Abstract
1. Intracellular records have been taken from cat motoneurones innervating flexor muscles of the hind limb. Contractions of the ankle flexors tibialis anterior and extensor digitorum longus were elicited by stimulation of the peripheral end of the cut L 7 ventral root and the reflex effects of these contractions were recorded in silent and repetitively firing motoneurones.
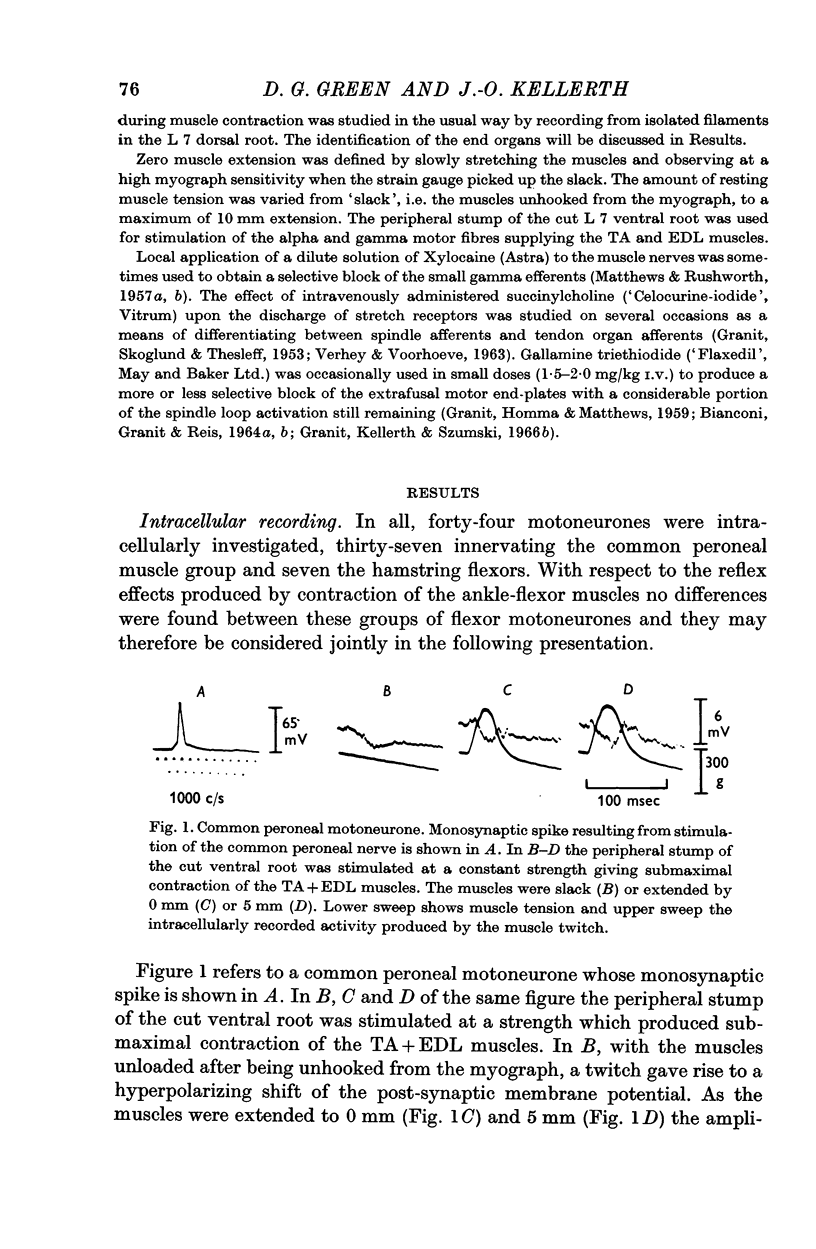
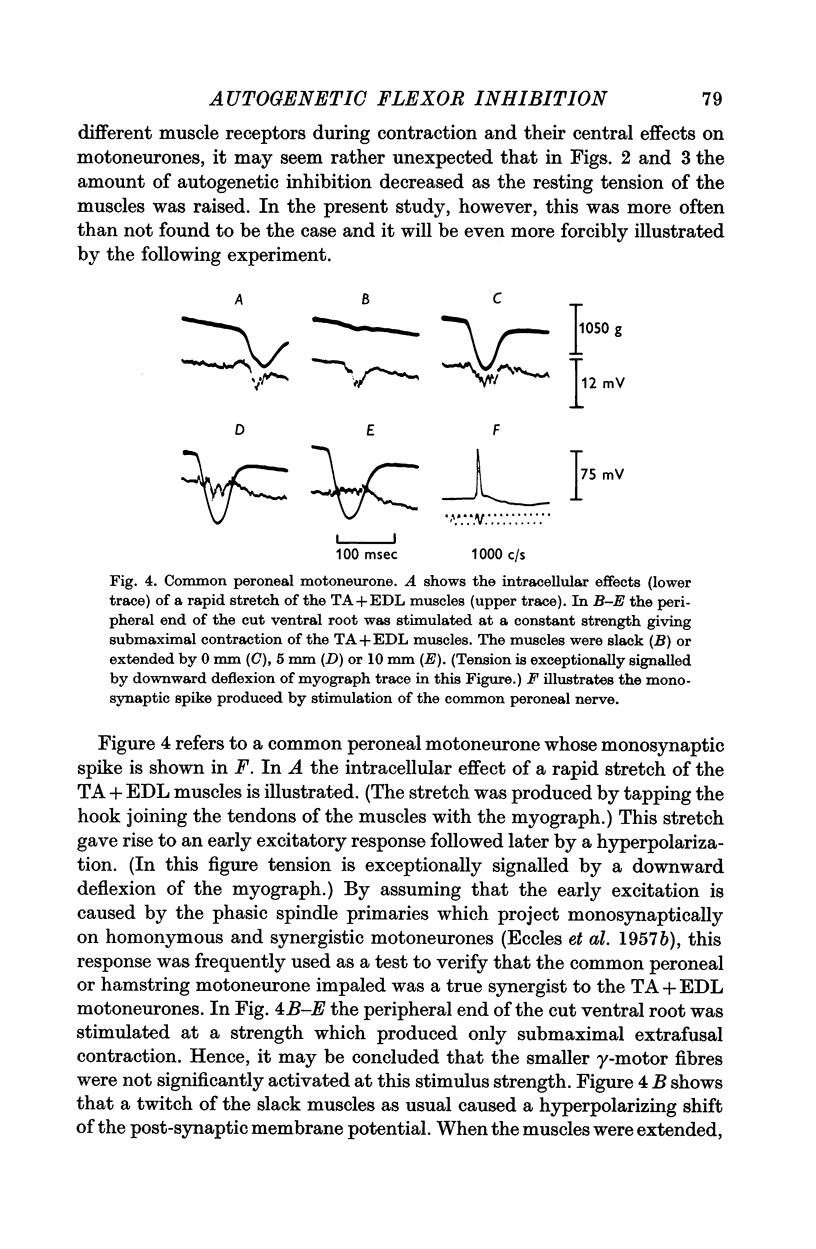
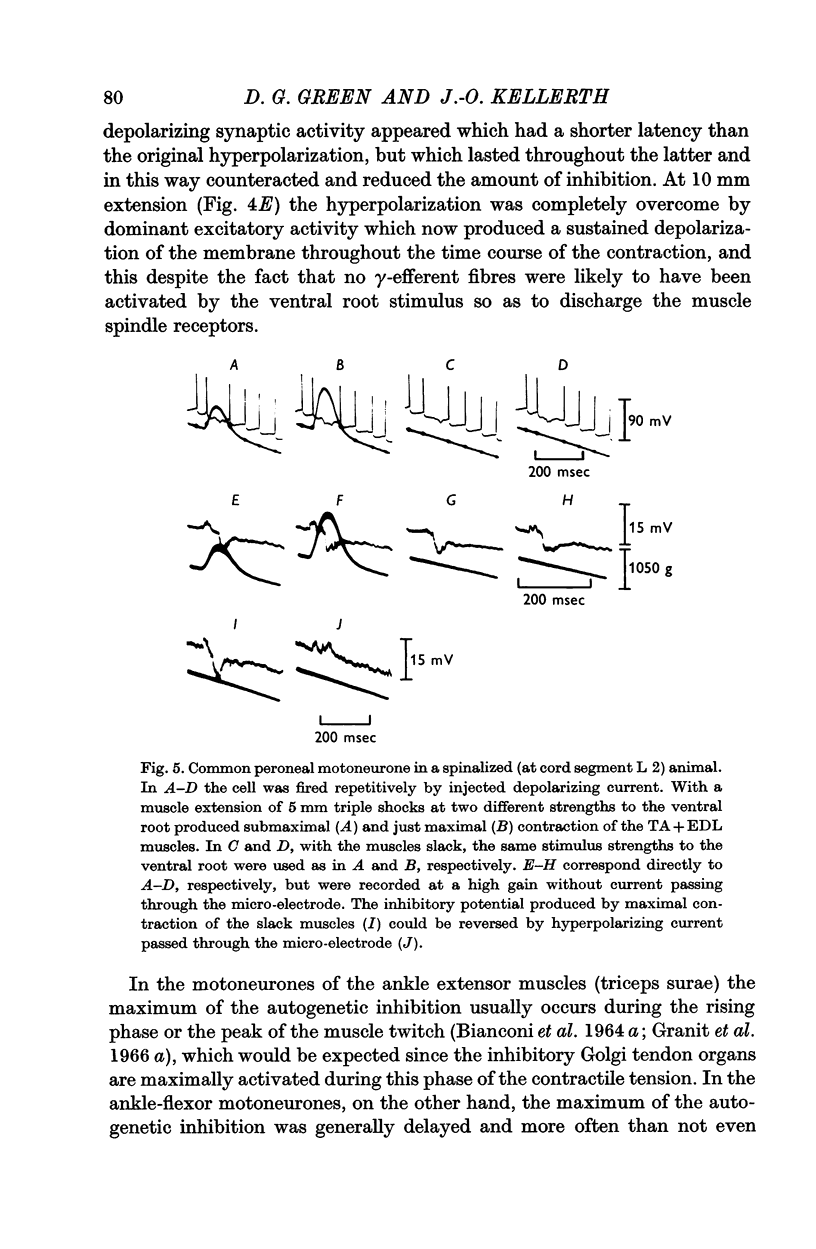
2. Contraction usually produces a hyperpolarizing response inside flexor motoneurones. This hyperpolarization is tension-sensitive in the sense that when, at constant muscle extension, the strength of the contraction is increased, the magnitude of the inhibitory response is augmented.
