Abstract
1. A study has been made of the effect of Na replacement in the incubation media on the hexose-dependent potential and the capacity of sacs of rat intestine to transfer fluid and galactose.
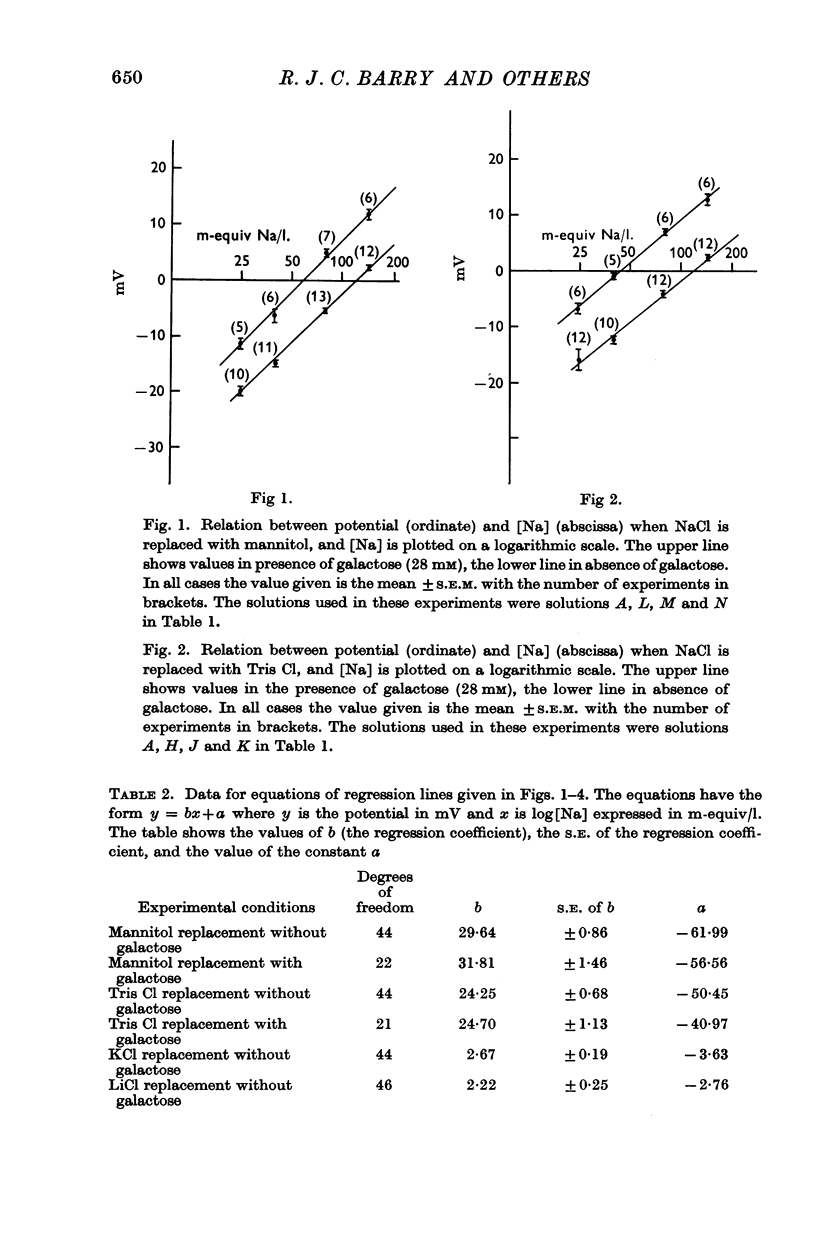
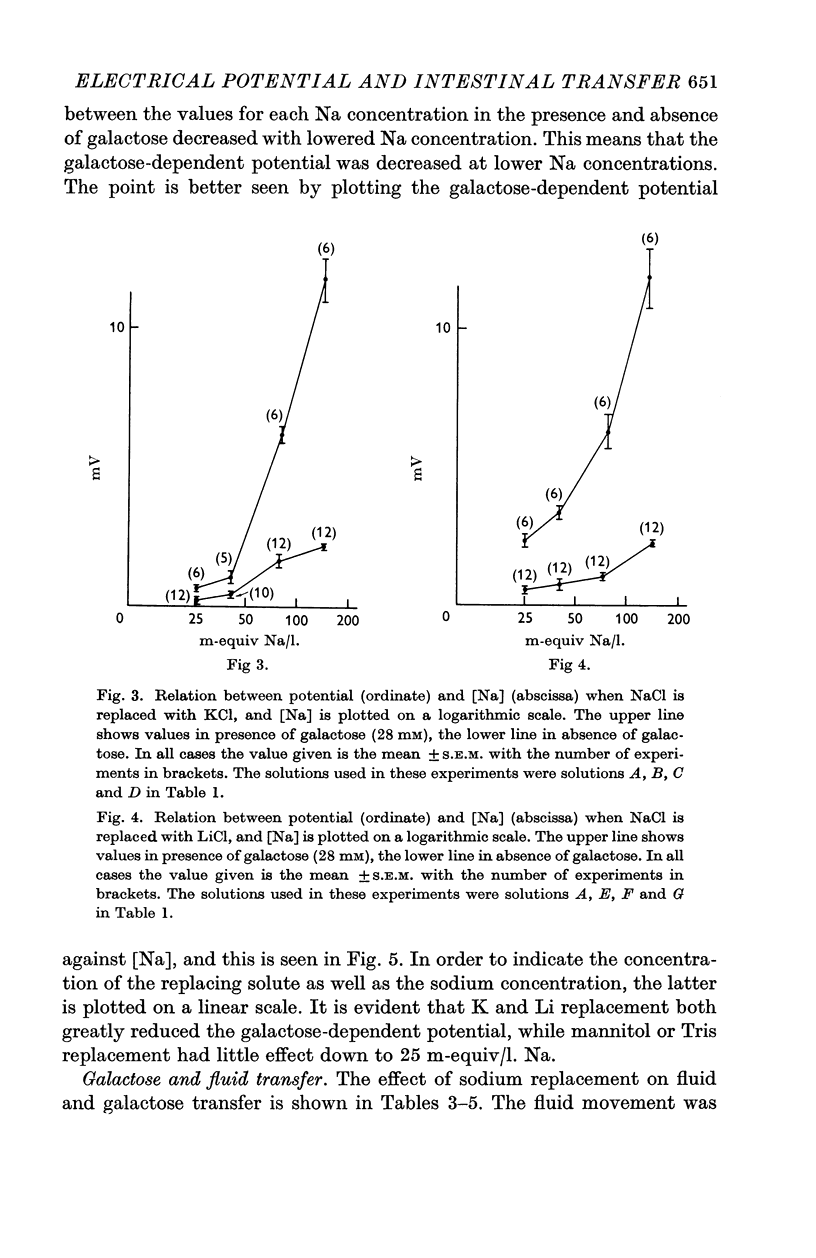
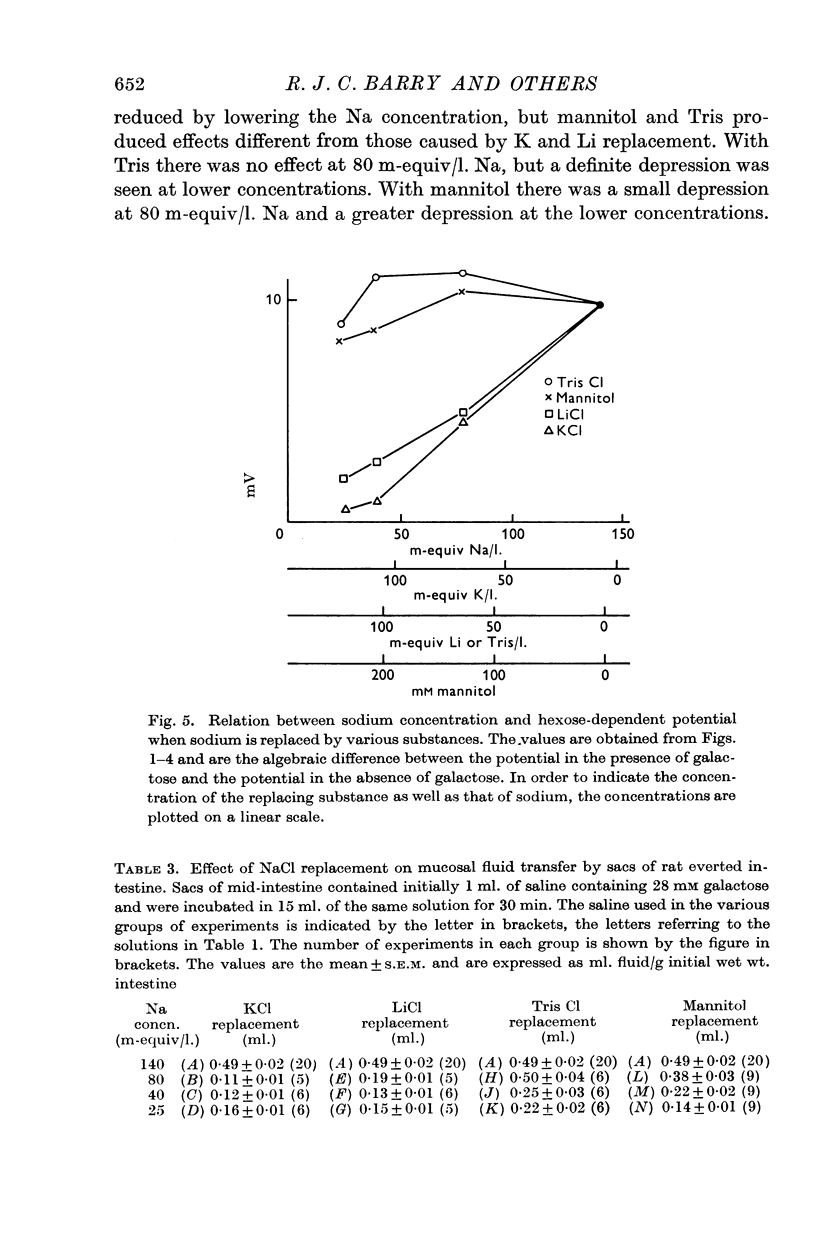
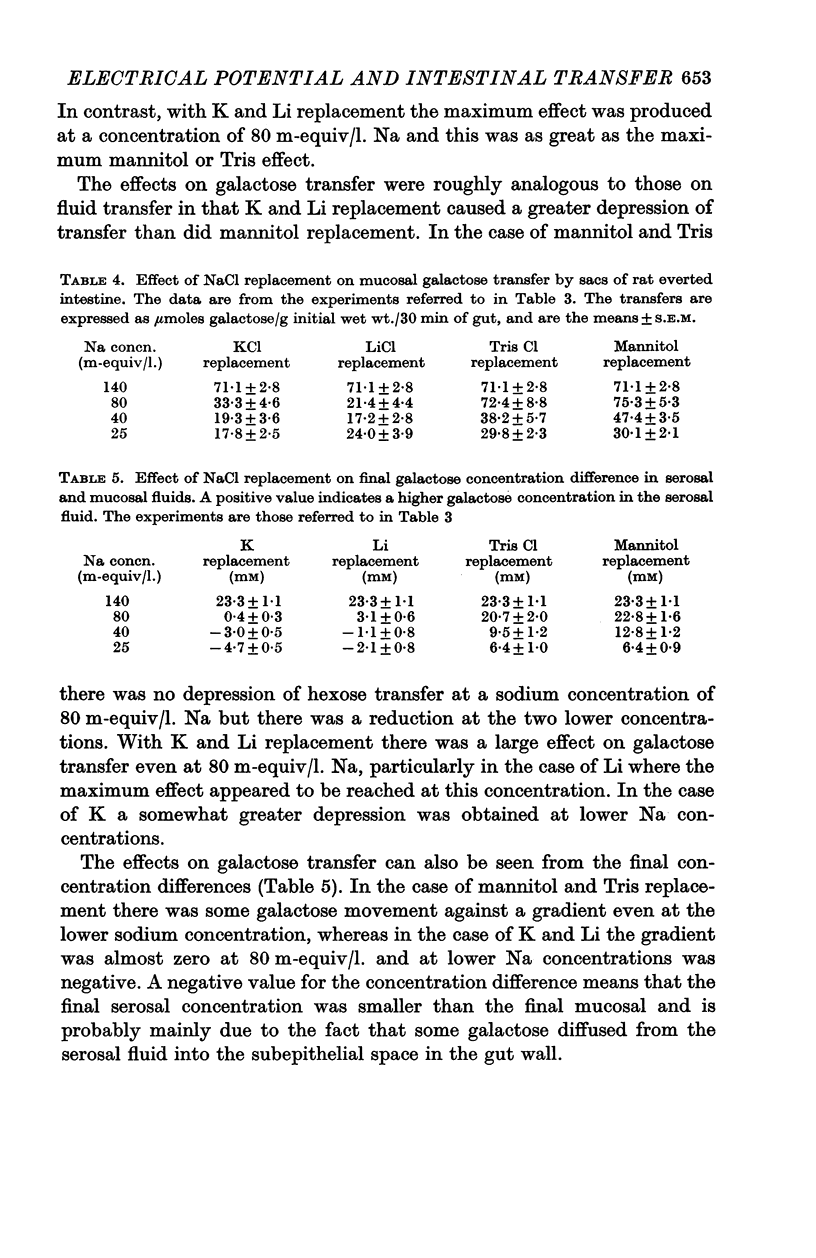
2. Replacement of NaCl with mannitol or Tris Cl had little effect on the hexose-dependent potential, while replacement with LiCl and KCl had an inhibitory effect on the potential.
