Abstract
Cellular proteins that regulate apoptotic cell death can modulate the outcome of Sindbis virus (SV) encephalitis in mice. Both endogenous and overexpressed BCL-2 and BAX proteins protect newborn mice from fatal SV infection by blocking apoptosis in infected neurons. To determine the effects of these cellular factors on the course of infection in older animals, a more neurovirulent SV vector (dsNSV) was constructed from a viral strain that causes both prominent spinal cord infection with hind-limb paralysis and death in weanling mice. This vector has allowed assessment of the effects of BCL-2 and BAX on both mortality and paralysis in these hosts. Similar to newborn hosts, weanling mice infected with dsNSV encoding BCL-2 or BAX survived better than animals infected with control viruses. This finding indicates that BCL-2 and BAX both protect neurons that mediate host survival. Neither cellular factor, however, could suppress the development of hind-limb paralysis or prevent the degeneration of motor neurons in the lumbar spinal cord. Infection of BAX knockout mice with dsNSV demonstrated that endogenous BAX also enhances the survival of animals but has no effect on paralysis. These findings for the spinal cord are consistent with earlier data showing that dying lumbar motor neurons do not exhibit an apoptotic morphology. Thus, divergent cell death pathways are activated in different target populations of neurons during neurovirulent SV infection of weanling mice.
The pathogenesis of central nervous system (CNS) viral infection is strongly influenced by variables that determine which cells become infected and how these cells respond to infection. Neurons are the principal cell type infected by Sindbis virus (SV), an alphavirus that causes acute encephalomyelitis in mice. Host and viral factors both influence the degree to which SV-infected neurons die (5, 11, 13), a process which in young mice occurs almost entirely via apoptosis (5, 12-14, 16, 19). Indeed, the neurovirulence of different strains of SV in young mice is dictated largely by the degree to which infected neurons undergo this form of programmed cell death (13). A neuroadapted strain of SV (NSV) causes severe, often fatal encephalomyelitis in adult animals that is accompanied by prominent hind-limb paralysis (8, 9). This paralysis develops as a result of infection spreading to motor neurons in the lumbosacral spinal cord that directly innervate the hind-limb musculature (8). Motor neurons degenerate following infection with NSV, but the morphology of these cells does not show features typical of apoptosis, suggesting that a different cell death pathway is activated in this target cell population (7). Investigating the mechanisms by which motor neurons die following NSV infection may elucidate more general pathways through which these cells respond to exogenous stressors.
Previous studies showed that a variety of genes known to modulate neuronal apoptosis can alter the pathogenesis of SV encephalitis in vivo. Many of these investigations were conducted with an attenuated strain of SV engineered to carry exogenous genes expressed from a second copy of the subgenomic promoter. This double-subgenome SV (dsSV) allows for the generation of viruses that can induce cell death and also serve as a vehicle to express proteins within cells that could modify the cellular response to infection (1). Thus, newborn mice infected with recombinant viruses that overexpress certain BCL-2 family members within neurons are much more likely to survive than littermates infected with viruses expressing control genes (12, 14). A limitation of this approach, however, has been the inability of dsSV-derived viruses to induce cellular damage and lethal disease in older animals. Since cDNA clones of SV that incorporate NSV genes were recently generated, insertion of the same subgenomic promoter and unique cloning site has allowed the generation of a more neurovirulent SV vector (dsNSV).
Here, we report that dsNSV-derived viruses reproduce many features of wild-type NSV following intracerebral inoculation into weanling mice. These include spread to motor neurons in the lumbar spinal cord, producing hind-limb paralysis, and the induction of lethal disease in a significant proportion of infected hosts. Furthermore, mice infected with dsNSV-derived viruses that overexpress the BCL-2 family members BCL-2 and BAX (designated dsNSV-BCL-2 and dsNSV-BAX, respectively) are protected from death compared to mice infected with dsNSV expressing green fluorescent protein (GFP) (designated dsNSV-GFP). It is likely that BCL-2 and BAX promote the survival of mice infected with dsNSV by inhibiting virus-induced cell death in neuronal populations that determine the fate of the host. In particular, these studies confirm a protective role of BAX in SV-infected neurons (14). Conversely, neither BCL-2 nor BAX confers any protection against the development of hind-limb paralysis or the degeneration of lumbar motor neurons in dsNSV-infected animals. This discrepancy reinforces previous data suggesting that a divergent neuronal cell death pathway becomes activated in this target cell population following NSV infection of older mice (7).
MATERIALS AND METHODS
Viruses.
