Abstract
1. Longitudinal vibration was applied to the de-efferented soleus muscle of anaesthetized cats while recording the discharge of single afferent fibres from the proprioceptors within the muscle. Conditions were defined under which vibration can be used to excite selectively the primary endings of muscle spindles without exciting the secondary endings of muscle spindles or Golgi tendon organs.
2. Frequencies of vibration of 100-500 c/s were used. The maximum amplitude of vibration which the vibrator could produce fell with increasing frequency; it was 250 μ (peak to peak) for 100 c/s and 20 μ for 500 c/s.
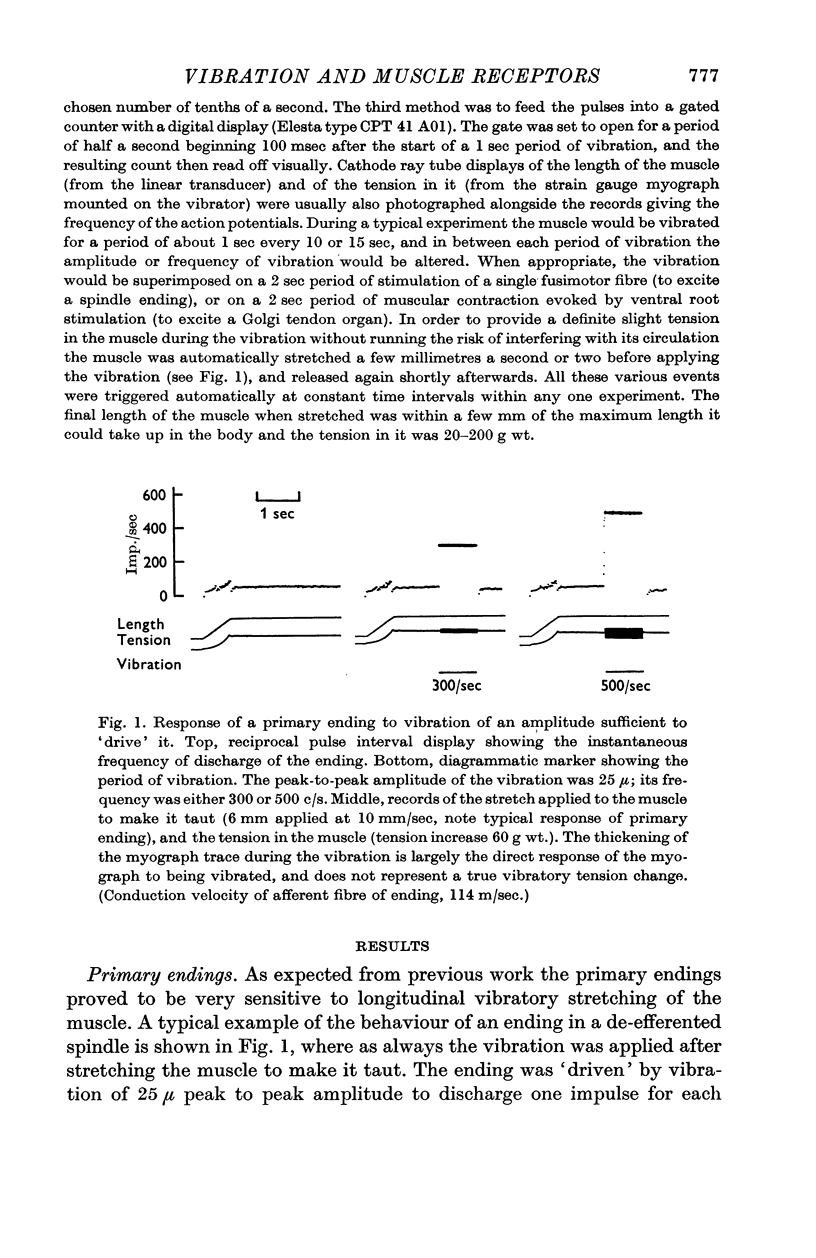
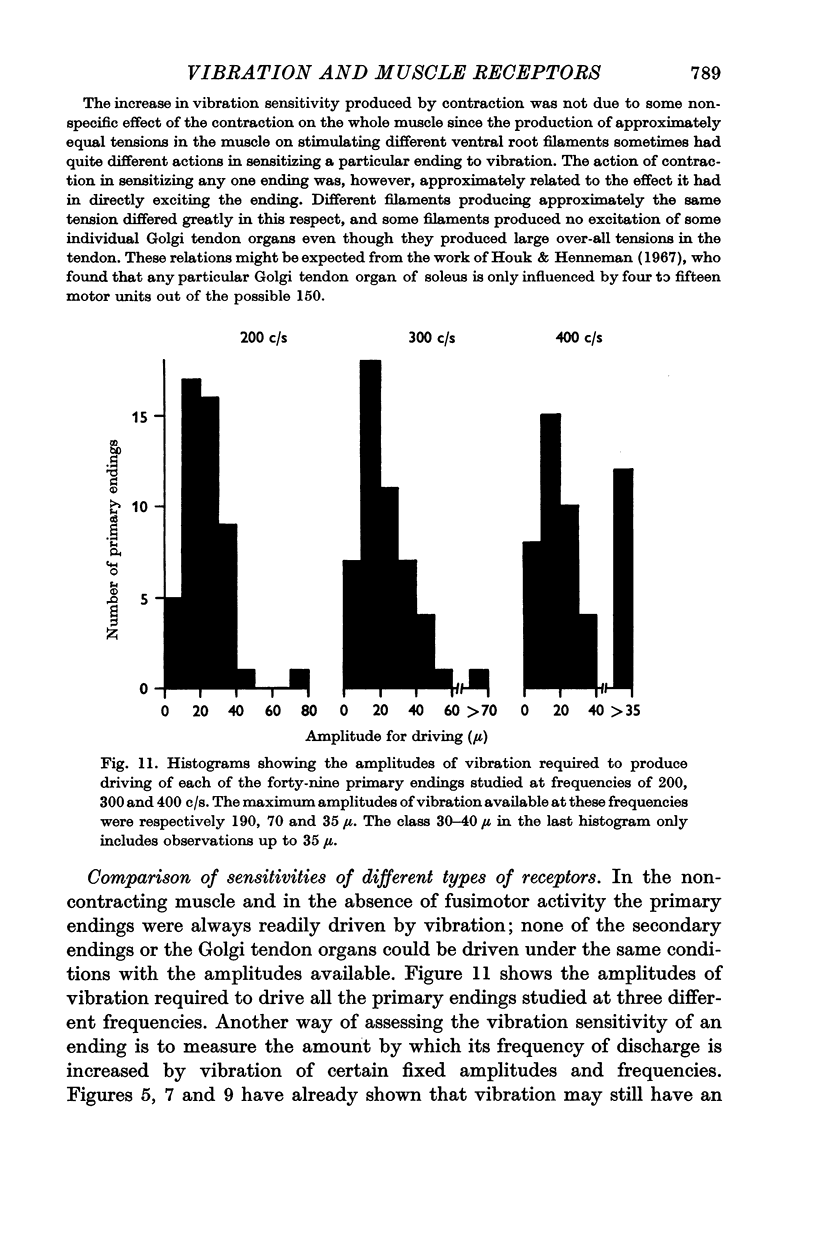
3. Primary endings of muscle spindles were very sensitive to vibration. Most could be `driven' to discharge one impulse for each cycle of vibration over the whole of the above