Abstract
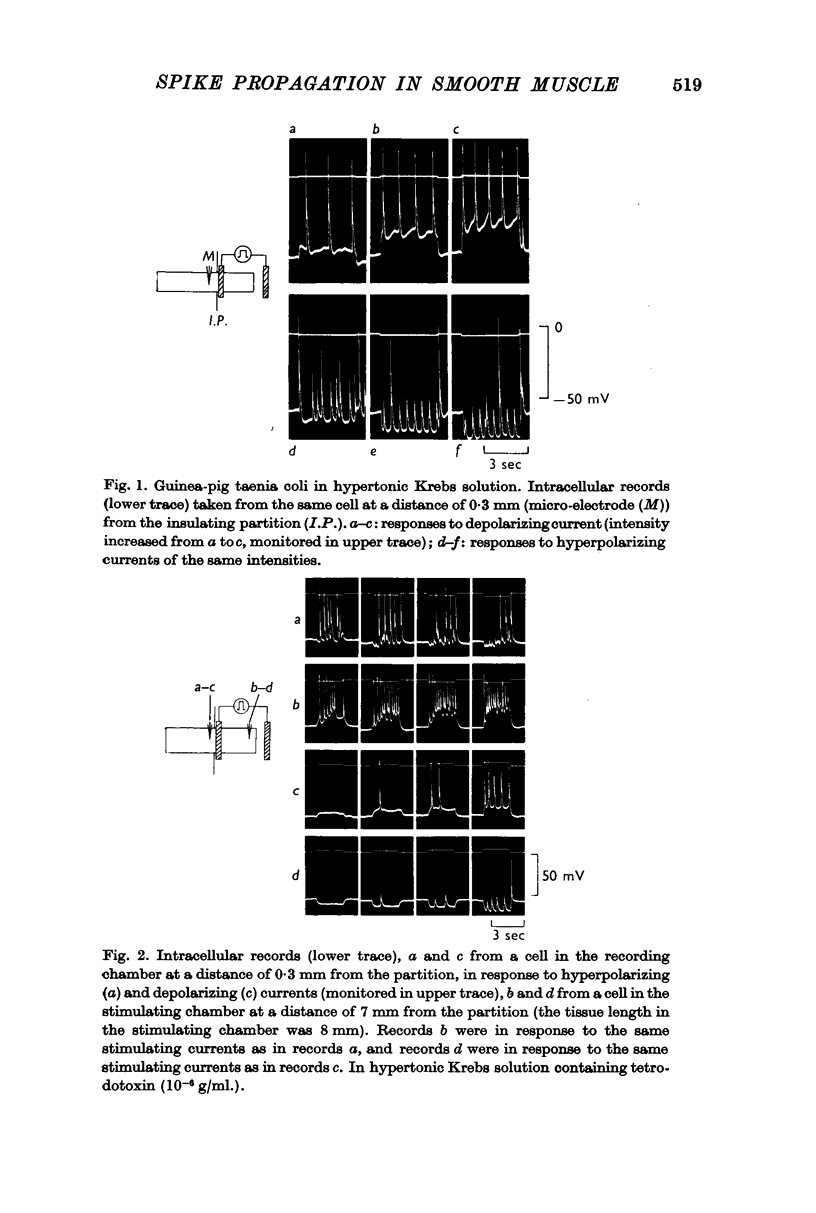
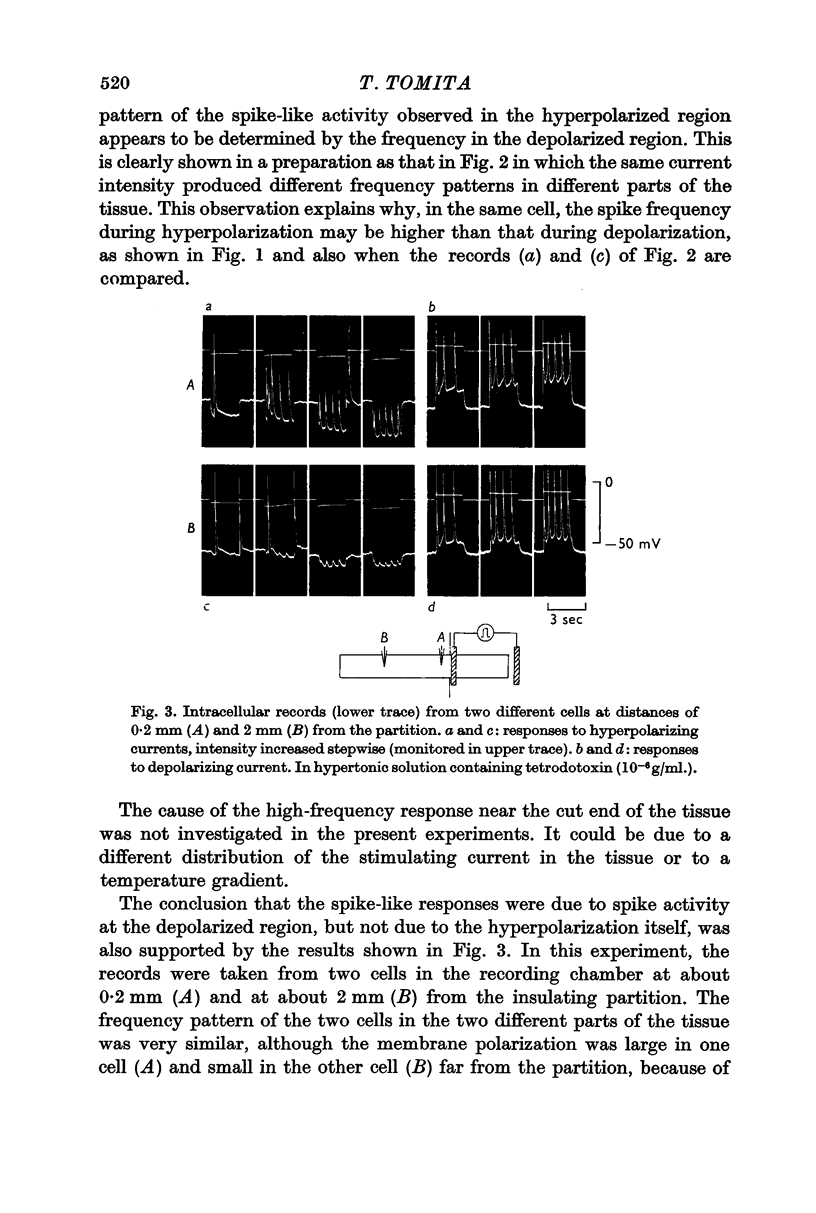
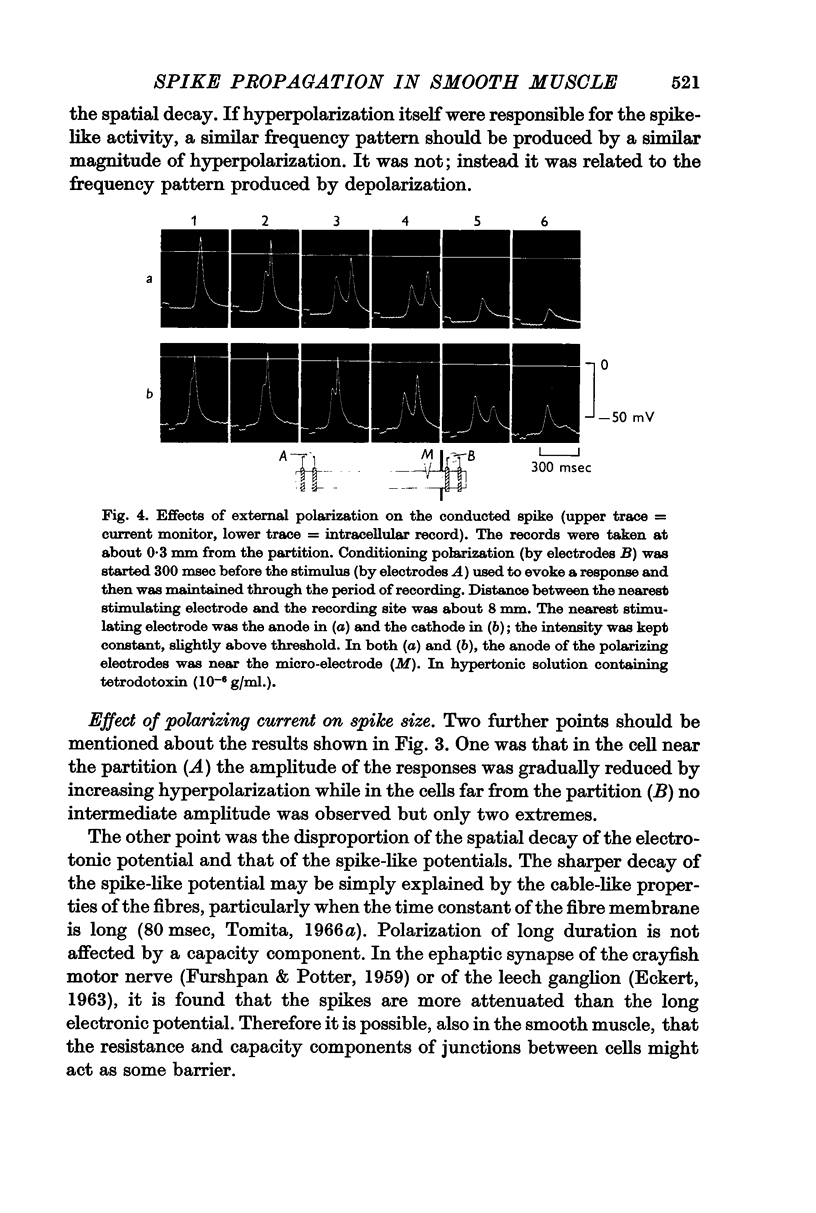
1. Spike activity was produced by external field stimulation of the guinea-pig taenia coli. Spikes were evoked by depolarization of the muscle membrane. Though the activity was usually also observed during hyperpolarization, this was shown to be conducted activity from the depolarized region of the tissue. Spike conduction was blocked when hyperpolarization exceeded 10 mV.
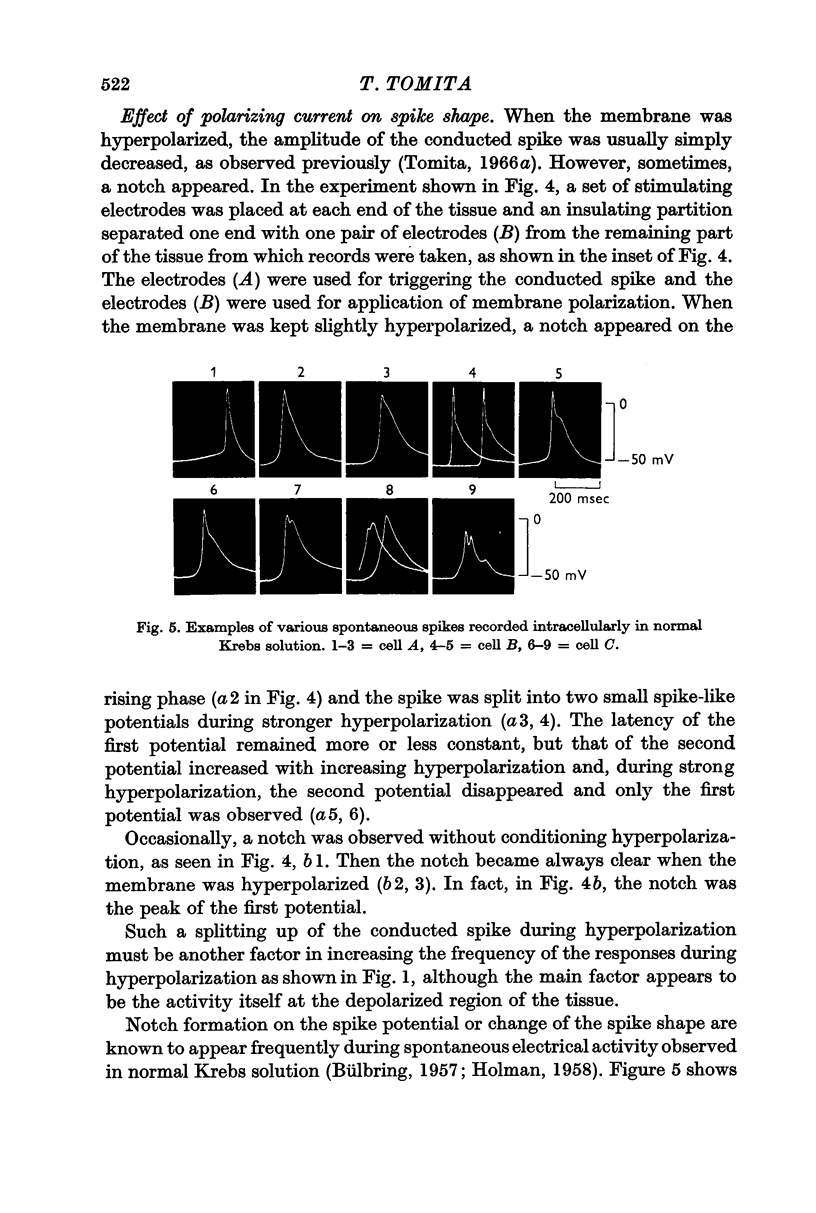
2. The shape of the conducted spike was influenced by membrane polarization. Sometimes a notch appeared on the spike and sometimes