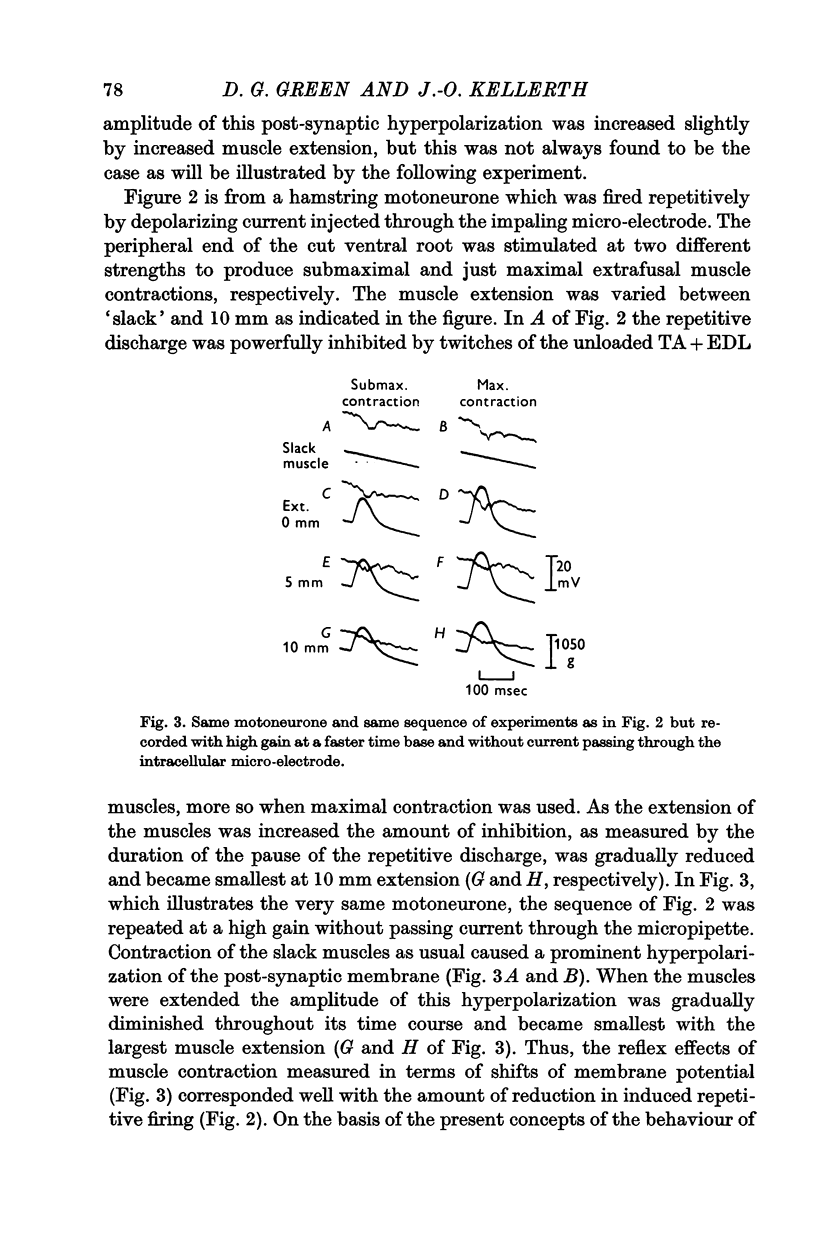
3. Increasing the resting length of the muscles, while using a stimulus of constant strength to the ventral root, causes this inhibitory response to increase in some cells. More often, however, the hyperpolarization caused by contraction is gradually reduced in duration and/or amplitude as the muscles are extended.
4. Even with the muscles slackened, so that they develop no tension at their ends, contraction usually produces prominent hyperpolarization of the motoneurones.