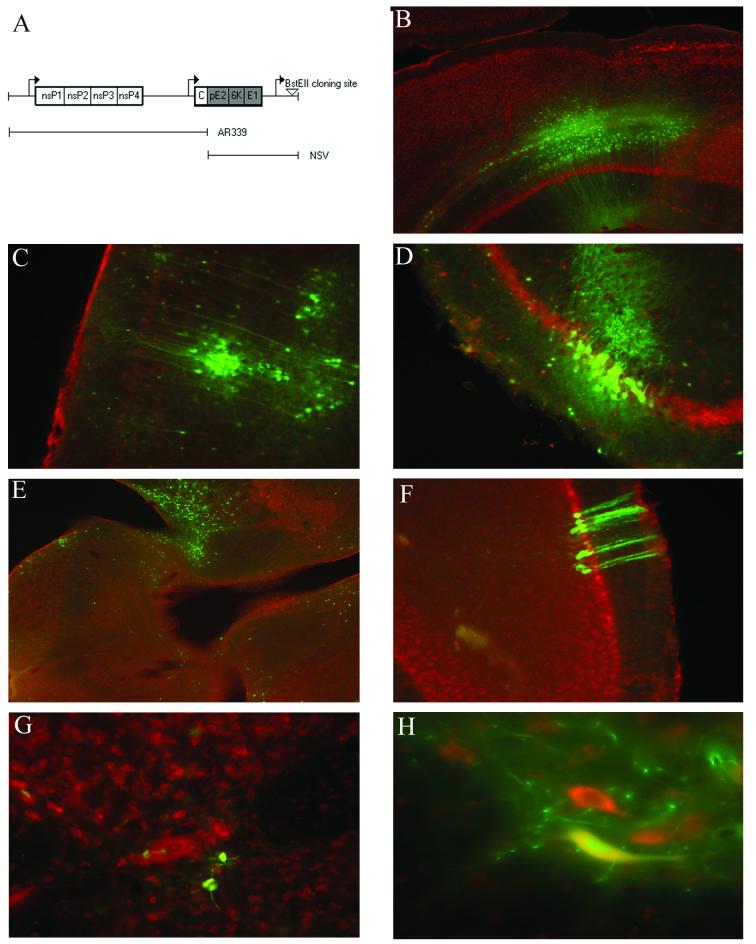
An NSV-like vector was generated from a cDNA clone (pTRSB) of a virus similar to wild-type SV following a series of modifications (17). The StuI/XhoI fragment of pTE12 containing the structural genes of NSV was first used to replace the identical region of pTRSB, creating pTE12/TRSB. Thereafter, the BsiWI/SacI fragment of dsTE12Q, another engineered strain of SV carrying a double subgenomic promoter, containing a second copy of the subgenomic viral promoter and a unique BstEII cloning site downstream of the structural genes was inserted into the same region of pTE12/TRSB (1, 12). This procedure generated the cDNA for a new recombinant virus (dsNSV) containing the nonstructural genes of SV, the structural genes of NSV, and a second subgenomic promoter with a unique cloning site (Fig. 1A).
FIG. 1.
Construction and in vivo tropism of NSV recombinants derived from dsNSV. (A) Schematic of dsNSV constructs. (B to H) Expression of functional GFP in infected cells of the cortex (B and C), hippocampus (D), brain stem (E), cerebellum (F), and lumbar spinal cord (G and H) 4 days after intracerebral inoculation of 4-week-old mice with 103 PFU of dsNSV-GFP (Nissl red counterstain) (magnifications, ×20 in panels B and E; ×40 in panels C, D, F, and G; and ×60 in panel H).
To then make recombinant NSV-like viruses that express exogenous genes, murine BAX (564 bp), murine BCL-2 (719 bp), the GFP gene (781 bp), and the GFP gene in reverse orientation (GFP-R; 781 bp) were individually inserted into dsNSV by PCR amplification from their respective templates by using primers with BstEII restriction sites. All open reading frames inserted into dsNSV were sequenced to confirm accurate PCR amplification. 5′-Capped RNA transcripts were synthesized from cDNA clones linearized with XhoI and transcribed in vitro at 38°C for 60 min with SP6 DNA-dependent RNA polymerase from an SP6 promoter located immediately 5′ of the viral genome sequence. Stocks of recombinant viruses were generated by transfecting 200 ng of RNA into BHK-21 cells with Lipofectin (Gibco/BRL) according to the manufacturer's instructions. Virus particle-containing supernatant fluids were collected from transfected cell monolayers after 24 to 48 h and frozen at −80°C. The amounts of viruses in supernatant fluids or in tissue homogenates were determined by plaque formation on BHK-21 cells.
Animal manipulations.
All animal procedures had received prior approval from our institutional animal care and use committee and were performed under isoflurane anesthesia. Four-week-old male C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Wild-type (BAX+/+) and BAX-deficient (BAX−/−) mice, both in a C57BL/6 background, were derived from litters produced by heterozygous breeding pairs also obtained from Jackson Laboratories. Mice of both genders were genotyped as previously described (14, 21).