the spike was split into two by hyperpolarization. This is probably due to the fact that functional bundles form a network and that the branches between bundles are more susceptible to the membrane polarization.
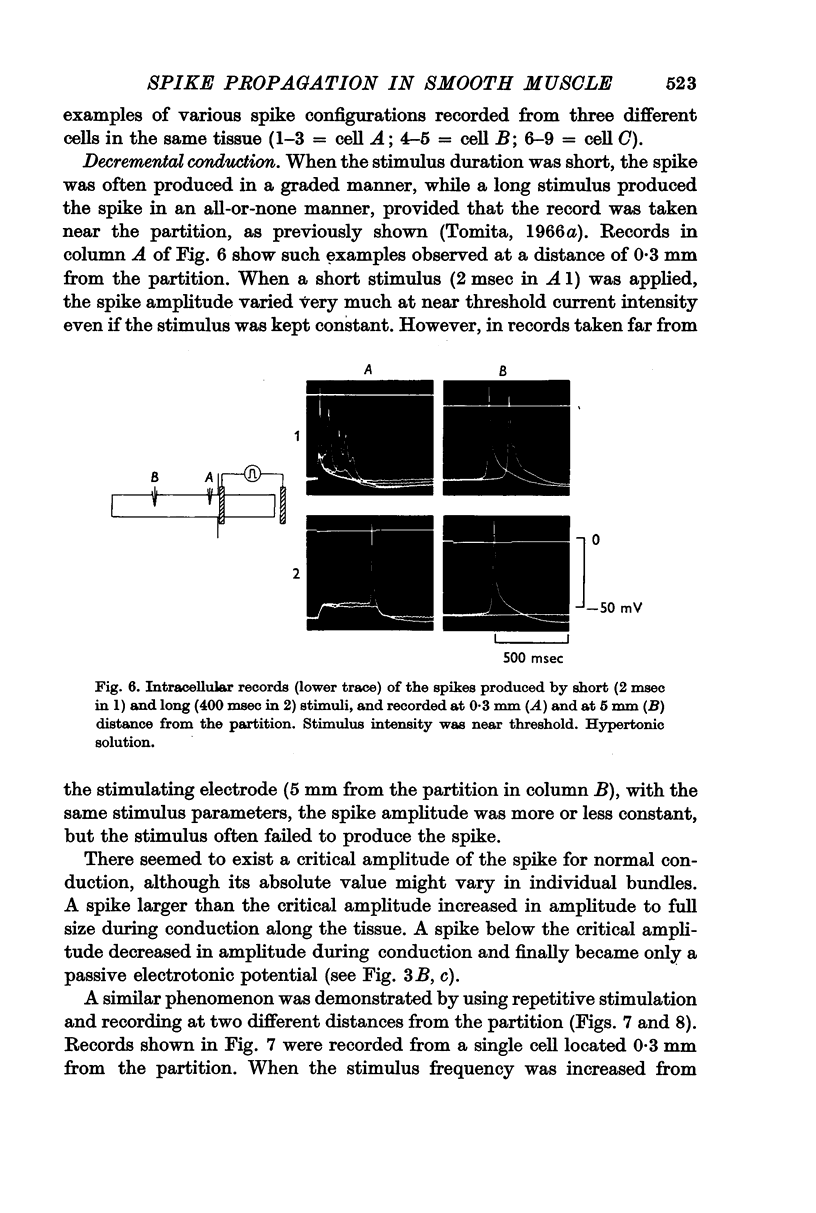
3. There was a critical spike amplitude for normal propagation. Therefore, different spike amplitudes were observed near the stimulating electrode, but only spikes of nearly full size amplitude were recorded far from the stimulating electrode, i.e. at a distance of more than 5 mm.
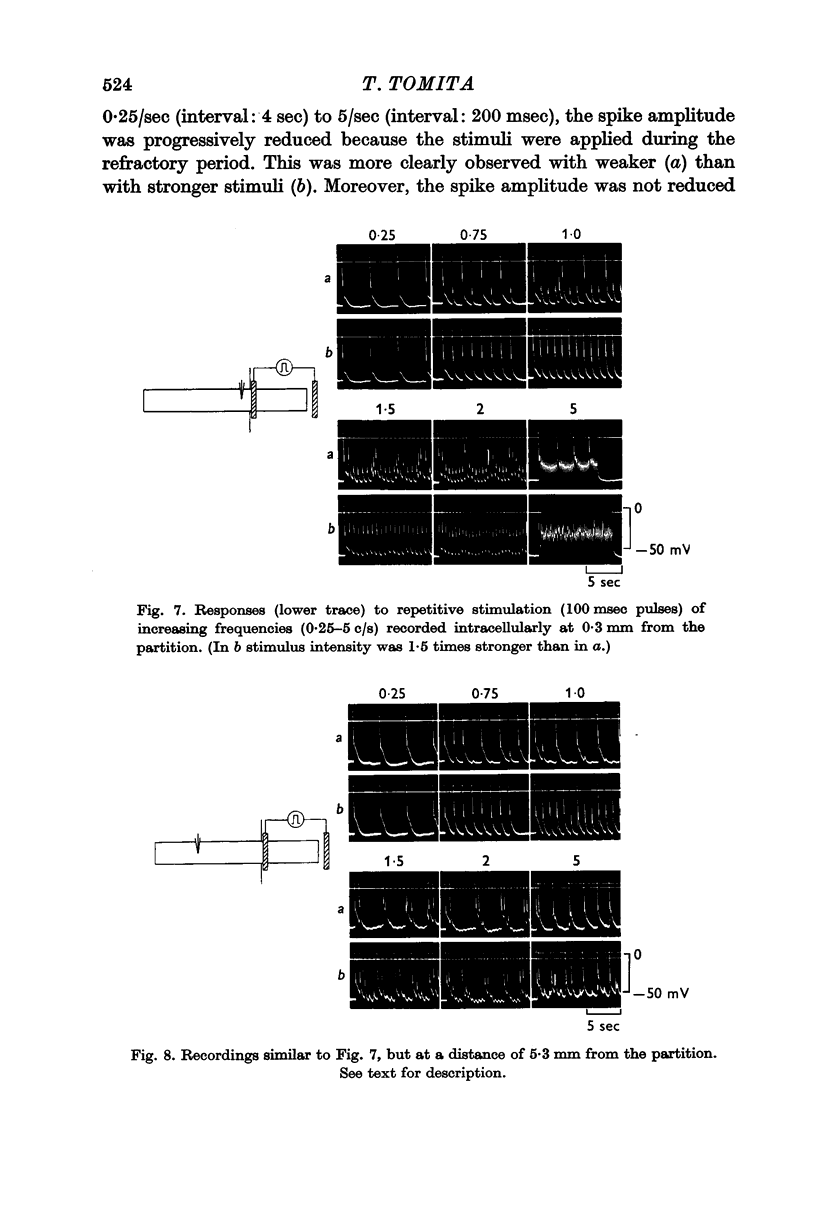
4. When repetitive stimulation was applied, the spike amplitude decreased with increasing frequency of stimulation. No steady level was reached, however, but the spike amplitude fluctuated at about 0·3 c/s.