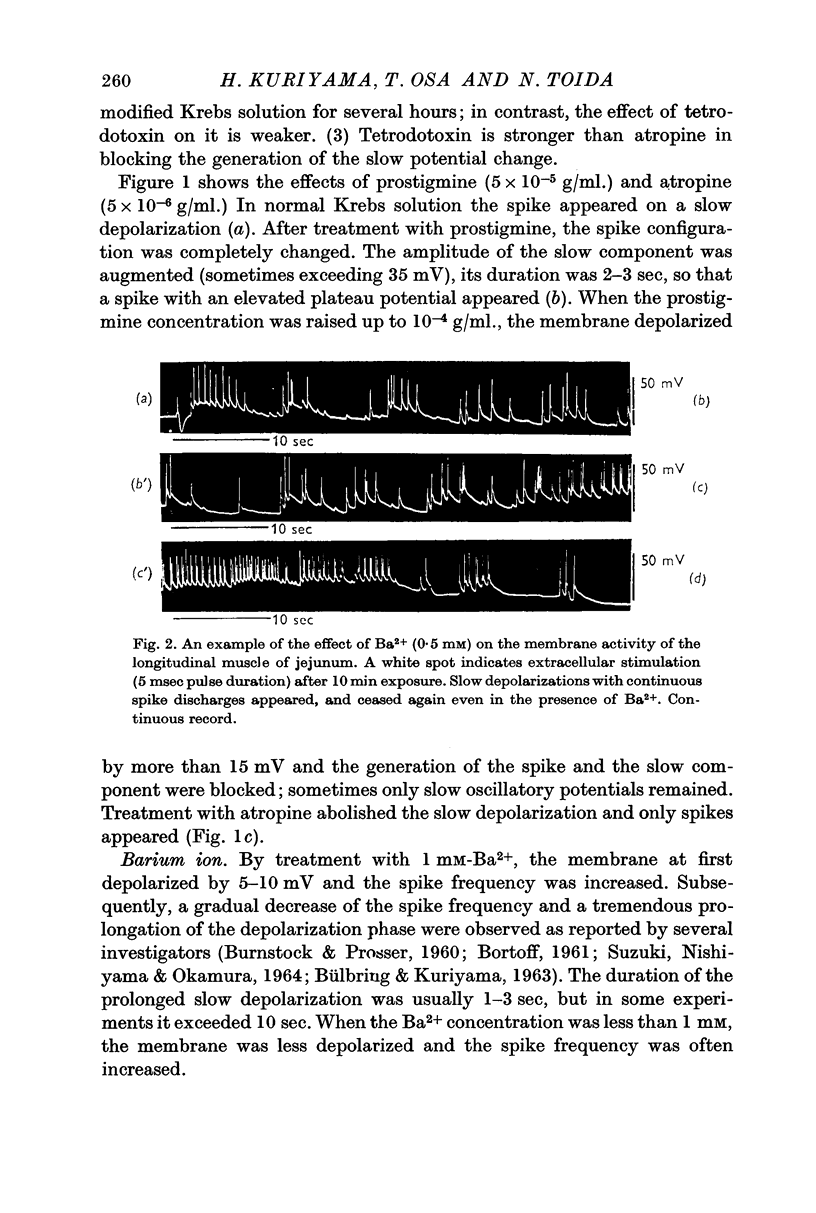
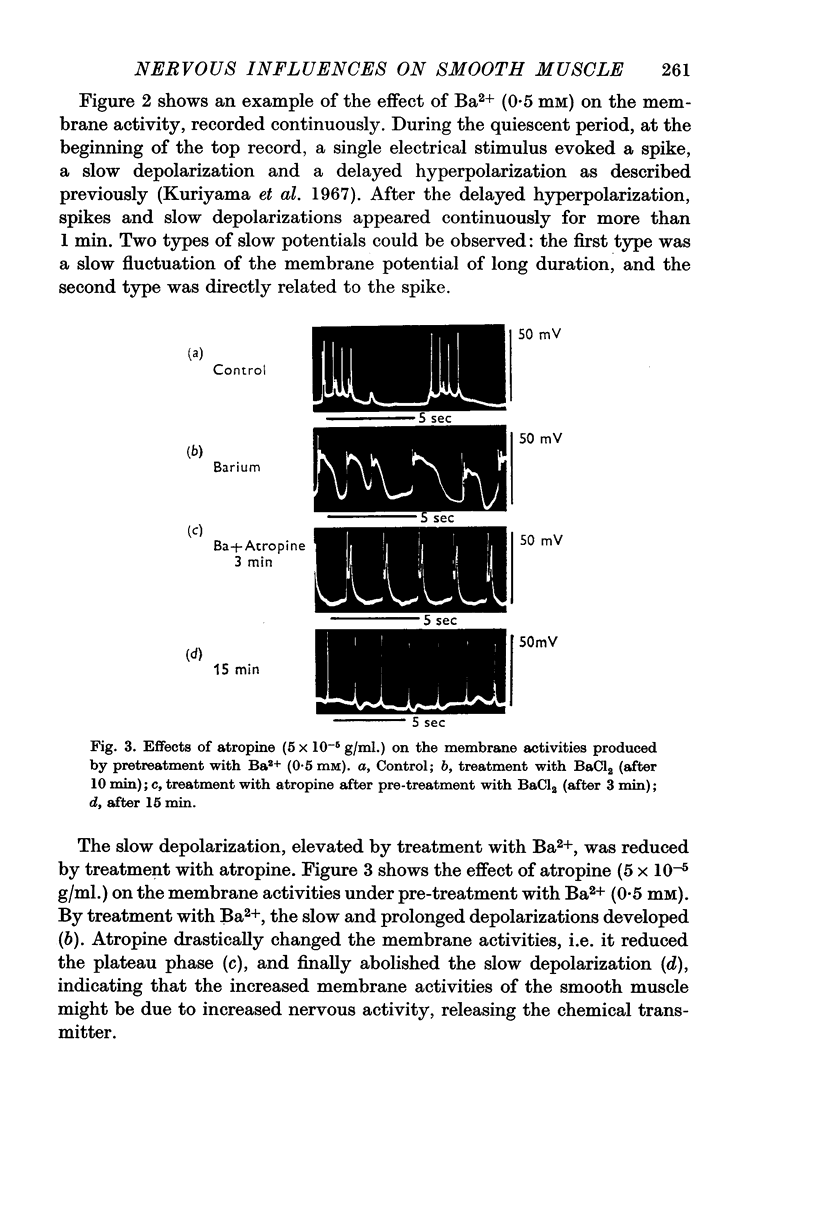
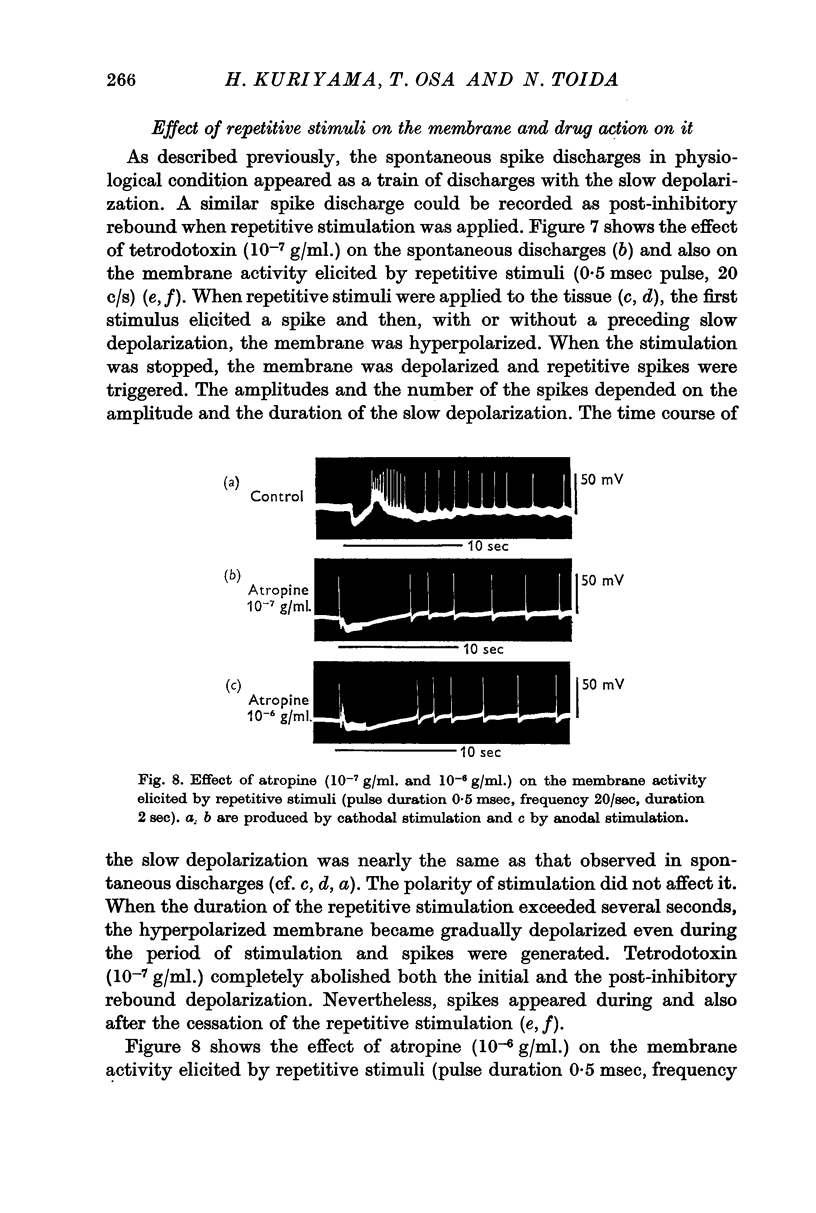
1. The effects of various chemicals on the spontaneously active membrane might be summarized as follows. (a) Cholinergic agents; atropine slightly hyperpolarized the membrane and reduced the amplitude of slow potential changes even in aged preparations. Prostigmine depolarized the membrane, and enhanced the amplitude and prolonged the duration of the slow potential changes. Atropine prevented the actions of prostigmine on the membrane. (b) Ba2+ depolarized the membrane, and enhanced the amplitude and prolonged the duration of the slow potential changes. The spike frequency was initially increased, then reduced. Atropine and tetrodotoxin partially prevented the action of Ba2+ on the membrane activities.
