Abstract
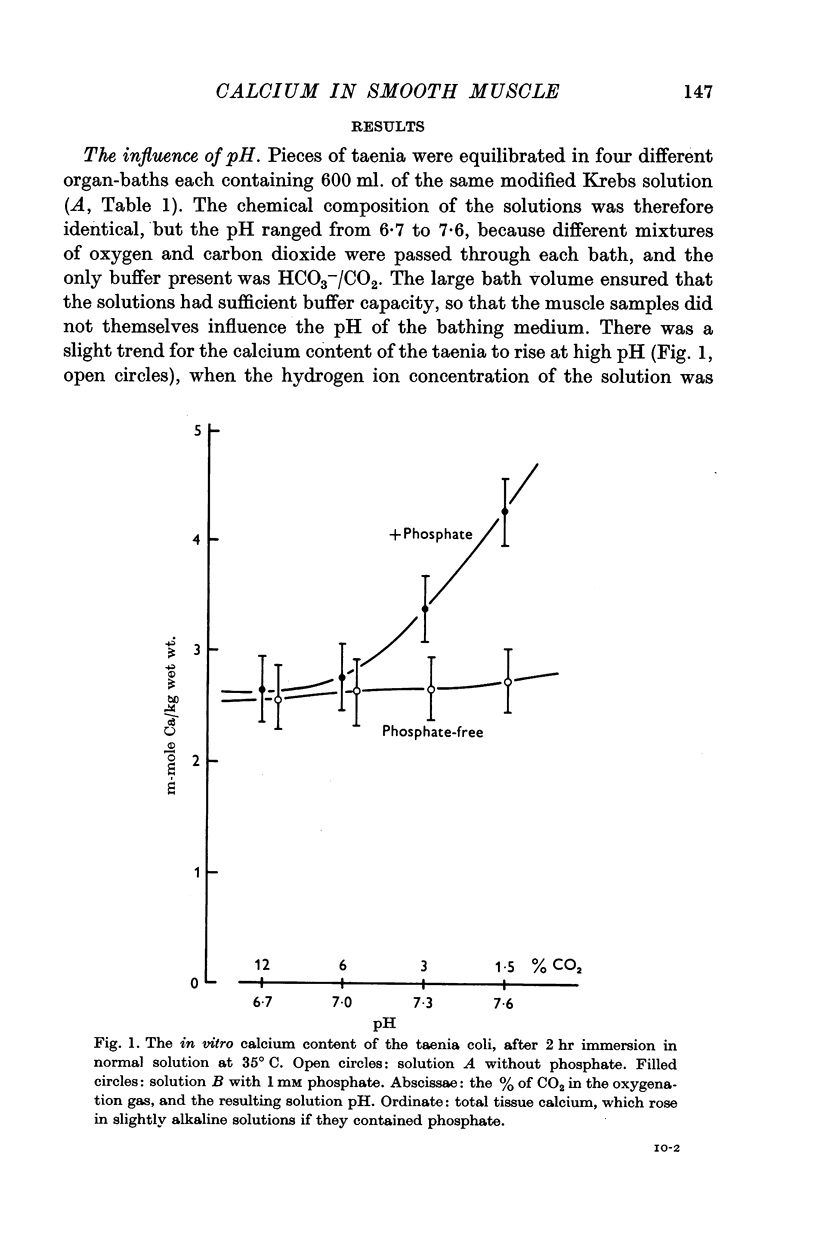
1. The in vitro calcium content of the smooth muscle of the guinea-pig taenia coli was 3·0 m-mole Ca/kg wet wt. when phosphate was omitted from the bathing medium, and was almost independent of pH changes in the range 6·7-7·6.
2. The calcium content was not changed when 1 mM phosphate was included in the medium, if the pH was 6·7 or 7·0. However, when the pH was 7·6, the calcium content increased by 1·5 m-mole Ca/kg wet wt. in the presence of phosphate.
3. The calcium content rose by 1·1 m-mole Ca/kg wet wt. when NaCl in the bathing medium was replaced by isotonic sucrose, and rose by 0·7 m-mole Ca/kg wet wt. when MgCl2 in the bathing medium was replaced. These increases may reflect a competition between Ca2+ and other cations for fixed negative sites in the tissue.