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBRING E., BURNSTOCK G., HOLMAN M. E. Excitation and conduction in the smooth muscle of the isolated taenia coli of the guinea-pig. J Physiol. 1958 Aug 6;142(3):420–437. doi: 10.1113/jphysiol.1958.sp006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Changes in configuration of spontaneously discharged spike potentials from smooth muscle of the guinea-pig's taenia coli; the effect of electrotonic currents and of adrenaline, acetylcholine and histamine. J Physiol. 1957 Feb 15;135(2):412–425. doi: 10.1113/jphysiol.1957.sp005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Membrane potentials of smooth muscle fibres of the taenia coli of the guinea-pig. J Physiol. 1954 Aug 27;125(2):302–315. doi: 10.1113/jphysiol.1954.sp005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. Membrane potentials recorded with high-resistance micro-electrodes; and the effects of changes in ionic environment on the electrical and mechanical activity of the smooth muscle of the taenia coli of the guineapig. J Physiol. 1958 May 28;141(3):464–488. doi: 10.1113/jphysiol.1958.sp005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMAN M. E. The effect of changes in sodium chloride concentration on the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1957 May 23;136(3):569–584. doi: 10.1113/jphysiol.1957.sp005782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSHIKO T., SPERELAKIS N. Prepotentials and unidirectional propagation in myocardium. Am J Physiol. 1961 Nov;201:873–880. doi: 10.1152/ajplegacy.1961.201.5.873. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H. The influence of potassium, sodium and chloride on the membrane potential of the smooth muscle of taenia coli. J Physiol. 1963 Apr;166:15–28. doi: 10.1113/jphysiol.1963.sp007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. Applications of Hodgkin-Huxley equations to excitable tissues. Physiol Rev. 1966 Jan;46(1):1–50. doi: 10.1152/physrev.1966.46.1.1. [DOI] [PubMed] [Google Scholar]
- Tomita T. Current spread in the smooth muscle of the guinea-pig vas deferens. J Physiol. 1967 Mar;189(1):163–176. doi: 10.1113/jphysiol.1967.sp008161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Membrane capacity and resistance of mammalian smooth muscle. J Theor Biol. 1966 Nov;12(2):216–227. doi: 10.1016/0022-5193(66)90114-7. [DOI] [PubMed] [Google Scholar]
- Weidmann S. The diffusion of radiopotassium across intercalated disks of mammalian cardiac muscle. J Physiol. 1966 Nov;187(2):323–342. doi: 10.1113/jphysiol.1966.sp008092. [DOI] [PMC free article] [PubMed] [Google Scholar]