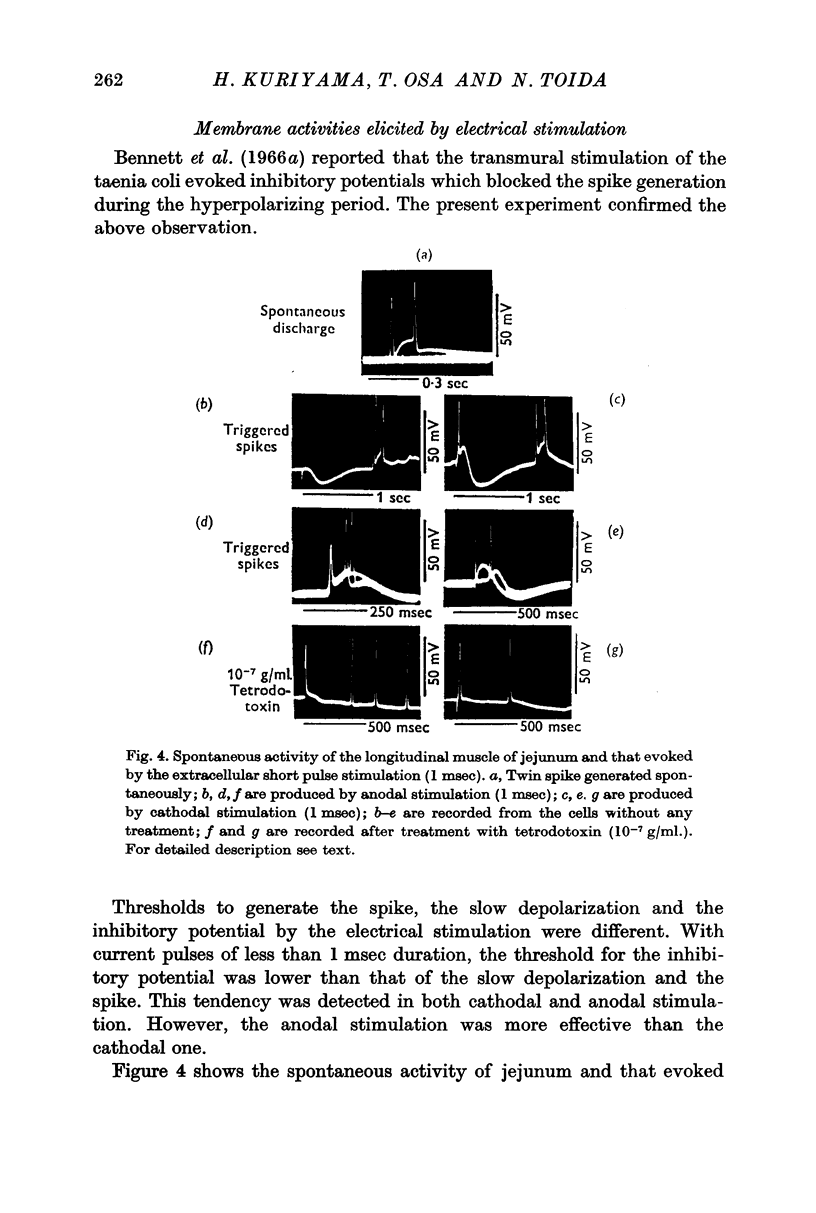
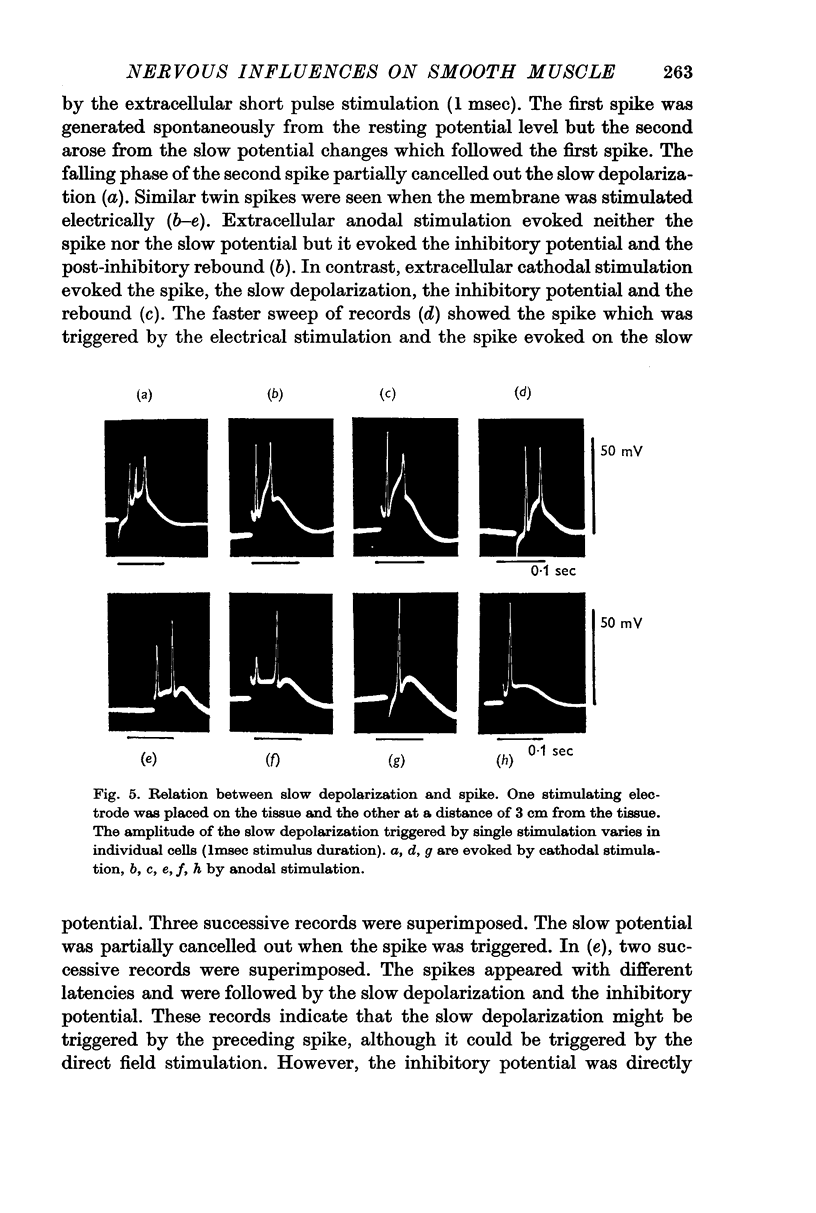
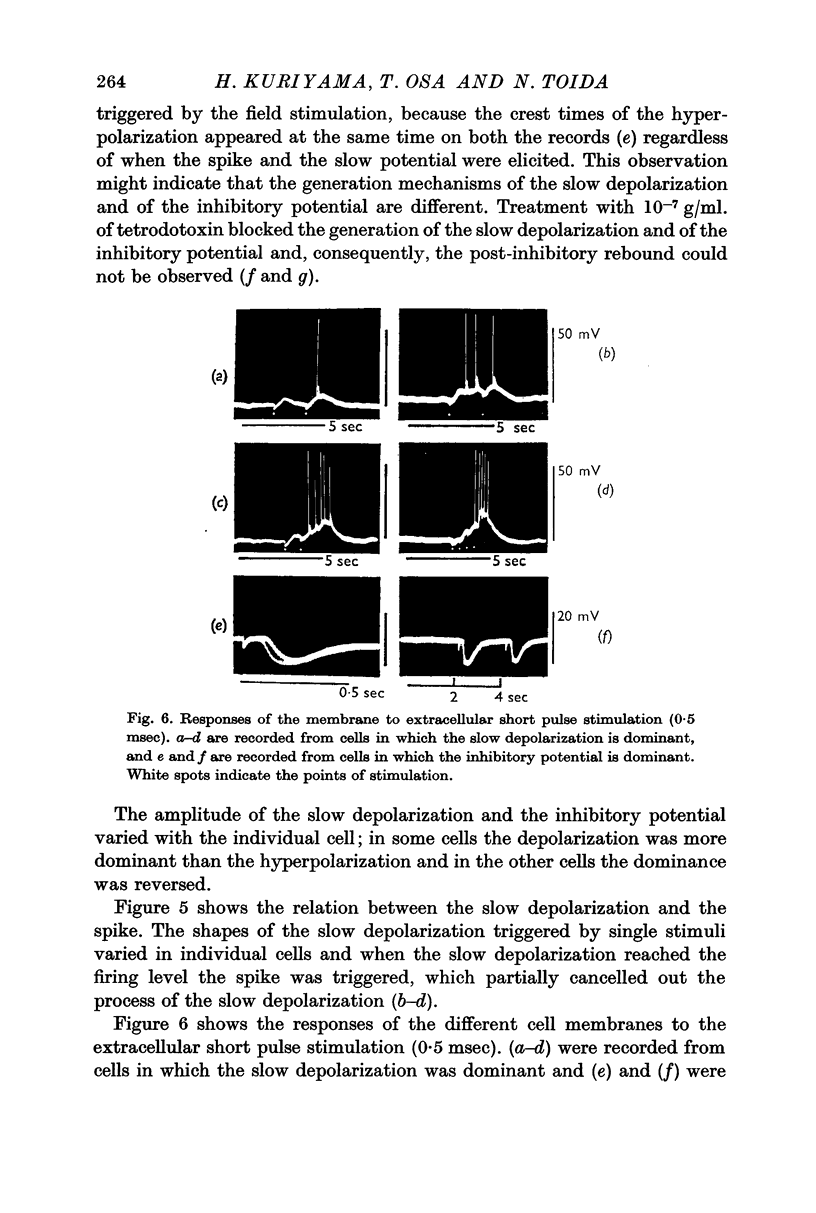
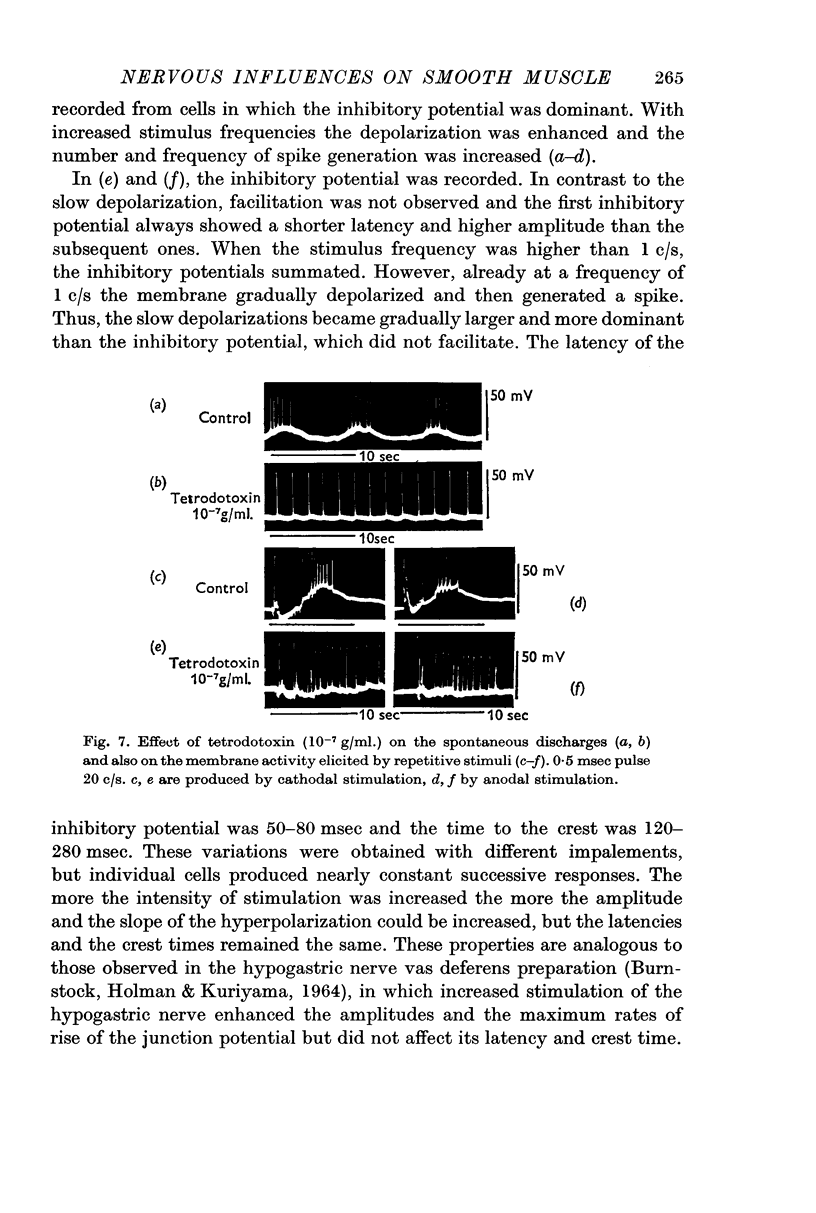
2. Effects of chemical agents on the membrane activity elicited by electrical stimulation might be summarized as follows. (a) Short pulse stimulation (0·5-1 msec) generated the spike as a direct response of the muscle cell membrane, then it was followed by slow depolarization, delayed hyperpolarization, i.e. the `inhibitory potential', and post-inhibitory rebound successively. (b) The slow depolarization and the post-inhibitory rebound were reduced in amplitude by treatment with atropine, and enhanced by treatments with prostigmine and Ba2+. Tetrodotoxin blocked all activities except the spike.