range of frequencies, provided the initial tension was moderate (20-200 g wt.). The amplitude of vibration required to produce driving usually varied by less than a factor of two over the whole range of frequencies. The most sensitive endings could be driven by vibrations of below 10 μ amplitude.
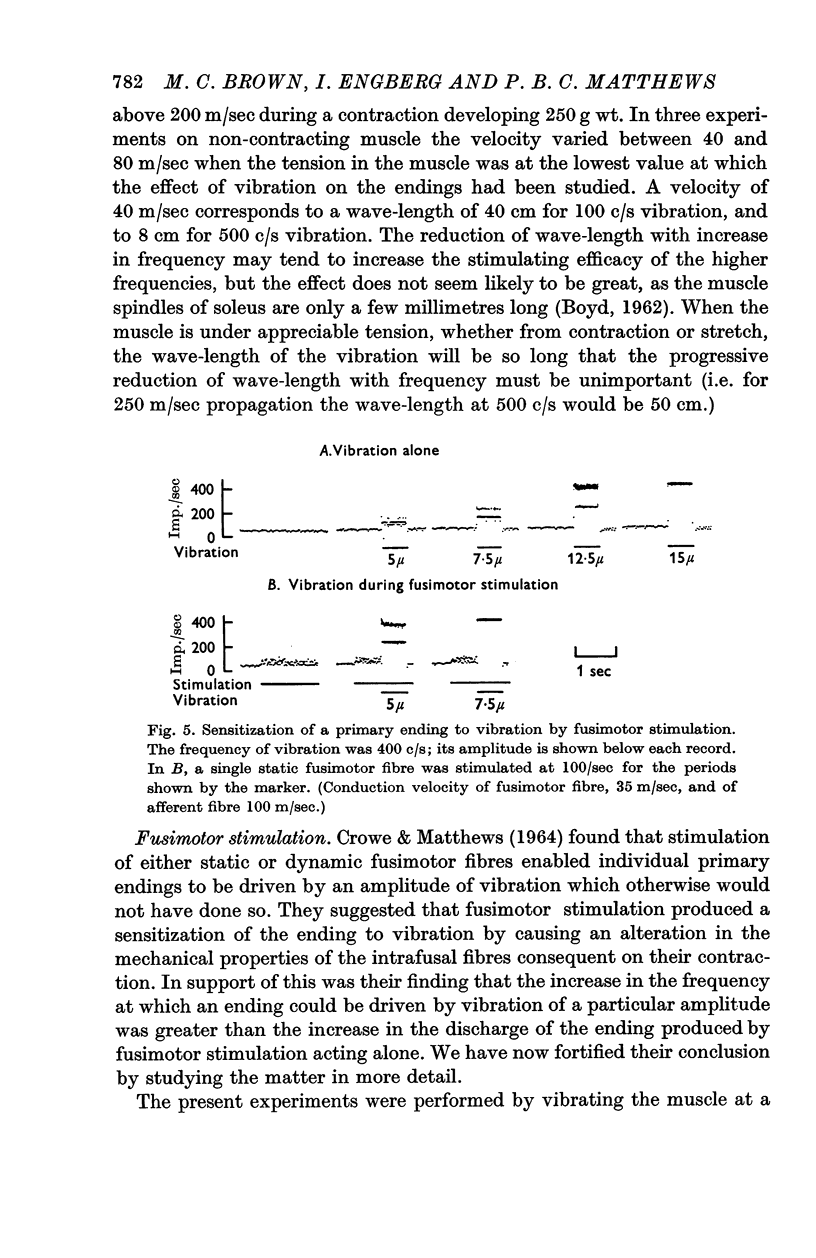
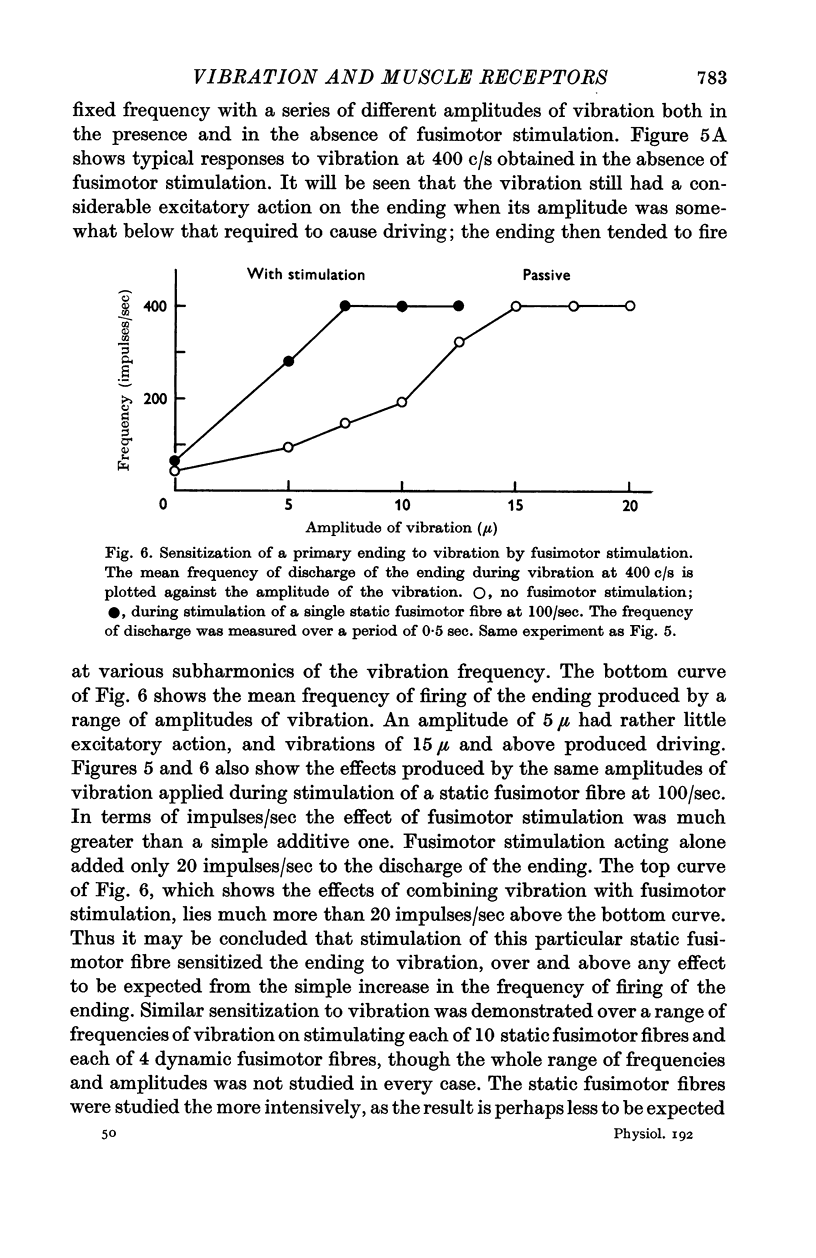
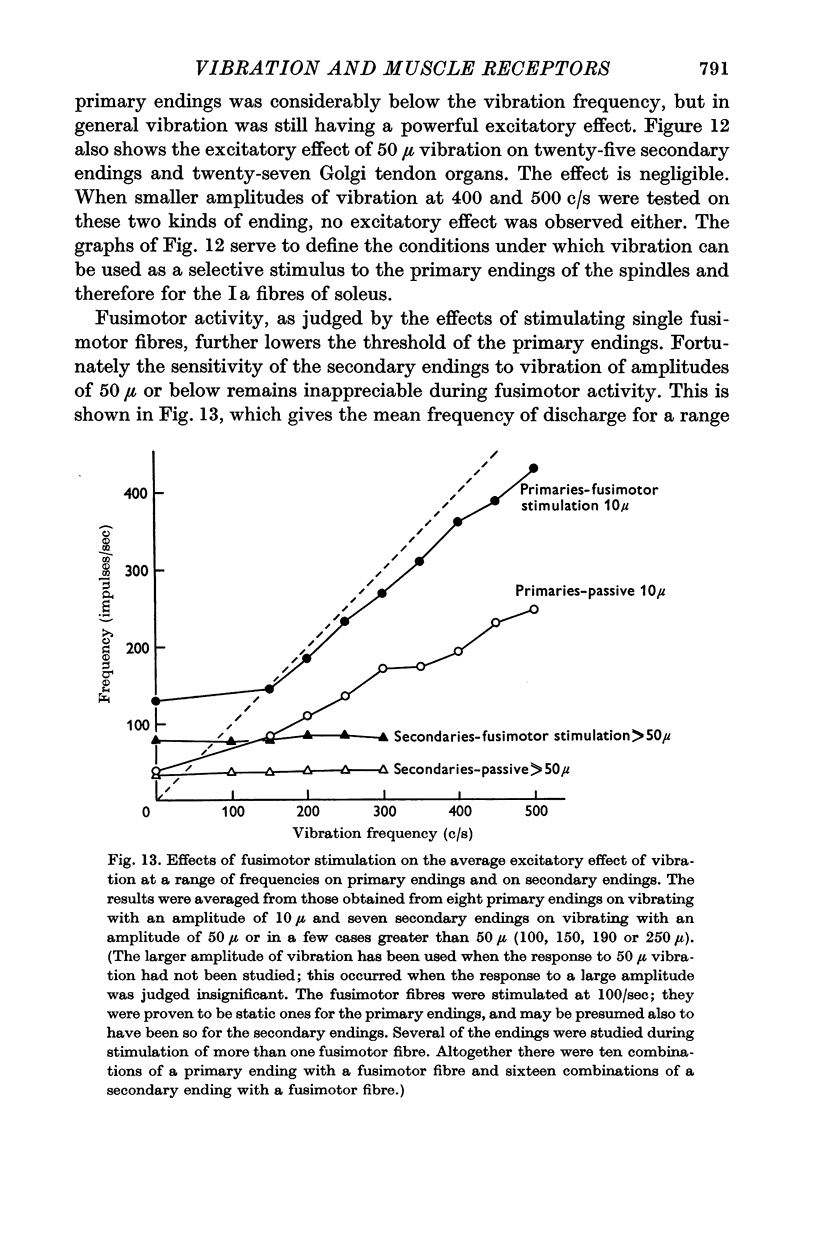