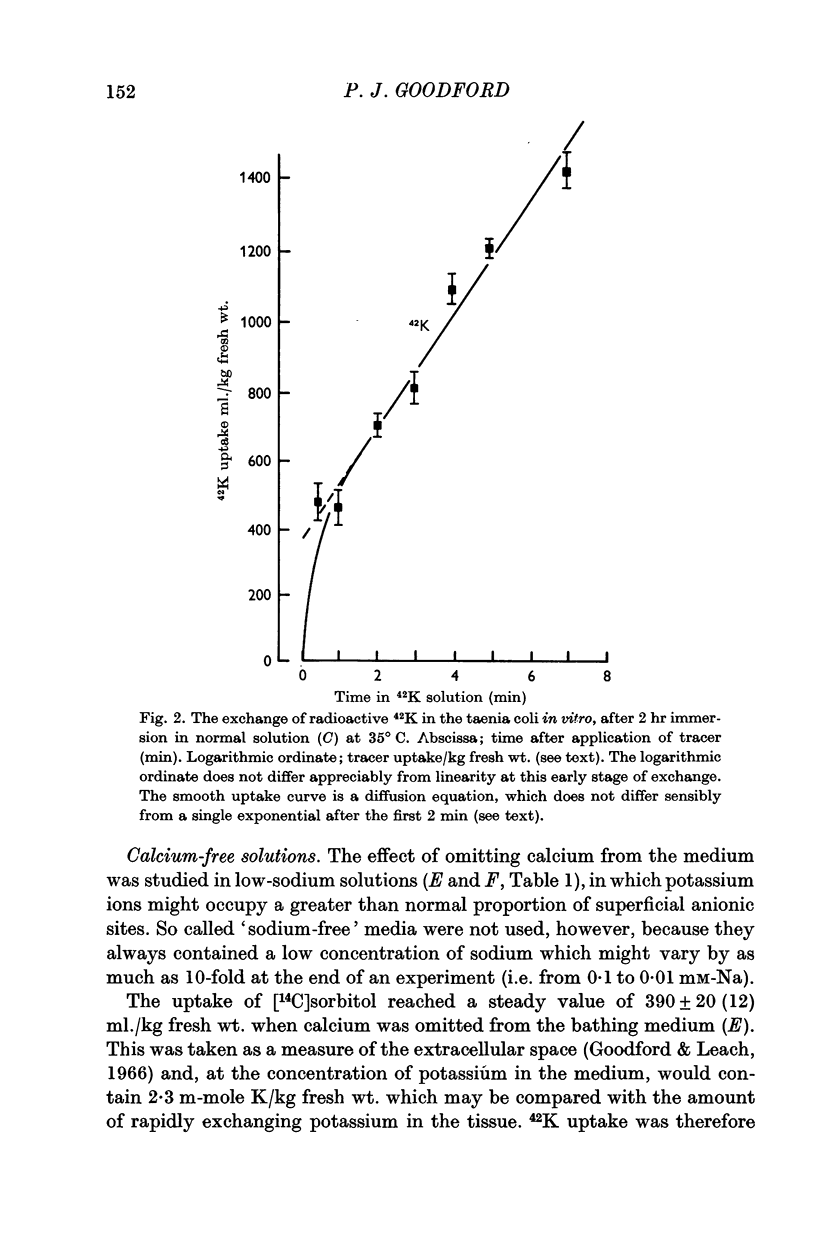
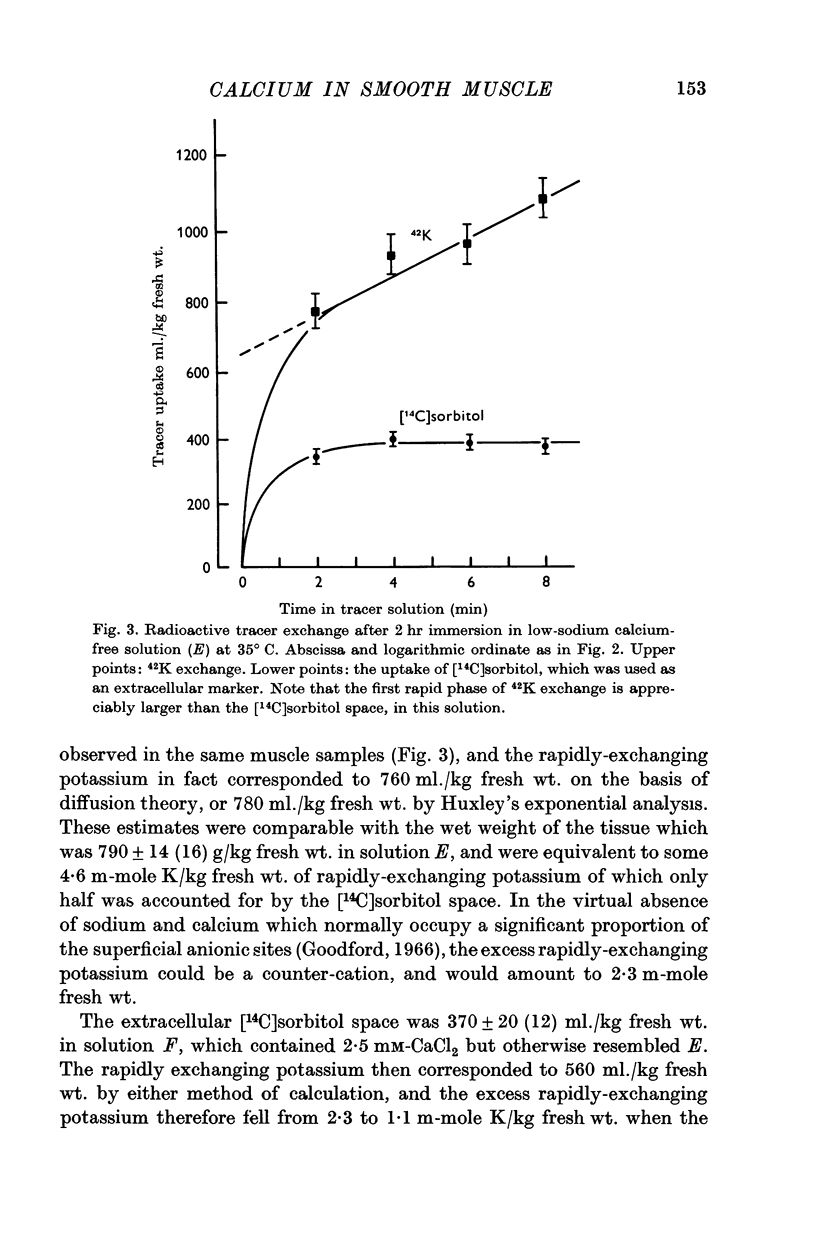
4. The initial rapid phase of 42K exchange corresponded to an `extra-cellular 42K-space' of 470 ml./kg fresh wt. in normal solution, rising to 560 ml./kg. fresh wt. in low-sodium solution and to 760 ml./kg fresh wt. in calcium-free low-sodium solution. In this last medium the extra-cellular [14C]sorbitol space was only 390 ml./kg fresh wt., so that there was a large excess of rapidly-exchanging potassium which may have been competing at fixed negative sites.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER H., GOODFORD P. J., HUETER J. THE CALCIUM CONTENT AND 45-CALCIUM UPTAKE OF THE SMOOTH MUSCLE OF THE GUINEA-PIG TAENIA COLI. J Physiol. 1965 Jan;176:163–179. doi: 10.1113/jphysiol.1965.sp007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOZLER E. Osmotic phenomena in smooth muscle. Am J Physiol. 1962 Jul;203:201–205. doi: 10.1152/ajplegacy.1962.203.1.201. [DOI] [PubMed] [Google Scholar]
- CHUJYO N., HOLLAND W. C. Potassium-induced contracture and calcium exchange in the guinea pig's taenia coli. Am J Physiol. 1963 Jul;205:94–100. doi: 10.1152/ajplegacy.1963.205.1.94. [DOI] [PubMed] [Google Scholar]
- FREEMAN-NARROD M., GOODFORD P. J. Sodium and potassium content of the smooth muscle of the guinea-pig taenia coli at different temperatures and tensions. J Physiol. 1962 Oct;163:399–410. doi: 10.1113/jphysiol.1962.sp006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J., HERMANSEN K. Sodium and potassium movements in the unstriated muscle of the guinea-pig taenia coli. J Physiol. 