3. Replacement with KCl, LiCl, mannitol and Tris Cl all reduced the capacity to transfer fluid and galactose, but the effects of Li and K replacement were greater than mannitol or Tris replacement.
4. There is not a fixed relation between the magnitude of the potential and the amount of galactose transferred.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY B. A., MATTHEWS J., SMYTH D. H. Transfer of glucose and fluid by different parts of the small intestine of the rat. J Physiol. 1961 Jul;157:279–288. doi: 10.1113/jphysiol.1961.sp006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY R. J., DIKSTEIN S., MATTHEWS J., SMYTH D. H., WRIGHT E. M. ELECTRICAL POTENTIALS ASSOCIATED WITH INTESTINAL SUGAR TRANSFER. J Physiol. 1964 Jun;171:316–338. doi: 10.1113/jphysiol.1964.sp007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. J., Smyth D. H., Wright E. M. Short-circuit current and solute transfer by rat jejunum. J Physiol. 1965 Nov;181(2):410–431. doi: 10.1113/jphysiol.1965.sp007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosacková J., Crane R. K. Studies on the mechanism of intestinal absorption of sugars. IX. Intracellular sodium concentrations and active sugar transport by hamster small intestine in vitro. Biochim Biophys Acta. 1965 Jul 22;102(2):436–441. doi: 10.1016/0926-6585(65)90133-0. [DOI] [PubMed] [Google Scholar]
- CLARKSON T. W., ROTHSTEIN A. Transport of monovalent cations by the isolated small intestine of the rat. Am J Physiol. 1960 Nov;199:898–906. doi: 10.1152/ajplegacy.1960.199.5.898. [DOI] [PubMed] [Google Scholar]
- Lyon I., Crane R. K. Studies on transmural potentials in vitro in relation to intestinal absorption. I. Apparent Michaelis constants for Na+dependent sugar transport. Biochim Biophys Acta. 1966 Feb 7;112(2):278–291. doi: 10.1016/0926-6585(66)90327-x. [DOI] [PubMed] [Google Scholar]
- PARSONS D. S., WINGATE D. L. The effect of osmotic gradients on fluid transfer across rat intestine in vitro. Biochim Biophys Acta. 1961 Jan 1;46:170–183. doi: 10.1016/0006-3002(61)90660-6. [DOI] [PubMed] [Google Scholar]
- RIKLIS E., QUASTEL J. H. Effects of cations on sugar absorption by isolated surviving guinea pig intestine. Can J Biochem Physiol. 1958 Mar;36(3):347–362. [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F., Wright E. M. Interpretation of hexose-dependent electrical potential differences in small intestine. Nature. 1967 Apr 29;214(5087):509–510. doi: 10.1038/214509a0. [DOI] [PubMed] [Google Scholar]
- Wright E. M. Diffusion potentials across the small intestine. Nature. 1966 Oct 8;212(5058):189–190. doi: 10.1038/212189a0. [DOI] [PubMed] [Google Scholar]