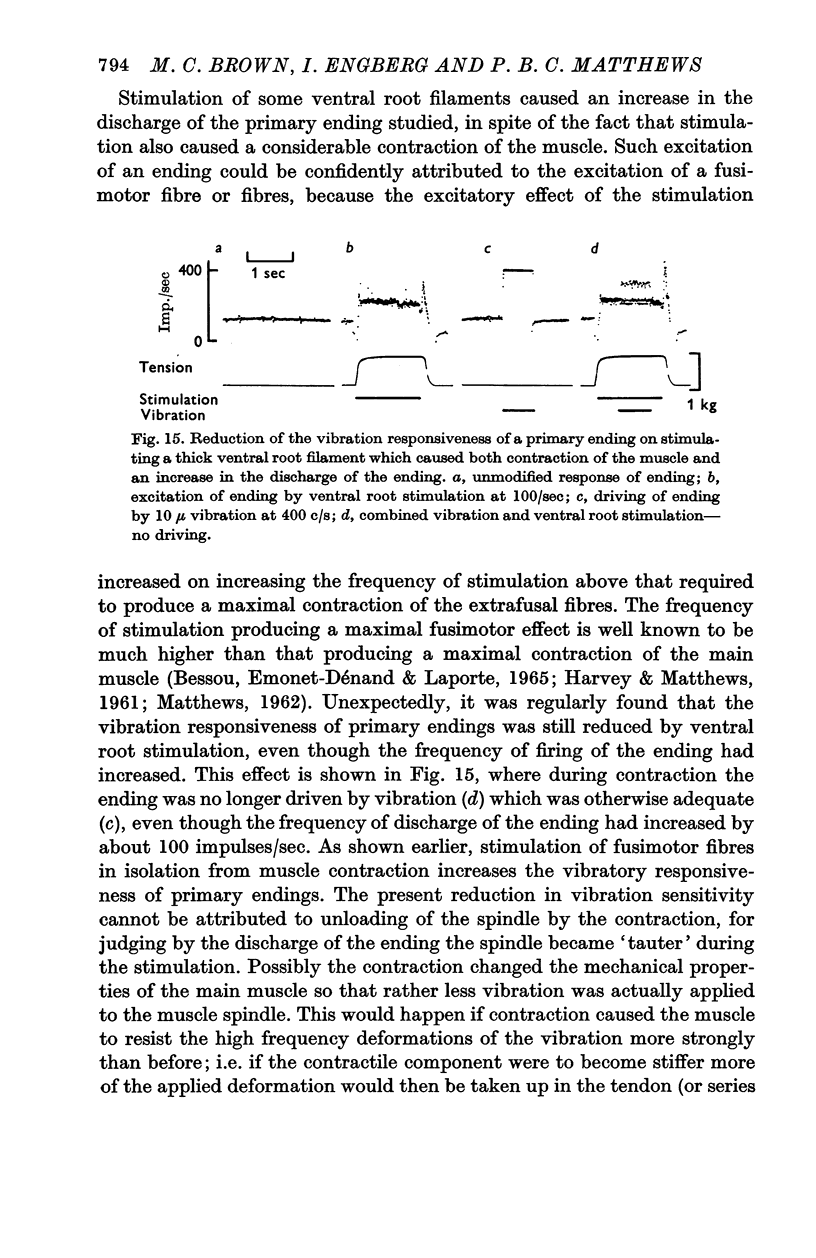
4. Stimulation of single fusimotor fibres, whether static or dynamic fusimotor fibres, increased the sensitivity of primary endings to vibration. Contraction of the main muscle, produced by stimulating α motor fibres, reduced the sensitivity of primary endings even when fusimotor fibres were also being stimulated.
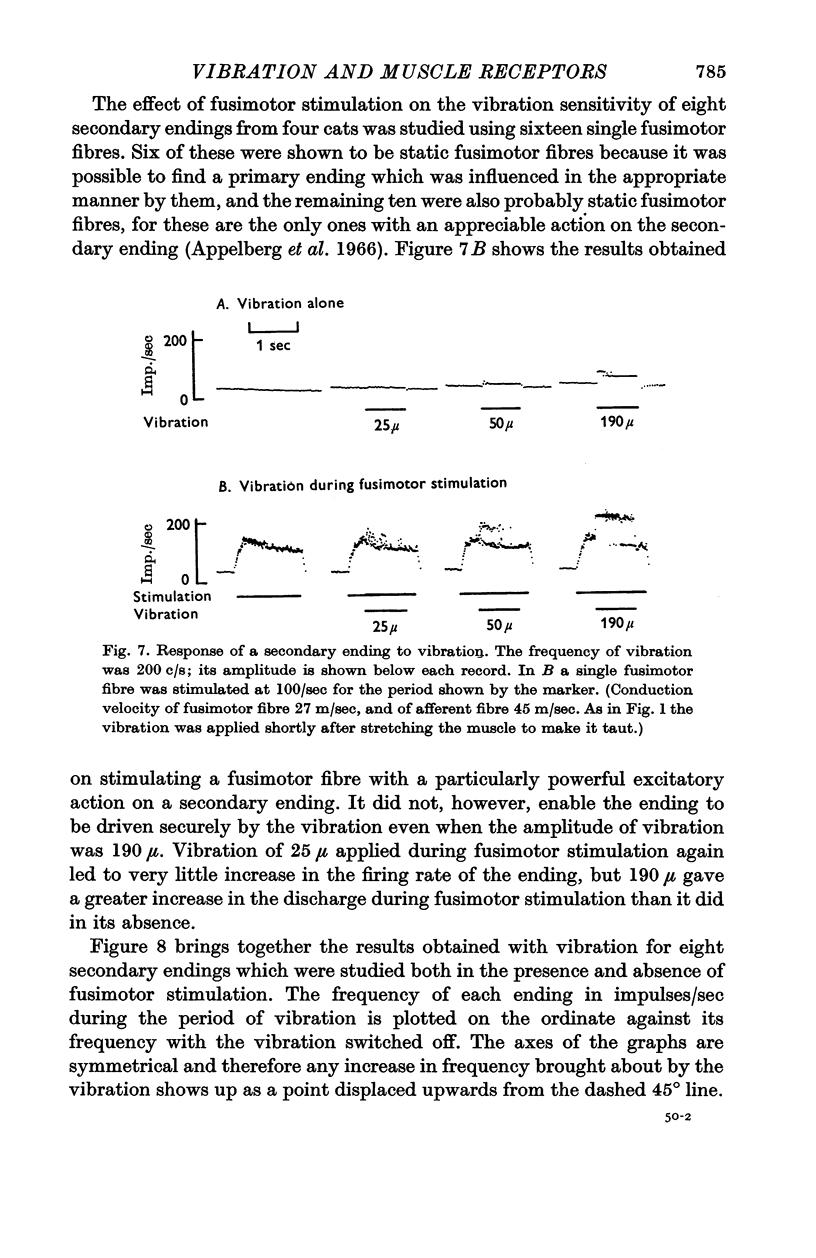
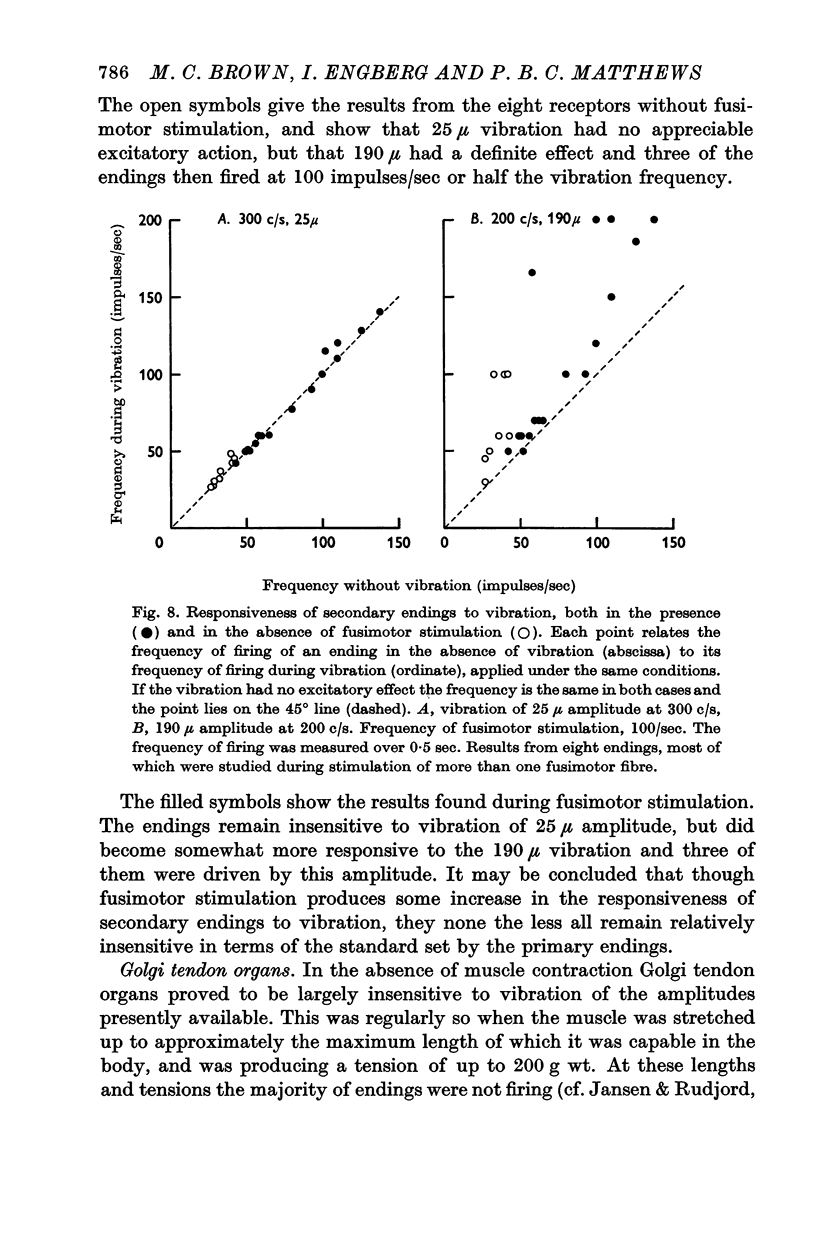
5. The secondary endings were very insensitive to longitudinal vibration and with the amplitudes available not one of twenty-five endings could be driven at 150 c/s or above; one ending could be driven at 100 c/s by vibration of 250 μ amplitude. Stimulation of single fusimotor fibres, probably all of which were static fusimotor fibres, made them slightly more sensitive to vibration but none of them approached the sensitivity of the primary endings.