5. By passing polarizing currents or injecting chloride ions through the intracellular micro-electrode, the hyperpolarizing potentials produced by contraction of the slack and extended muscles are shown to be, at least in part, genuinely post-synaptic inhibitory events.
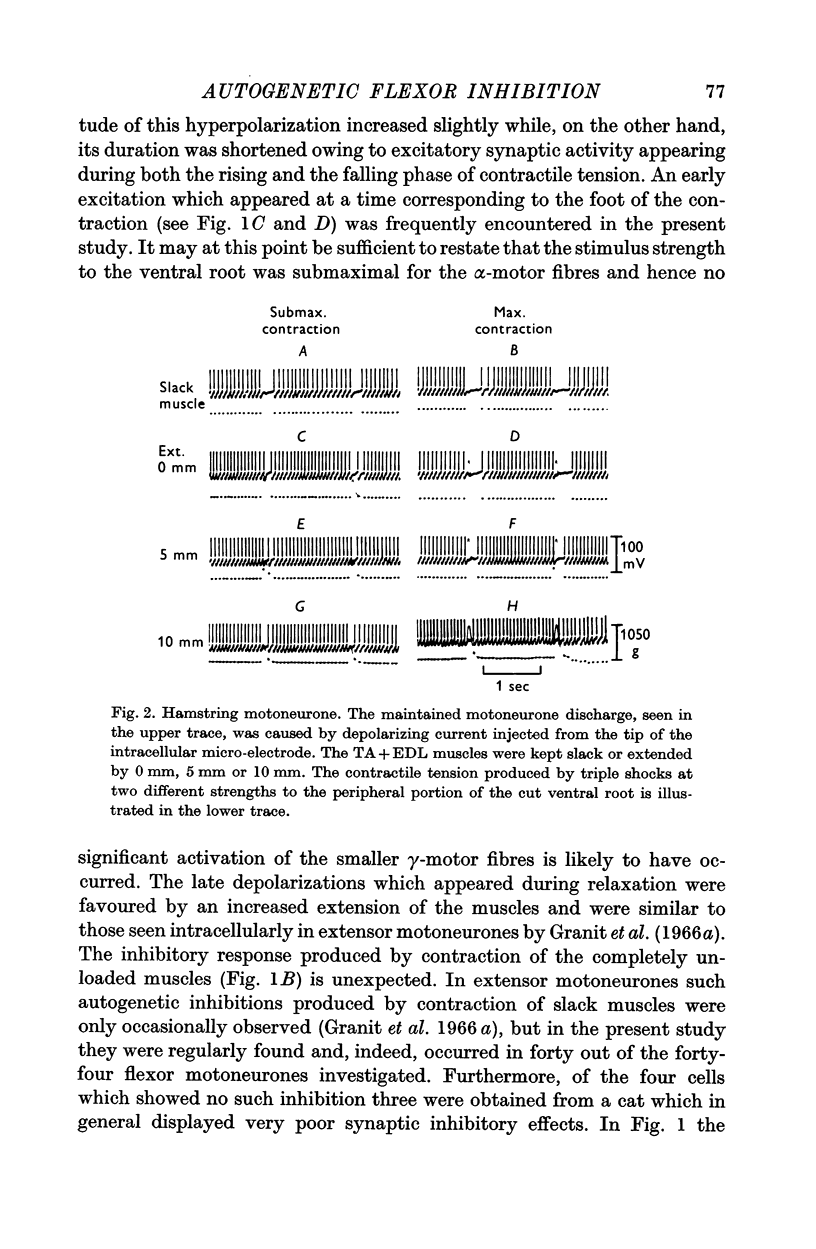
6. When the neurone is fired repetitively by injected current, the `silent period' in contraction corresponds to the hyperpolarization of the post-synaptic membrane.