All animals were infected with 103 PFU of the various NSV recombinants in 20 μl of phosphate-buffered saline (PBS) via direct intracerebral inoculation. Infected animals were either (i) scored daily for survival and the presence of hind-limb paralysis, (ii) perfused with PBS for the determination of viral titers in homogenates of brains and spinal cords, or (iii) perfused with 4% paraformaldehyde in PBS for subsequent histological examination. Hind-limb paralysis, determined by an examiner blinded to the various experimental groups, was defined as obvious motor dysfunction involving either or both hind limbs during attempted ambulation across a laboratory bench top.
The statistical significance of survival differences was determined by using Kaplan-Meier analysis. Given some fluctuations in paralysis scoring among individual animals, the risk of developing paralysis was determined as a function of subject days free of paralysis in each group. A logistic regression model was then used to estimate the odds of developing paralysis over the course of the experiment for each study group relative to a reference group. Odds ratios (OR) and P values are reported for each paired comparison.
Histological analysis.
Paraformaldehyde-fixed brain tissue was embedded in paraffin, and 25-μm coronal sections taken at −0.5 mm relative to the bregma were stained with hematoxylin and eosin (H&E). Adjacent sections were taken for SV immunohistochemical analysis, which was performed as previously described (7, 9,14). The total numbers of normal and pyknotic neurons per hippocampus in H&E-stained sections and infected hippocampal neurons in immunostained sections were counted by a blinded examiner. Both hippocampi in three sections were scored from three different mice infected with each virus. Data are presented as the mean and standard error of the mean (SEM) percentage of nonpyknotic hippocampal neurons or the total number of infected hippocampal neurons.
To assess the fate of lumbar motor neurons, the entire lumbar spinal column (spinal cord plus adjacent nerve roots) was decalcified and embedded in paraffin, and 25-μm sections were stained either with H&E or via a modified Bielschowsky method to specifically label nerve axons (7, 24). Motor neuron cell bodies, defined as any cell greater than 20 μm in diameter within spinal cord grey matter ventral to the central canal, were counted in every 12th section between the L4 and L6 levels. The mean and SEM numbers of remaining lumbar motor neurons per animal are reported. Motor neuron axons were specifically quantified as they passed through the lumbar ventral nerve roots. Axonal density (the number of intact axons per cross-sectional area of each root in arbitrary units) was determined for each L4 and L5 ventral nerve root (four roots per animal) taken from mice 21 days after infection. This late stage of disease was studied because axonal loss is delayed following destruction of the cell body in the spinal cord. Data are presented as the mean and SEM axonal density per ventral nerve root. Differences between the means of the various neuron or axon counts were analyzed by using a two-tailed Student t test. Significant differences were defined as a P value of <0.05.
RESULTS
In vivo characterization of dsNSV-derived viruses.
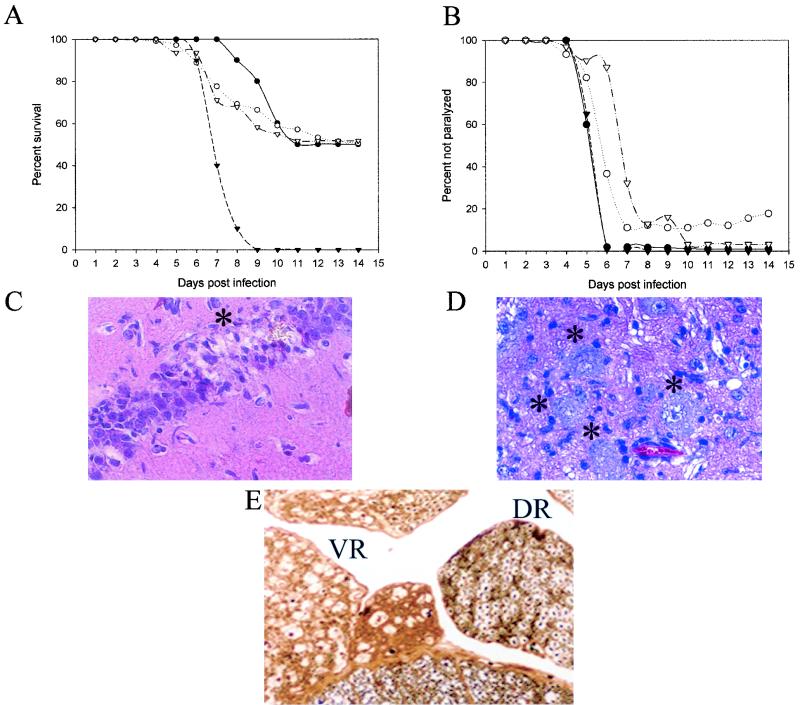
To characterize the tropism and exogenous gene expression of dsNSV-derived recombinant viruses, tissues from animals infected with dsNSV-GFP were analyzed 4 days after infection. Functional GFP was expressed within neurons of the cortex, hippocampus, brain stem, cerebellum, and lumbar spinal cord (Fig. 1B to H). This tissue tropism closely reproduces the distribution in wild-type NSV infection of adult mice (8, 9). dsNSV-derived viruses also reproduced many clinical features of wild-type NSV in 4-week-old animals, including the ability to produce significant hind-limb paralysis (Fig. 2B). However, the survival of mice infected with various control dsNSV-derived viruses, dsNSV alone, dsNSV-GFP, or dsNSV expressing GFP-R (dsNSV-GFP-R) was greater than the survival of age-matched animals infected with wild-type NSV (Fig. 2A). Despite this enhanced survival, dsNSV still caused significant destruction of both hippocampal and lumbar motor neurons in infected animals (Fig. 2C to E). Furthermore, insertion of the GFP gene into dsNSV did not attenuate its phenotype compared to that of the vector alone, nor was the virus toxic compared to a virus expressing the GFP-R gene (Fig. 2A and B). In sum, this system provides one of the first opportunities to investigate the molecular determinants of alphavirus-induced neuronal cell death in the fully developed nervous system.