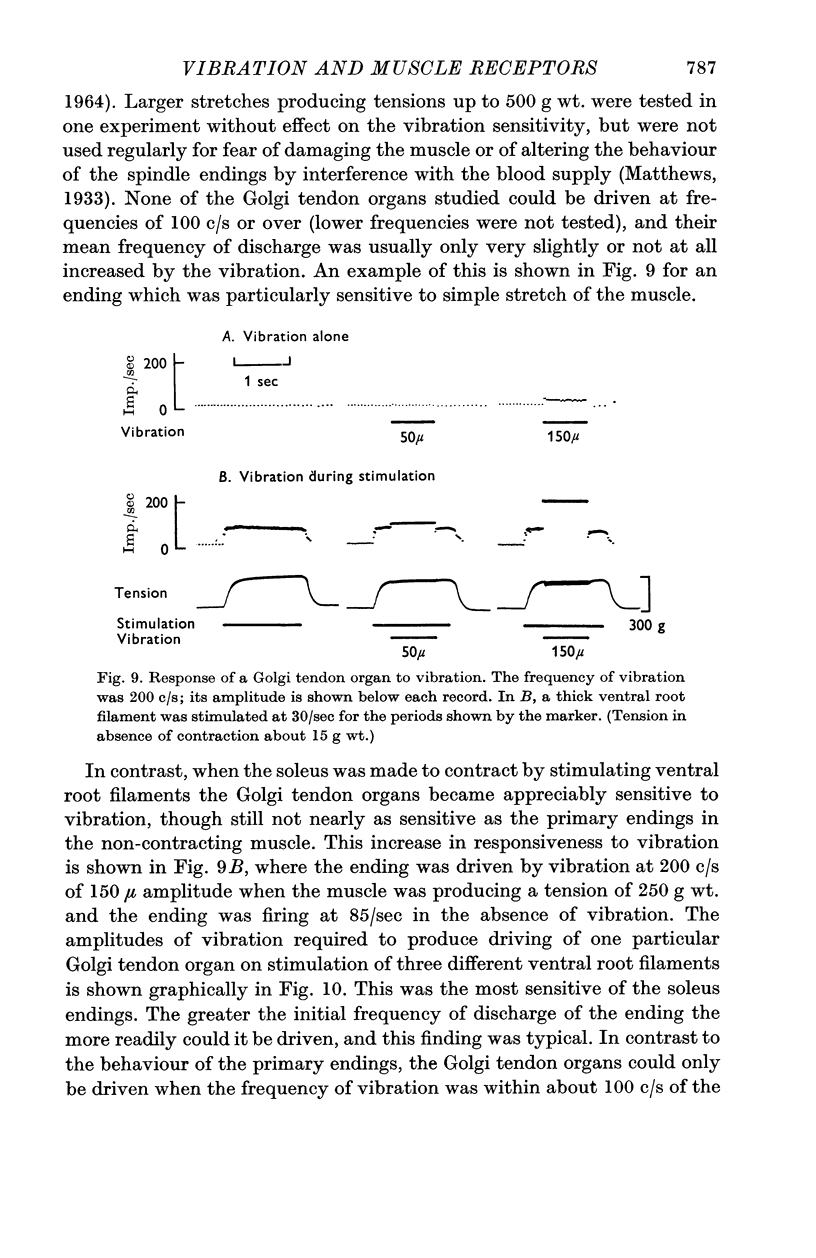
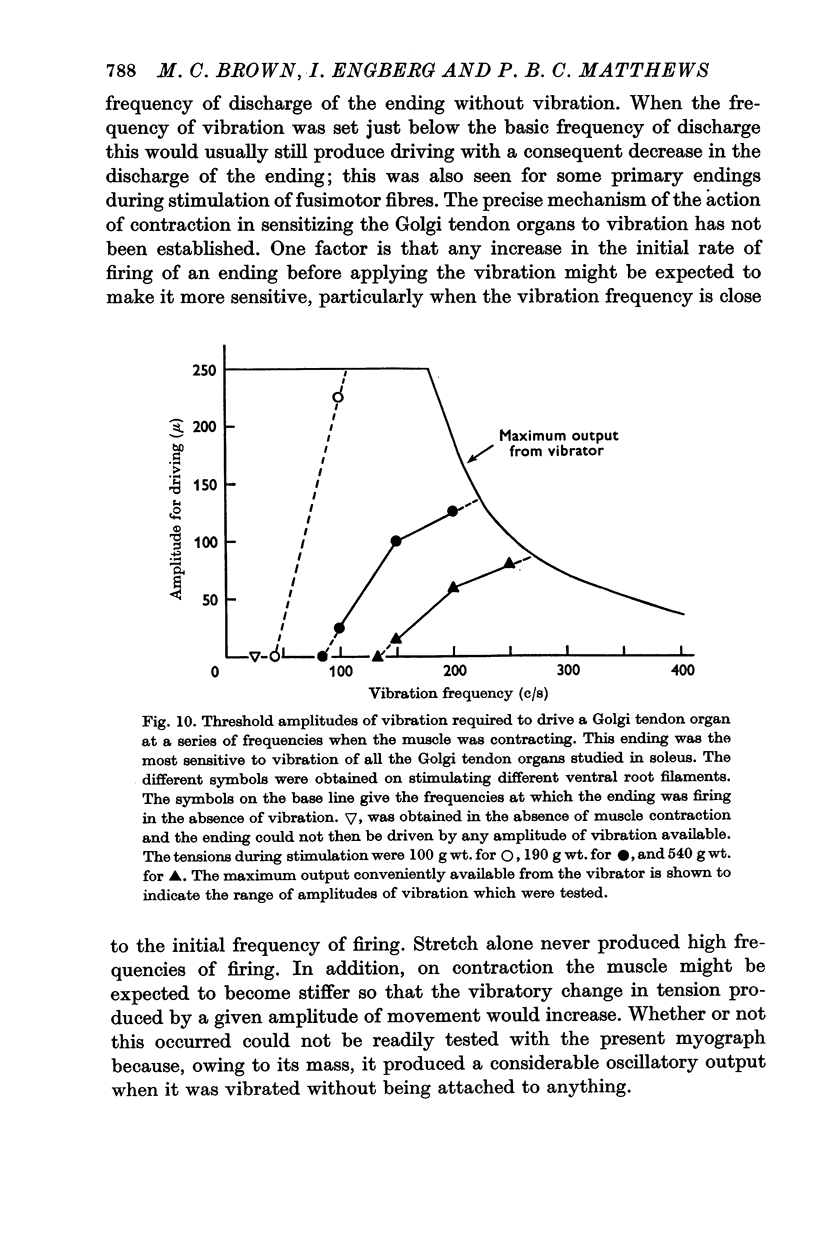
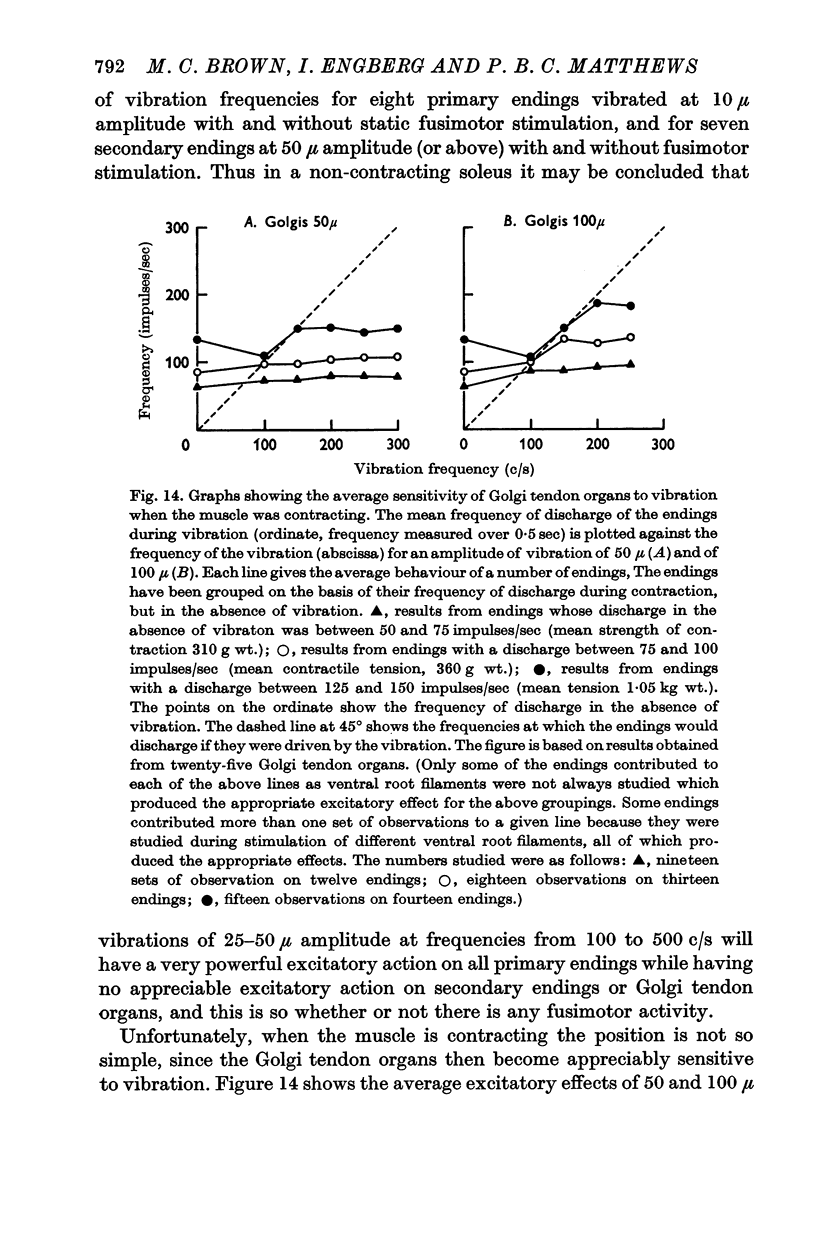
6. The Golgi tendon organs were as insensitive as the secondary endings when the muscle was not contracting and none could be driven at any frequency in spite of quite high tensions in the muscle. However, when the muscle was made to contract by stimulating α fibres in ventral root filaments the tendon organs became appreciably more sensitive, the degree of sensitization increasing approximately with the strength of the contraction. They never became as sensitive as the primary endings, and with the amplitudes of vibration available none was driven at frequencies of over 250 c/s.