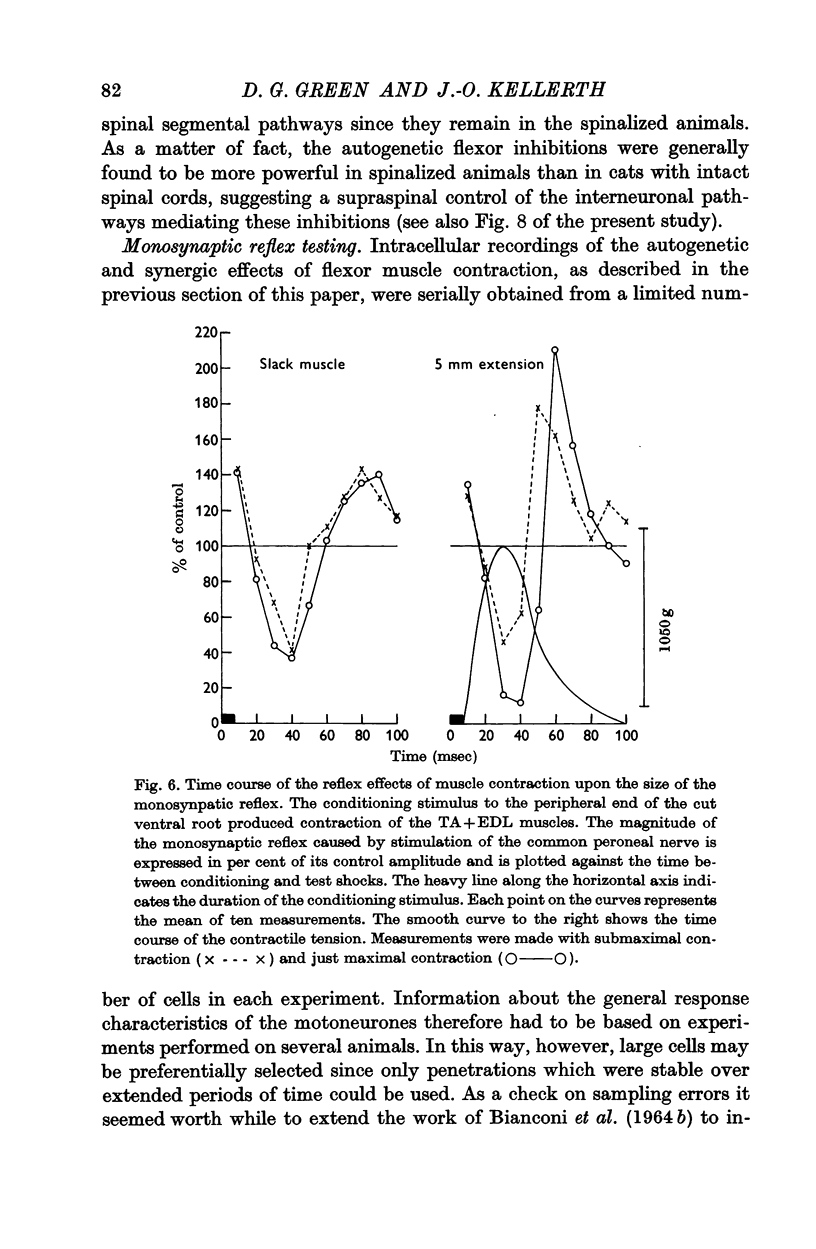
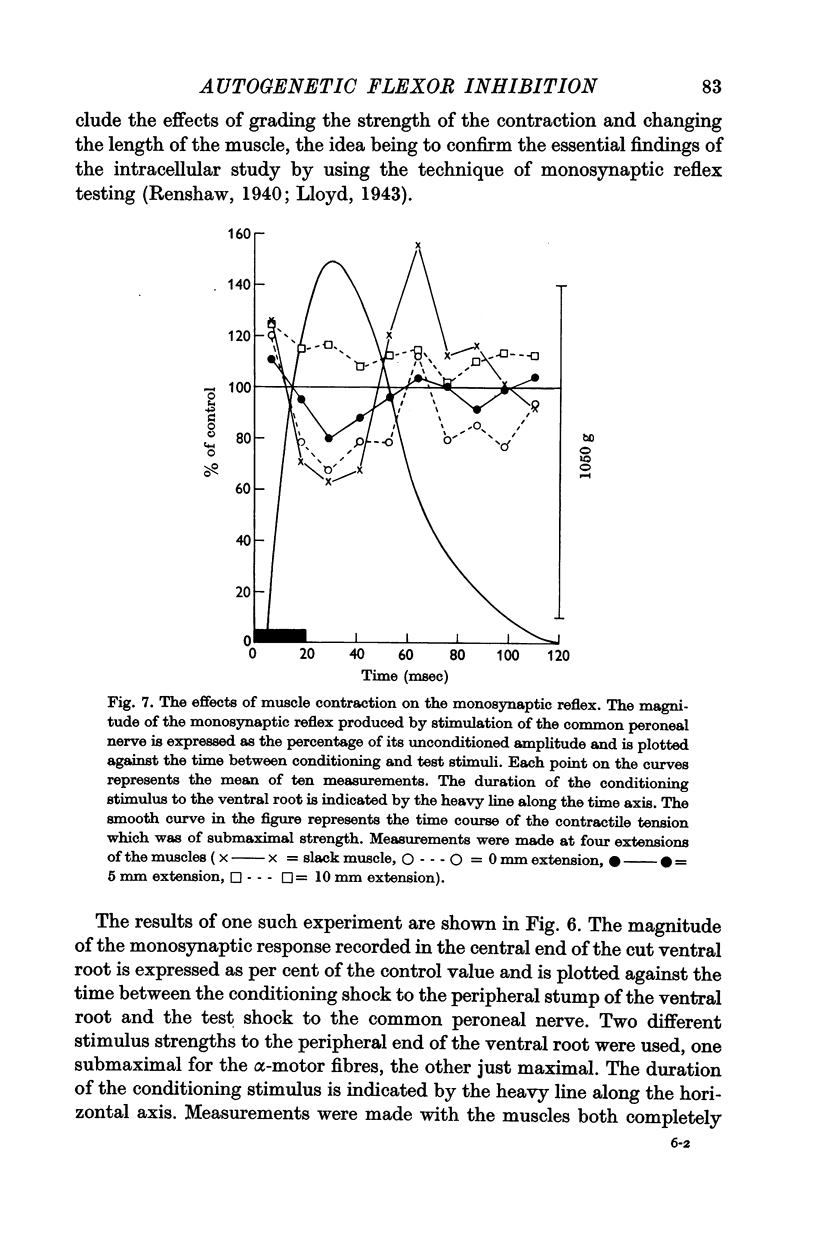
7. Monosynaptic testing of the flexor motoneurone pool has been used to confirm the essential features of the intracellularly recorded activity.