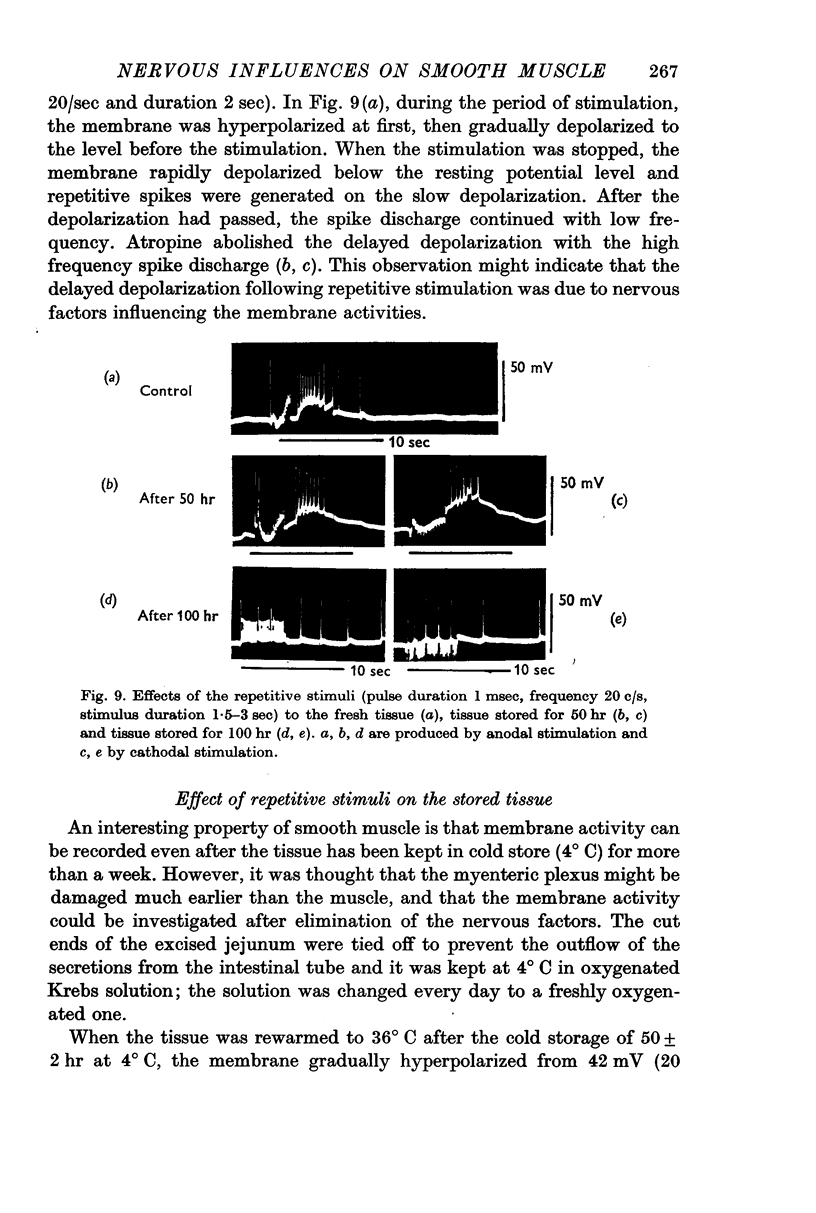
3. When repetitive stimulation (20 c/s) was applied to the membrane, the membrane hyperpolarized; then, after 3-5 sec, it gradually depolarized even if the stimulation was continued, and triggered spikes. The hyperpolarization always preceded depolarization. The duration and the amplitude of the delayed depolarization was proportionally increased by the increased intensity and duration of stimulation. Atropine and tetrodotoxin blocked the generation of the post-inhibitory rebound.