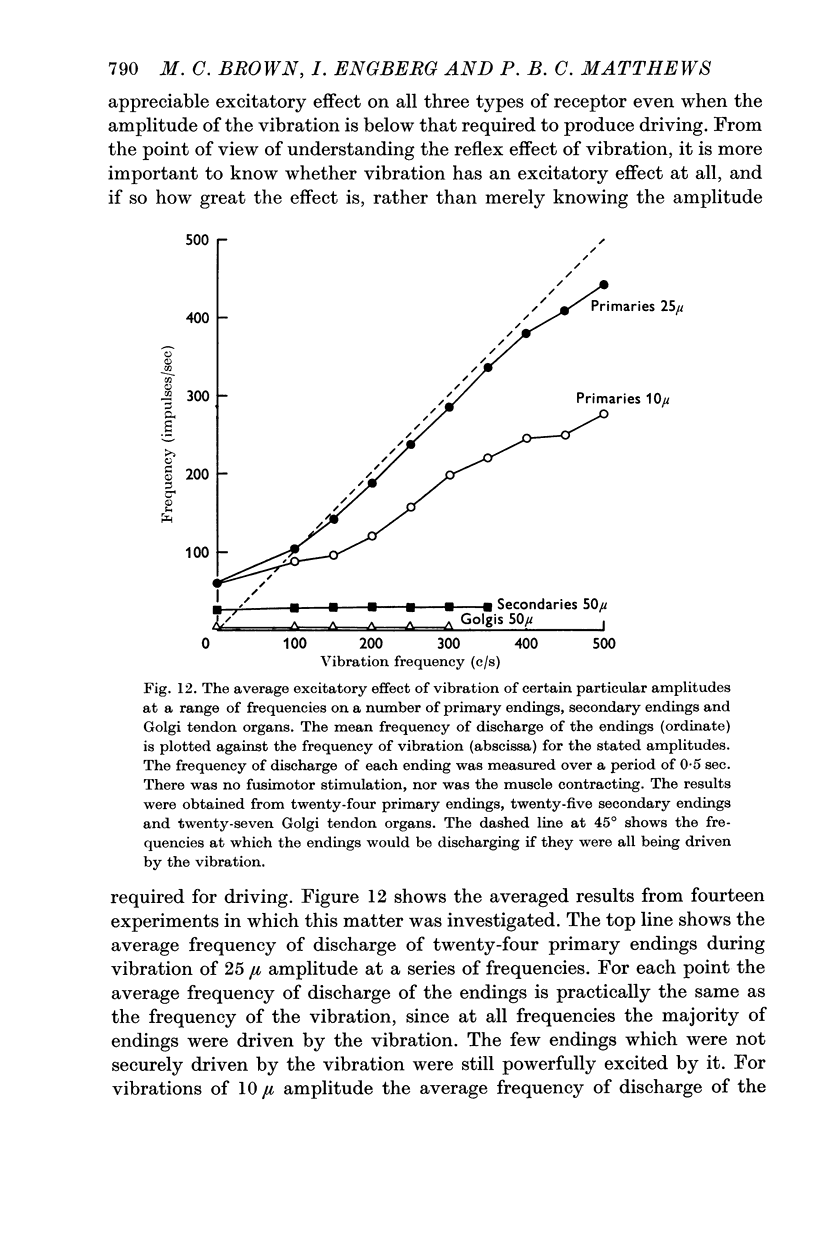
7. When the amplitude of vibration was somewhat below that required to produce driving of an ending it still produced some increase in its mean frequency of discharge. However, amplitudes of vibration of 25-50 μ applied to a non-contracting muscle, whether with or without fusimotor stimulation, produced driving of nearly all primary endings without any significant increase in the mean frequency of firing of secondary endings or Golgi tendon organs. Such vibration can therefore be used as a specific stimulus for the primary endings in order to investigate the central effects or repetitive discharge of the Ia afferent fibres from them.