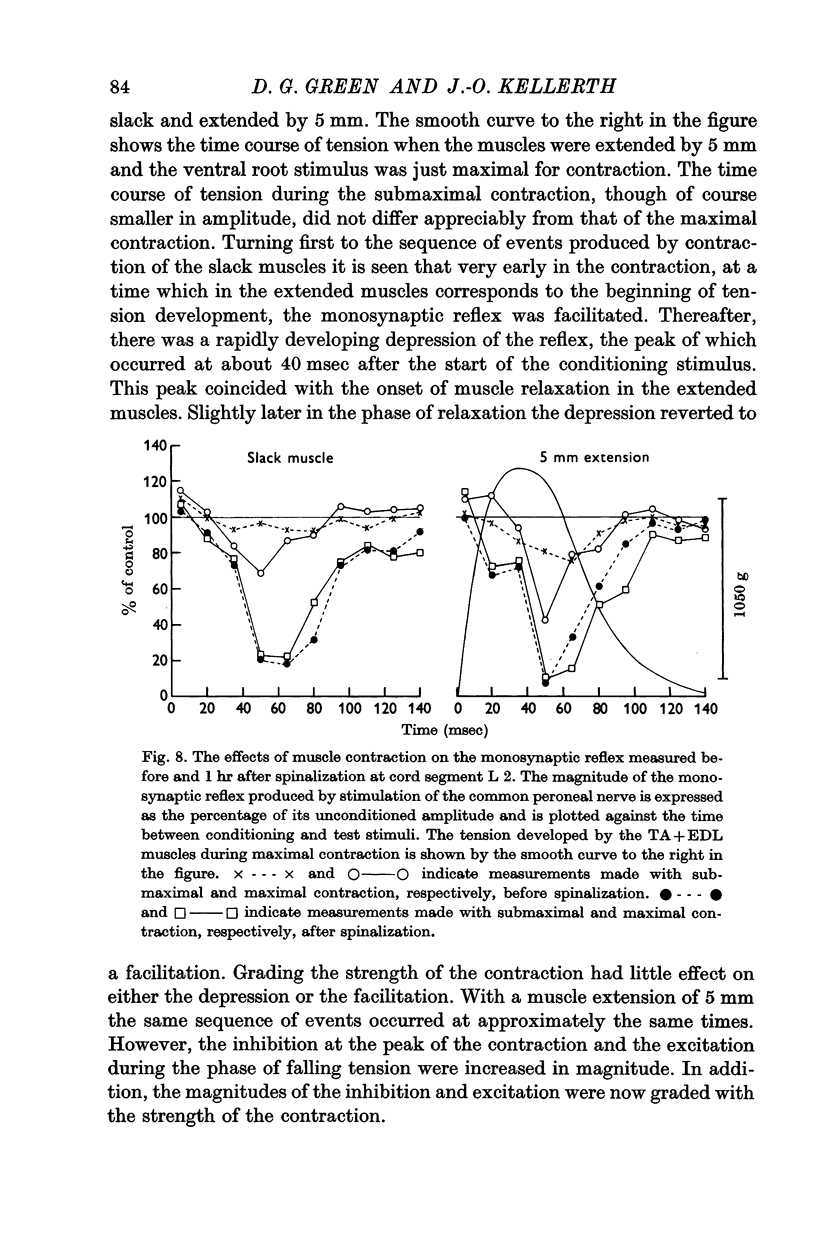
8. Acutely spinalizing the animal increases the magnitude of the inhibitory responses caused by contraction.
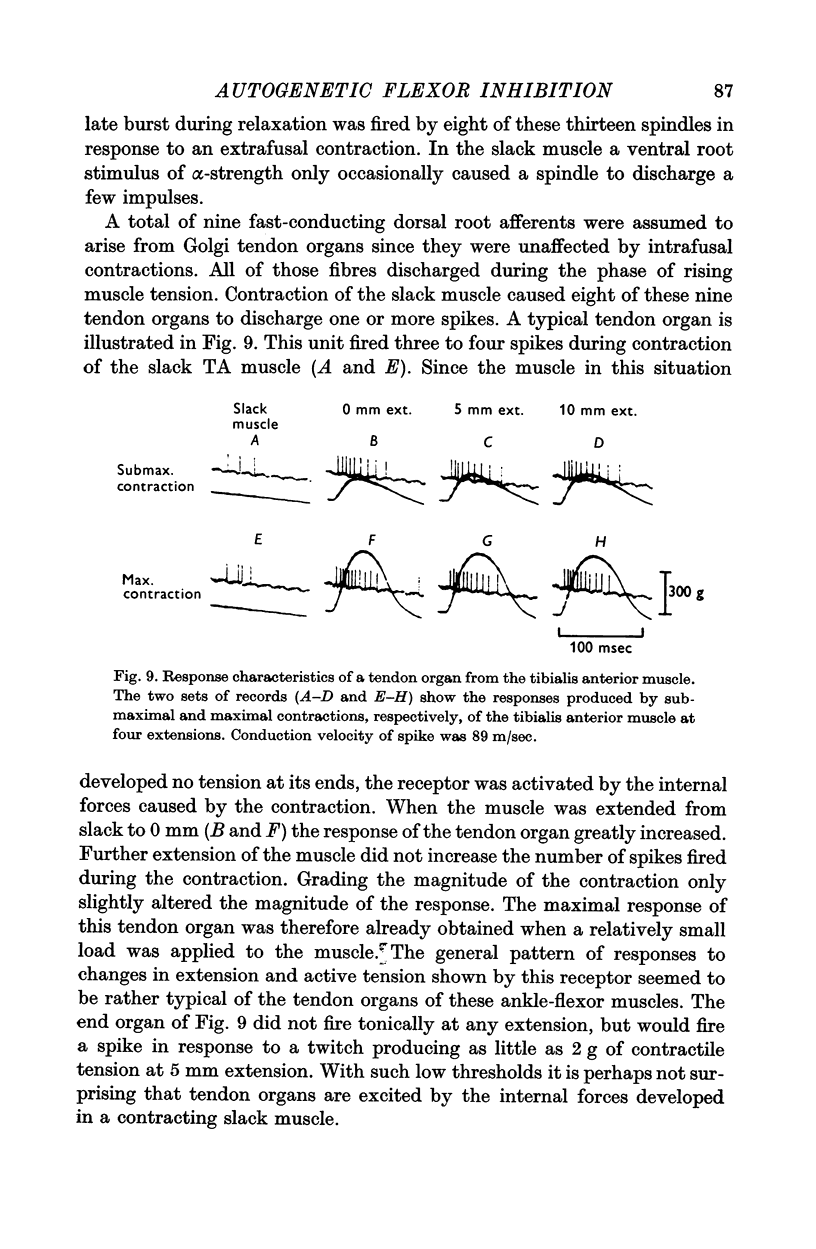
9. Recordings from dorsal root fibres show that Golgi tendon organs of the ankle flexors are very sensitive to contraction and are indeed often activated by the internal forces developed in a contracting slack muscle.