FIG. 2.
Clinical and histological analyses of disease caused by viruses generated from dsNSV in comparison to wild-type NSV. (A) Percentage of 4-week-old mice (at least 40 per group) that survived intracerebral inoculation with 103 PFU of dsNSV alone (•), dsNSV-GFP (○), dsNSV-GFP-R (▿), or wild-type NSV (▾). (B) Percentage of animals that failed to develop any hind-limb paralysis following challenge with the viruses listed in panel A. (C) Destruction of hippocampal neurons (asterisk) 4 days after infection with dsNSV (magnification, ∼×37). (D) Morphological features of degenerating lumbar motor neurons (asterisks) 4 days after infection with dsNSV (magnification, ∼×37). (E) Selective destruction of motor neuron axons (black dots) in lumbar ventral nerve roots (VR), with sparing of sensory axons (black dots) in lumbar dorsal nerve roots (DR), 21 days after infection with dsNSV (magnification, ∼×37).
Overexpression of BCL-2 family members protects weanling mice from death, but not hind-limb paralysis, induced by dsNSV infection.
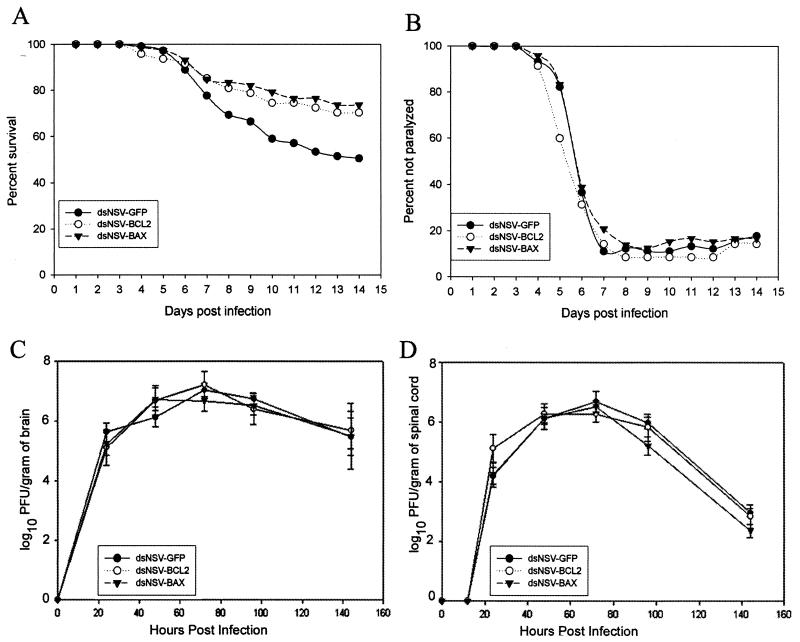
To determine whether the overexpression of BCL-2 family members altered the outcome of dsNSV infection in older mice, BCL-2 and BAX were individually inserted into dsNSV, and recombinant viruses were generated. At 14 days following intracerebral challenge, significantly more animals survived infection with either dsNSV-BAX (73.6%) or dsNSV-BCL-2 (70.2%) than with dsNSV-GFP (50.4%) (Fig. 3A). However, neither BAX nor BCL-2 overexpression could inhibit the development of hind-limb paralysis among dsNSV-infected animals (Fig. 3B). When replication of the recombinant viruses was examined, no differences were found in the titers of infectious viruses present in either the brains or the spinal cords of infected animals (Fig. 3C and D). These data imply that BCL-2 and BAX each can interrupt a dsNSV-induced cell death pathway in target neurons of weanling animals that determine the fate of the host. Other populations of infected neurons that are less directly involved in host survival, including lumbar motor neurons, appear not to be affected by the overexpression of these proteins.
FIG. 3.