1961 Oct;158:426–448. doi: 10.1113/jphysiol.1961.sp006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodford P. J. An interaction between potassium and sodium in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Sep;186(1):11–26. doi: 10.1113/jphysiol.1966.sp008017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodford P. J., Leach E. H. The extracellular space of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Sep;186(1):1–10. doi: 10.1113/jphysiol.1966.sp008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- MANERY J. F. Water and electrolyte metabolism. Physiol Rev. 1954 Apr;34(2):334–417. doi: 10.1152/physrev.1954.34.2.334. [DOI] [PubMed] [Google Scholar]
- NEEDHAM D. M., WILLIAMS J. M. THE PROTEINS OF THE DILUTION PRECIPITATE OBTAINED FROM SALT EXTRACTS OF PREGNANT AND NON-PREGNANT UTERUS. Biochem J. 1963 Dec;89:534–545. doi: 10.1042/bj0890534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R., LUTTGAU H. C. Antagonism between calcium and sodium ions. Nature. 1957 May 25;179(4569):1066–1067. doi: 10.1038/1791066a0. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTELIN G. [The effect of calcium and sodium on contraction of M. rectus abdominis]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;273:164–181. [PubMed] [Google Scholar]
- SCHATZMANN H. K. [Calcium uptake and excretion in intestinal muscle of guinea pigs]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1961;274:295–310. [PubMed] [Google Scholar]
- Tomita T. Membrane capacity and resistance of mammalian smooth muscle. J Theor Biol. 1966 Nov;12(2):216–227. doi: 10.1016/0022-5193(66)90114-7. [DOI] [PubMed] [Google Scholar]
- WEATHERALL M. Quantitative analysis of movements of potassium in rabbit auricles. Proc R Soc Lond B Biol Sci. 1962 May 15;156:57–82. doi: 10.1098/rspb.1962.0028. [DOI] [PubMed] [Google Scholar]
- WEBER A., HERZ R. The binding of calcium to actomyosin systems in relation to their biological activity. J Biol Chem. 1963 Feb;238:599–605. [PubMed] [Google Scholar]