8. Experiments on endings in the peroneus longus muscle showed that these behaved similarly to those in soleus.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg B., Bessou P., Laporte Y. Action of static and dynamic fusimotor fibres on secondary endings of cat's spindles. J Physiol. 1966 Jul;185(1):160–171. doi: 10.1113/jphysiol.1966.sp007978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCONI R., van der MEULEN J. The response to vibration of the end organs of mammalian muscle spindles. J Neurophysiol. 1963 Jan;26:177–190. doi: 10.1152/jn.1963.26.1.177. [DOI] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Emonet-Dénand F., Laporte Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J Physiol. 1965 Oct;180(3):649–672. doi: 10.1113/jphysiol.1965.sp007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. FURTHER STUDIES OF STATIC AND DYNAMIC FUSIMOTOR FIBRES. J Physiol. 1964 Oct;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gail P., Lance J. W., Neilson P. D. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry. 1966 Feb;29(1):1–11. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D. Gamma control of dynamic properties of muscle spindles. J Neurophysiol. 1956 Jul;19(4):356–366. doi: 10.1152/jn.1956.19.4.356. [DOI] [PubMed] [Google Scholar]
- HARVEY R. J., MATTHEWS P. B. The response of de-efferented muscle spindle endings in the cat's soleus to slow extension of the muscle. J Physiol. 1961 Jul;157:370–392. doi: 10.1113/jphysiol.1961.sp006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSEN J. K., RUDJORD T. ON THE SILENT PERIOD AND GOLGI TENDON ORGANS OF THE SOLEUS MUSCLE OF THE CAT. Acta Physiol Scand. 1964 Dec;62:364–379. doi: 10.1111/j.1748-1716.1964.tb10435.x. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C., QUILLIAM J. P. Function of medullated small-nerve fibers in mammalian ventral roots; efferent muscle spindle innervation. J Neurophysiol. 1951 Jan;14(1):29–54. doi: 10.1152/jn.1951.14.1.29. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., BESSOU P. Distribution dans les sous-groupes rapide et lent du groupe I des fibres IA d'origine fusoriale et des fibres IB d'origine Golgienne. C R Seances Soc Biol Fil. 1957;151(1):178–182. [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Lindblom U. Properties of touch receptors in distal glabrous skin of the monkey. J Neurophysiol. 1965 Sep;28(5):966–985. doi: 10.1152/jn.1965.28.5.966. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. MUSCLE SPINDLES AND THEIR MOTOR CONTROL. Physiol Rev. 1964 Apr;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. THE RESPONSE OF DE-EFFERENTED MUSCLE SPINDLE RECEPTORS TO STRETCHING AT DIFFERENT VELOCITIES. J Physiol. 1963 Oct;168:660–678. doi: 10.1113/jphysiol.1963.sp007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Reflex activation of the soleus muscle of the decerebrate cat by vibration. Nature. 1966 Jan 8;209(5019):204–205. doi: 10.1038/209204b0. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966 May;184(2):450–472. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. Excitation of mechanoreceptor units in the skin of the rabbit ear. Arch Ital Biol. 1967 Jun;105(2):290–314. [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENKIN B. Z., VALLBO A. B. SIMULTANEOUS RESPONSES OF GROUPS I AND II CAT MUSCLE SPINDLE AFFERENTS TO MUSCLE POSITION AND MOVEMENT. J Neurophysiol. 1964 May;27:428–450. doi: 10.1152/jn.1964.27.3.429. [DOI] [PubMed] [Google Scholar]
- Rack P. M., Westbury D. R. The effects of suxamethonium and acetylcholine on the behaviour of cat muscle spindles during dynamics stretching, and during fusimotor stimulation. J Physiol. 1966 Oct;186(3):698–713. doi: 10.1113/jphysiol.1966.sp008063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO M. Response of Pacinian corpuscles to sinusoidal vibration. J Physiol. 1961 Dec;159:391–409. doi: 10.1113/jphysiol.1961.sp006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Euler C., Peretti G. Dynamic and static contributions to the rhythmic y activation of primary and secondary spindle endings in external intercostal muscle. J Physiol. 1966 Dec;187(3):501–516. doi: 10.1113/jphysiol.1966.sp008106. [DOI] [PMC free article] [PubMed] [Google Scholar]