Overexpression of BCL-2 family members alters survival but not paralysis caused by dsNSV infection. (A) Percentage of 4-week-old mice (at least 75 per group, in four separate experiments) that survived intracerebral inoculation with 103 PFU of either dsNSV-GFP, dsNSV-BCL-2, or dsNSV-BAX. BCL-2 and BAX are both protective compared to GFP (P < 0.04 and P < 0.004, respectively). (B) Percentage of animals that did not develop hind-limb paralysis following infection. The risk of paralysis among dsNSV-BCL-2-infected mice compared to dsNSV-GFP-infected mice generated an OR of 0.87 (P = 0.19). The risk of paralysis among dsNSV-BAX-infected mice compared to dsNSV-GFP-infected mice generated an OR of 1.09 (P = 0.32). (C and D) Replication of recombinant dsNSV-derived viruses in the brains (C) and spinal cords (D) of infected mice. Each point represents the mean and SEM log10 PFU per gram of tissue from at least three animals. None of the brain or spinal cord viral growth curves was statistically different from one another.
BCL-2 and BAX both protect hippocampal neurons but not lumbar motor neurons from dsNSV-induced cell death.
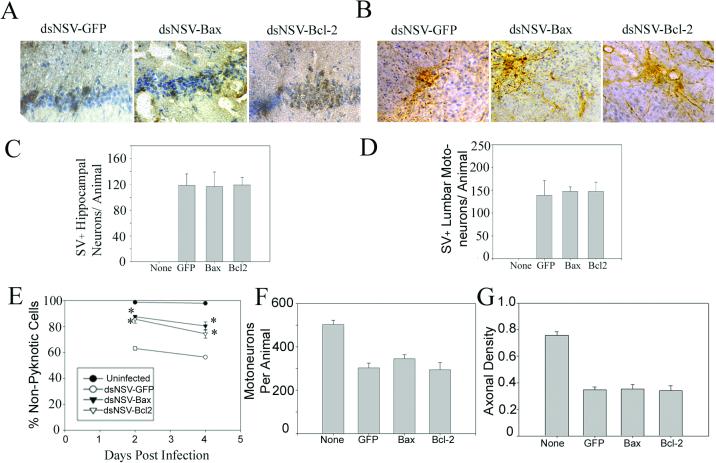
To determine whether the divergent effects of BCL-2 or BAX overexpression on dsNSV-induced hind-limb paralysis and host survival could be correlated with the fate of different neuronal populations in infected animals, tissue sections from various regions of the CNS were examined. Hippocampal neurons are highly susceptible to wild-type NSV infection, and many of these cells eventually undergo apoptosis (7, 9,16). Immunostaining showed that all three dsNSV-derived viruses infected this brain region to equivalent degrees (Fig. 4A and C). However, when this brain region was scored for the amount of cellular injury, significantly less neuronal damage was found in dsNSV-BCL-2- and dsNSV-BAX-infected samples than in dsNSV-GFP-infected samples (Fig. 4E). In the lumbar spinal cord, equivalent numbers of motor neurons were infected with these three viruses (Fig. 4B and D). To assess the fate of these cells, cell bodies and their axonal extensions passing through the lumbar ventral nerve roots were both counted. These axons invariably degenerate following destruction of the cell body in the spinal cord, and their loss is easily quantified following NSV infection (7). Not only did lumbar motor neurons degenerate in the ventral grey matter of dsNSV-BCL-2- and dsNSV-BAX-infected animals to the same degree as in dsNSV-GFP-infected mice (Fig. 4F), but also the effects of this cellular destruction were identical among axons in lumbar ventral nerve roots at a later time point (Fig. 4G). Thus, overexpression of BCL-2 and BAX protects hippocampal neurons but not lumbar motor neurons from dsNSV-induced cell death.
FIG. 4.
Fate of neurons in the hippocampus and lumbar spinal cord of 4-week-old mice infected intracerebrally with 103 PFU of dsNSV-GFP, dsNSV-BCL-2, or dsNSV-BAX compared to uninfected animals. Immunostaining for viral antigen shows equivalent involvement of hippocampal (A and C) and lumbar spinal cord (B and D) neurons 3 days postinfection. (E) More nonpyknotic hippocampal neurons (mean and SEM percentage) were counted in dsNSV-BCL-2- or dsNSV-BAX-infected animals 2 and 4 days postinfection than in dsNSV-GFP-infected animals. Values for both dsNSV-BCL-2 and dsNSV-BAX were significantly greater than values for dsNSV-GFP at both time points (asterisks) (P < 0.05). (F and G) Conversely, the number of lumbar motor neuron cell bodies 4 days postinfection (F) and the density of axons in lumbar ventral nerve roots 21 days postinfection (G) were not statistically different among animals infected with any of the three viruses. Error bars in panels C, D, F, and G indicate SEMs.
Endogenous BAX protects weanling mice from death but not hind-limb paralysis induced by dsNSV infection.