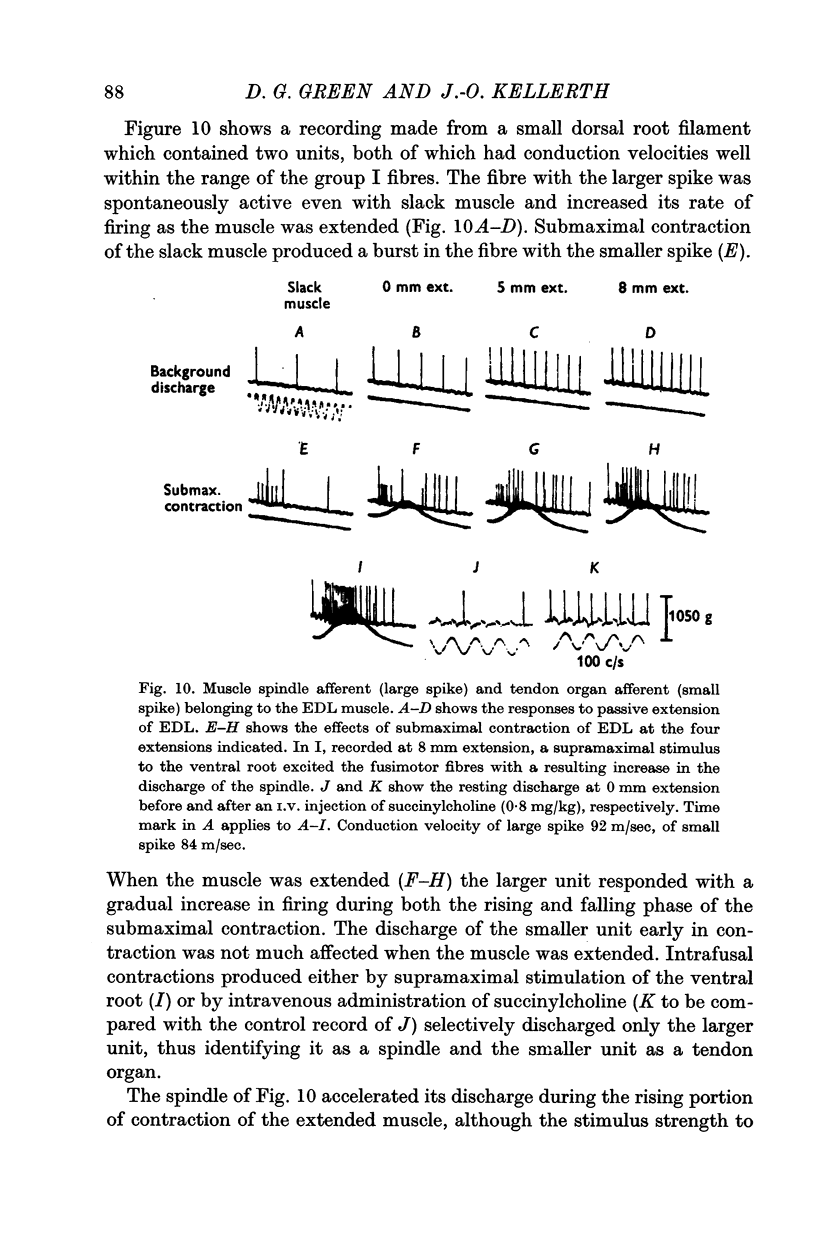
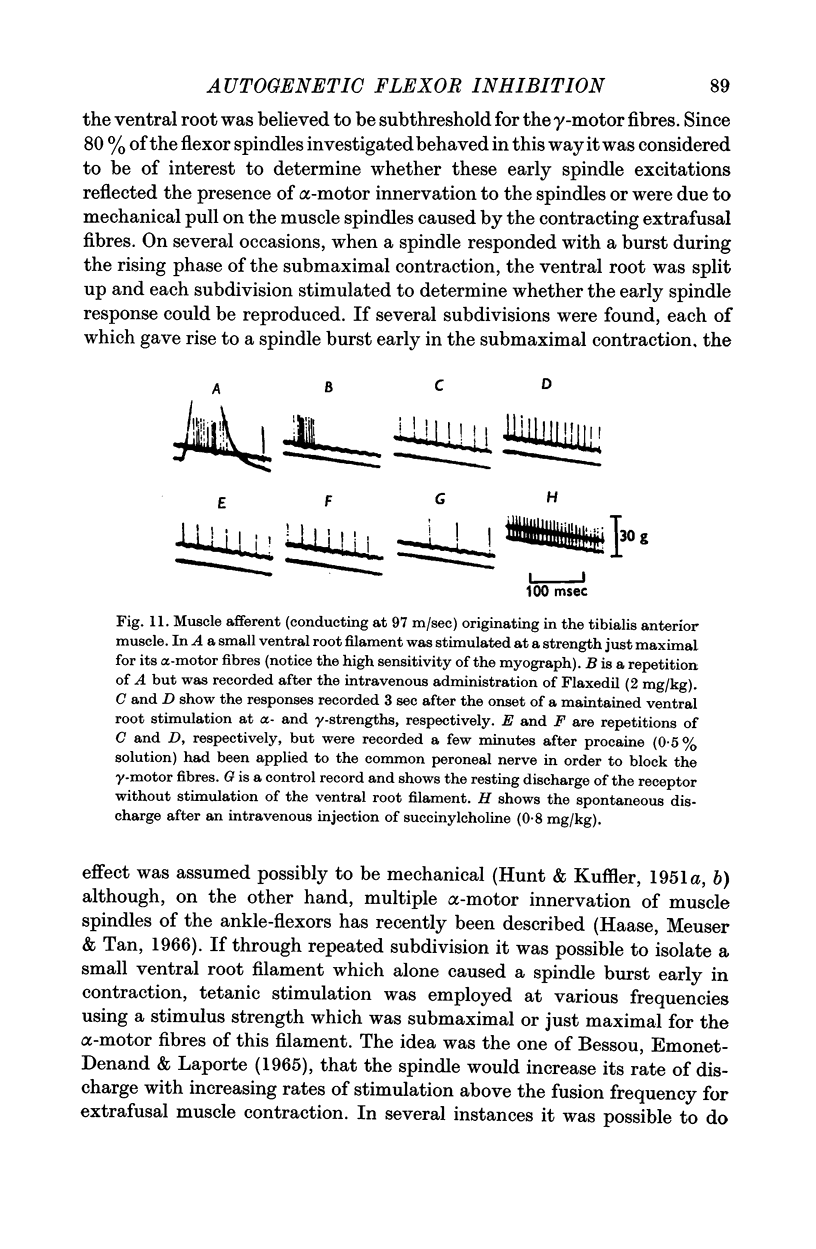
10. A number of muscle spindles of the ankle flexors are activated by stimulation of the ventral root at a strength submaximal or just maximal for the α-motor fibres, despite the simultaneous unloading effect of the contracting extrafusal fibres. This spindle activation, which occurs mainly during the phase of tension development in contraction, is favoured by an increased extension of the muscle. Attempts were made to establish whether it is due to α-motor innervation of the receptors or to some mechanical interaction between the intra- and extrafusal muscle fibres.