4. Effects of repetitive stimulation on the stored tissues were observed. The responses to repetitive stimulation of the membrane of muscles which had been stored 50 hr at 4 °C, were the same as those observed in the fresh tissue. The response of the tissue which had been stored 100 hr was the same as that observed in the fresh tissue treated with tetrodotoxin, i.e. all activities except the spikes were blocked.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E., KURIYAMA H. Effects of changes in the external sodium and calcium concentrations on spontaneous electrical activity in smooth muscle of guinea-pig taenia coli. J Physiol. 1963 Apr;166:29–58. doi: 10.1113/jphysiol.1963.sp007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., BENNETT M., HOLMAN M. E. INHIBITION OF THE SMOOTH MUSCLE ON THE TAENIA COLI. Nature. 1963 Nov 9;200:581–582. doi: 10.1038/200581a0. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E., KURIYAMA H. FACILITATION OF TRANSMISSION FROM AUTONOMIC NERVE TO SMOOTH MUSCLE OF GUINEA-PIG VAS DEFERENS. J Physiol. 1964 Jul;172:31–49. doi: 10.1113/jphysiol.1964.sp007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., PROSSER C. L. Delayed repolarization in smooth muscles. Proc Soc Exp Biol Med. 1960 Feb;103:269–270. doi: 10.3181/00379727-103-25484. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. E. Transmission from perivascular inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):527–540. doi: 10.1113/jphysiol.1966.sp007835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Rand M. J. The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol. 1966 Feb;182(3):504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. Nerve-mediated excitation of the taenia of the guinea-pig caecum. J Physiol. 1966 Jul;185(1):148–159. doi: 10.1113/jphysiol.1966.sp007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE J. S. Spontaneous mechanical and electrical activity of stretched and unstretched intestinal smooth muscle cells and their response to sympathetic-nerve stimulation. J Physiol. 1962 Jun;162:54–75. doi: 10.1113/jphysiol.1962.sp006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUKUHARA T., YAMAGAMI M., NAKAYAMA S. On the intestinal intrinsic reflexes. Jpn J Physiol. 1958 Mar 30;8(1):9–20. doi: 10.2170/jjphysiol.8.9. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Electrophysiological study of the intestinal smooth muscle of the guinea-pig. J Physiol. 1967 Jul;191(2):239–255. doi: 10.1113/jphysiol.1967.sp008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI T., INOMATA H. THE INHIBITORY POST-SYNAPTIC POTENTIAL IN INTESTINAL SMOOTH MUSCLE INVESTIGATED WITH INTRACELLULAR MICROELECTRODE. Tohoku J Exp Med. 1964 Feb 25;82:48–51. doi: 10.1620/tjem.82.48. [DOI] [PubMed] [Google Scholar]
- SUZUKI T., NISHIYAMA A., OKAMURA K. THE EFFECTS OF BARIUM ION ON THE RESTING AND ACTION POTENTIAL OF INTESTINAL SMOOTH MUSCLE CELL. Tohoku J Exp Med. 1964 Feb 25;82:87–92. doi: 10.1620/tjem.82.87. [DOI] [PubMed] [Google Scholar]