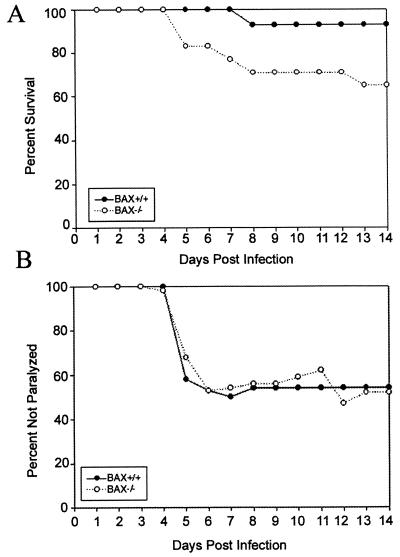
To confirm that BAX is protective in weanling mice infected with dsNSV, BAX+/+ and BAX−/− mice were inoculated with dsNSV-GFP and monitored for the development of hind-limb paralysis and death. Consistent with the enhanced survival of wild-type mice infected with dsNSV-BAX (Fig. 3A), BAX−/− mice were significantly more susceptible to fatal dsNSV-GFP infection than their wild-type littermates (Fig. 5A). In contrast, the presence or absence of endogenous BAX had no effect on whether infected animals developed hind-limb paralysis (Fig. 5B). The improved survival of animals in this experiment compared to the results for dsNSV-GFP-infected animals reported earlier (Fig. 2A) may be explained by several factors. First, both male and female animals were used in this experiment (50% of each gender in both groups), whereas only male mice were used in earlier assays. The inclusion of females in this experiment allowed us to maximize the use of mice from each litter, even though female gender is known to be a variable that independently increases resistance to wild-type NSV infection (D. E. Griffin, unpublished data). Second, host genetic background may be a factor; increased resistance may reflect an effect of the original hosts despite “adequate” backcrossing of the gene defect to the present inbred strain. Regardless of differences between experiments, however, these data confirm that BAX protects weanling mice from the lethal effects of dsNSV, presumably by inhibiting virus-induced apoptosis in target neurons that determine the fate of the host. In contrast, the development of paralysis is not affected by endogenous BAX, implying that lumbar motor neurons degenerate in a completely BAX-independent manner.
FIG. 5.
Cumulative survival (A) and development of hind-limb paralysis (B) in 4-week-old BAX+/+ and BAX−/− mice following infection with dsNSV-GFP (20 mice per group). BAX+/+ mice were protected from death compared to BAX−/− animals (P < 0.007). However, the risk of paralysis in BAX+/+ mice was not different from that in BAX−/− animals (OR, 1.19; P = 0.35).
DISCUSSION
The construction of dsNSV and its use to generate NSV-like recombinants permit further investigation into factors that control neuronal cell death during alphavirus infection of the mature CNS. These data reveal that the pathogenesis of this infection is complex, with the destruction of some neuronal populations but not others being inhibited by the apoptosis-modulating proteins BCL-2 and BAX. Such findings are in keeping with previous morphological and biochemical studies showing evidence of apoptosis in degenerating neurons of the hippocampus but not the lumbar spinal cord of wild-type NSV-infected animals (7).
The protective effects of various BCL-2 family members and caspase inhibitors, along with the documented correlation between neurovirulence and the histochemical extent of cellular DNA fragmentation in brain tissue sections, strongly implicate neuronal apoptosis as the cause of fatal SV infection in newborn mice (12-15, 19). Neuronal apoptosis can also be detected throughout the hippocampus of 4- to 6-week-old animals infected with NSV by using similar morphological and histochemical approaches (7, 16). Nevertheless, emerging data also suggest that nonapoptotic neuronal cell death can be detected in the brains of NSV-infected mice, sometimes immediately adjacent to where apoptotic neurons are found (J. L. Nargi-Aizenman and D. E. Griffin, unpublished data). Furthermore, degenerating motor neurons in the lumbar spinal cord of wild-type NSV-infected animals do not have an apoptotic morphology (7). At present, the relative contributions of apoptotic versus nonapoptotic neuronal cell death in the pathogenesis of NSV infection and the molecular pathways underlying nonapoptotic neuronal cell death remain somewhat unclear.
Although BCL-2 was found to inhibit the lethal effects of dsNSV among adult animals in the present study, others have reported a failure of BCL-2 to protect mice from death caused by a related dsSV strain (15). Here, 10-day-old CD1 mice succumbed equally to infection caused by dsTE12, a related SV vector that incorporates some NSV genes, whether or not BCL-2 was expressed (15). It has been shown, however, that neurons become more resistant to apoptosis induced by SV as the age of the host increases or when they differentiate in vitro for longer periods of time (11, 13). This age-related resistance of cultured neurons correlates with an up-regulation of endogenous cellular inhibitors of apoptosis (including BCL-2), suggesting that the fate of SV-infected neurons is determined by the relative overall intracellular balance between prodeath and antideath signals (11). It is possible that the failure of dsTE12 expressing BCL-2 to protect 10-day-old CD1 mice can be explained by the immaturity of endogenous neuronal defenses to block enough dsTE12-induced apoptosis even when supplemented with the protective effects of exogenous BCL-2. According to this model, one would predict that overexpressed BCL-2 would protect slightly older CD1 mice once their endogenous neuronal resistance had increased to a sufficient degree.
The BAX protein is widely known as a prodeath member of the BCL-2 family that triggers apoptosis in multiple cell types (3, 21). In the nervous system, BAX plays an important role in the physiological elimination of unnecessary neurons that occurs during normal development (3, 23). However, BAX is a potent inhibitor of SV-induced neuronal apoptosis in newborn mice, even surpassing the protective effects of BCL-2 (14). Nevertheless, BAX-mediated inhibition of SV-induced cell death is also highly dependent on neuronal subtype. While BAX potently protects hippocampal neurons, it is prodeath in infected dorsal root ganglion neurons, suggesting that its death-modulating effects are influenced by host factors that differ in neuronal populations (14). Given the relative protection of hippocampal neurons in mice infected with dsNSV-BAX, the enhanced survival of wild-type versus BAX-deficient animals is likely the result of endogenous BAX expression in target neurons important for survival. Whether these cells constitute a discrete population of neurons in a single region of the CNS or are representative of neurons found throughout multiple brain regions is not known. As such, the exact mechanism of protection remains unclear. In contrast, while the lack of protection conferred by BAX and BCL-2 in dsNSV-infected lumbar motor neurons does not explain the mechanism through which these cells degenerate, this finding supports previous data which suggested that these cells do not undergo typical apoptosis (7). Thus, NSV-infected lumbar motor neurons do not have an apoptotic morphology or show any evidence of DNA fragmentation (7, 8). Furthermore, unlike hippocampal neurons, these cells appear to lack the expression of caspase 3, an important cellular effector of apoptosis (7). We believe that dsNSV-derived viruses will allow us to explore the function of a variety of other factors potentially involved in motor neuron survival and death. In this way, we expect to be able to characterize the molecular mechanisms through which these cells degenerate following infection with dsNSV.
Further investigation into dsNSV-induced motor neuron cell death may help elucidate more general mechanisms through which these particular cells respond to other viruses as well as to nonviral stressors. In transgenic mice that express the human poliovirus receptor, poliovirus infection results in paralysis and degeneration of motor neurons, many of which show biochemical and morphological evidence of apoptosis (4). These findings imply that differentiated motor neurons may retain the capacity to undergo programmed cell death in some situations. In contrast, transgenic mice carrying a mutant superoxide dismutase 1 gene spontaneously develop progressive motor neuron degeneration akin to amyotrophic lateral sclerosis (6). Similar to what is seen with NSV (7), degenerating motor neurons in these animals show massive vacuolation and formation of intracytoplasmic protein aggregates but no evidence of DNA fragmentation or caspase activation (2, 18). A recently identified alternative, nonapoptotic form of programmed cell death characterized by prominent cytoplasmic vacuolation in cultured cells has morphological similarities to this motor neuron cell death (22). The contribution of classical apoptosis to various human neurodegenerative disorders that affect motor neurons still remains poorly established. Understanding cell death pathways in this population of neurons will have important potential implications for unraveling the pathogenesis of these diseases.
In conclusion, the construction of dsNSV and the generation of recombinant viruses that express exogenous genes within infected neurons have allowed us to extend our studies of the role of neuronal apoptosis in alphavirus pathogenesis. In newborn animals, apoptosis appears to be the predominant mechanism underlying neuronal cell death following SV infection. In older mice, however, neurovirulent strains of SV appear to activate multiple cell death pathways in different neuronal populations. The neurons of newborn mice may simply be more prone to activate an apoptotic cascade as a general response to stress because the intracellular machinery for such events is already in place; the programmed cell death of neurons is a crucial part of normal nervous system development (10, 20). Neurons of more mature mice, on the other hand, may have down-regulated these cell death pathways once CNS development is complete and the physiological elimination of neurons is no longer necessary. In this setting, pathological stressors such as viruses may activate other cell death pathways. We believe that an improved understanding of how various populations of neurons die following acute viral infection of the CNS may help to shed light on the pathogenesis of other neurological disorders. New recombinant viruses will serve to probe the molecular basis of how discrete populations of differentiated neurons respond to the same death stimulus.
Acknowledgments
This work was supported by Public Health Service grants NS02130 (to D.A.K.), NS34175 (to J.M.H.), and NS18597 (to D.E.G) from the National Institutes of Health.
We thank Chitra Krishnan for assistance with the statistical interpretation of our results.
REFERENCES
- 1.Cheng, E. H.-Y., B. Levine, L. H. Boise, C. B. Thompson, and J. M. Hardwick. 1996. Bax-independent inhibition of apoptosis by Bcl-XL. Nature 379:554-556. [DOI] [PubMed] [Google Scholar]
- 2.Dal Canto, M. C., and M. E. Gurney. 1994. Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am. J. Pathol. 145:1271-1279. [PMC free article] [PubMed] [Google Scholar]
- 3.Deckwerth, T. L., J. L. Elliott, C. M. Knudson, E. M. Johnson, Jr., W. D. Snider, and S. J. Korsmeyer. 1996. Bax is required for neuronal death after trophic factor deprivation and during development. Neuron 17:401-411. [DOI] [PubMed] [Google Scholar]
- 4.Girard, S., T. Couderc, J. Destombes, D. Thiesson, F. Delpeyroux, and B. Blondel. 1999. Poliovirus induces apoptosis in the mouse central nervous system. J. Virol. 73:6066-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin, D. E., B. Levine, S. Ubol, and J. M. Hardwick. 1994. The effects of alphavirus infection on neurons. Ann. Neurol. 35:S23-S27. [DOI] [PubMed] [Google Scholar]
- 6.Gurney, M. E., H. Pu, A. Y. Chiu, M. C. Dal Canto, C. Y. Polchow, D. D. Alexander, J. Caliendo, A. Hentati, Y. W. Kwon, H. X. Deng, W. Cheng, P. Zhai, R. L. Sufit, and T. Siddique. 1994. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264:1772-1775. [DOI] [PubMed] [Google Scholar]
- 7.Havert, M. B., B. Schofield, D. E. Griffin, and D. N. Irani. 2000. Activation of divergent neuronal cell death pathways in different target cell populations during neuroadapted Sindbis virus infection of mice. J. Virol. 74:5352-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson, A. C., T. R. Moench, D. E. Griffin, and R. T. Johnson. 1987. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab. Investig. 56:418-423. [PubMed] [Google Scholar]
- 9.Jackson, A. C., T. R. Moench, B. D. Trapp, and D. E. Griffin. 1988. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab. Investig. 58:503-509. [PubMed] [Google Scholar]
- 10.Kuan, C. Y., K. A. Roth, R. A. Flavell, and P. Rakic. 2000. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 23:291-297. [DOI] [PubMed] [Google Scholar]
- 11.Levine, B., Q. Huang, J. T. Issacs, J. C. Reed, D. E. Griffin, and J. M. Hardwick. 1993. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 361:739-742. [DOI] [PubMed] [Google Scholar]
- 12.Levine, B., J. E. Goldman, H. H. Jiang, D. E. Griffin, and J. M. Hardwick. 1996. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc. Natl. Acad. Sci. USA 93:4810-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis, J., S. L. Wesselingh, D. E. Griffin, and J. M. Hardwick. 1996. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J. Virol. 70:1828-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis, J., G. A. Oyler, K. Ueno, Y.-R. Fannjiang, B. N. Chau, J. Vornov, S. J. Korsmeyer, S. Zou, and J. M. Hardwick. 1999. Inhibition of virus-induced neuronal apoptosis by Bax. Nat. Med. 5:832-835. [DOI] [PubMed] [Google Scholar]
- 15.Liang, X. H., L. K. Kleeman, H. H. Jiang, G. Gordon, J. E. Goldman, G. Berry, B. Herman, and B. Levine. 1998. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 72:8586-8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang, X. H., J. E. Goldman, H. H. Jiang, and B. Levine. 1999. Resistance of interleukin-1β-deficient mice to fatal Sindbis virus encephalitis. J. Virol. 73:2563-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKnight, K. L., D. A. Simpson, S.-C. Lin, T. A. Knott, J. M. Polo, D. F. Pence, D. B. Johannsen, H. W. Heidner, N. L. Davis, and R. E. Johnston. 1996. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J. Virol. 70:1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migheli, A., C. Atzori, R. Piva, M. Tortarolo, M. Girelli, D. Schiffer, and C. Bendotti. 1999. Lack of apoptosis in mice with ALS. Nat. Med. 5:966-967. [DOI] [PubMed] [Google Scholar]
- 19.Nava, V. E., A. Rosen, M. A. Veliuona, R. J. Clem, B. Levine, and J. M. Hardwick. 1998. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J. Virol. 72:452-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijhawan, D., N. Honarpour, and X. Wang. 2000. Apoptosis in neural development and disease. Annu. Rev. Neurosci. 23:73-87. [DOI] [PubMed] [Google Scholar]
- 21.Oltvai, Z., C. Milliman, and S. J. Korsmeyer. 1993. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609-619. [DOI] [PubMed] [Google Scholar]
- 22.Sperandio, S., I. de Belle, and D. E. Bredesen. 2000. An alternative, nonapoptotic form of programmed cell death. Proc. Natl. Acad. Sci. USA 97:14376-14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White, F. A., C. R. Keller-Peck, C. M. Knudson, S. J. Korsmeyer, and W. D. Snider. 1998. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J. Neurosci. 18:1428-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto, T., and A. Hirano. 1986. A comparative study of modified Bielschowsky, Bodian and thioflavin S stains on Alzheimer's neurofibrillary tangles. Neuropathol. Appl. Neurobiol. 23:16-25. [DOI] [PubMed] [Google Scholar]