11. The possible central and peripheral causes of the changes in motoneurone excitability produced by flexor muscle contraction are discussed. It is suggested that tendon organs of flexor muscles strongly inhibit flexor motoneurones and that α-motor innervation of muscle spindles is likely to play a more prominent role in flexors than in extensor muscles.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., OTANI T. Response of single motoneurons to direct stimulation in toad's spinal cord. J Neurophysiol. 1955 Sep;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- BIANCONI R., GRANIT R., REIS D. J. THE EFFECTS OF EXTENSOR MUSCLE SPINDLES AND TENDON ORGANS ON HOMONYMOUS MOTONEURONES IN RELATION TO GAMMA-BIAS AND CURARIZATION. Acta Physiol Scand. 1964 Aug;61:331–347. [PubMed] [Google Scholar]
- BIANCONI R., GRANIT R., REIS D. J. THE EFFECTS OF FLEXOR MUSCLE SPINDLES AND TENDON ORGANS ON HOMONYMOUS MOTONEURONES IN RELATION TO GAMMA-BIAS AND CURARIZATION. Acta Physiol Scand. 1964 Aug;61:348–356. [PubMed] [Google Scholar]
- Bessou P., Emonet-Dénand F., Laporte Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J Physiol. 1965 Oct;180(3):649–672. doi: 10.1113/jphysiol.1965.sp007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J., ECCLES R. M., ITO M. EFFECTS PRODUCED ON INHIBITORY POSTSYNAPTIC POTENTIALS BY THE COUPLED INJECTIONS OF CATIONS AND ANIONS INTO MOTONEURONS. Proc R Soc Lond B Biol Sci. 1964 May 19;160:197–210. doi: 10.1098/rspb.1964.0036. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HOMMA S., MATTHEWS P. B. Prolonged changes in the discharge of mammalian muscle spindles following tendon taps or muscle twitches. Acta Physiol Scand. 1959 Jun 24;46:185–193. doi: 10.1111/j.1748-1716.1959.tb01747.x. [DOI] [PubMed] [Google Scholar]
- GRANIT R., KELLERTH J. O., WILLIAMS T. D. 'ADJACENT' AND 'REMOTE' POST-SYNAPTIC INHIBITION IN MOTONEURONES STIMULATED BY MUSCLE STRETCH. J Physiol. 1964 Nov;174:453–472. doi: 10.1113/jphysiol.1964.sp007497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., KELLERTH J. O., WILLIAMS T. D. INTRACELLULAR ASPECTS OF STIMULATING MOTONEURONES BY MUSCLE STRETCH. J Physiol. 1964 Nov;174:435–452. doi: 10.1113/jphysiol.1964.sp007496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G., SKOGLUND S., STEG G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957 Sep;20(5):470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- GRANIT R., SKOGLUND S., THESLEFF S. Activation of muscle spindles by succinylcholine and decamethonium, the effects of curare. Acta Physiol Scand. 1953;28(2-3):134–151. doi: 10.1111/j.1748-1716.1953.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Granit B., Kellerth J. O., Szumski A. J. Intracellular recording from extensor motoneurons activated across the gamma loop. J Neurophysiol. 1966 May;29(3):530–544. doi: 10.1152/jn.1966.29.3.530. [DOI] [PubMed] [Google Scholar]
- Granit R., Kellerth J. O., Szumski A. J. Intracellular autogenetic effects of muscular contration on extensor motoneurones. The silent period. J Physiol. 1966 Feb;182(3):484–503. doi: 10.1113/jphysiol.1966.sp007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Further study of efferent small-nerve fibers to mammalian muscle spindles; multiple spindle innervation and activity during contraction. J Physiol. 1951 Apr;113(2-3):283–297. doi: 10.1113/jphysiol.1951.sp004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Stretch receptor discharges during muscle contraction. J Physiol. 1951 Apr;113(2-3):298–315. doi: 10.1113/jphysiol.1951.sp004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The effect of stretch receptors from muscle on the discharge of motorneurons. J Physiol. 1952 Jul;117(3):359–379. doi: 10.1113/jphysiol.1952.sp004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J., Meuser P., Tan U. Die Konvergenz fusimotorischer alpha-Impulse auf de-efferentierte Flexorspindeln der Katze. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;289(1):50–58. [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B., RUSHWORTH G. The relative sensitivity of muscle nerve fibres to procaine. J Physiol. 1957 Feb 15;135(2):263–269. doi: 10.1113/jphysiol.1957.sp005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B., RUSHWORTH G. The selective effect of proCaine on the stretch reflex and tendon jerk of soleus muscle when applied to its nerve. J Physiol. 1957 Feb 15;135(2):245–262. doi: 10.1113/jphysiol.1957.sp005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Participation by pressure-pain receptors of mammalian muscles in the flexion reflex. J Physiol. 1961 May;156:498–514. doi: 10.1113/jphysiol.1961.sp006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara N., Ohye C. Polysynaptic activation of extensor motorneurones from group Ia fibres in the cat spinal cord. Experientia. 1964 Nov 15;20(11):628–629. doi: 10.1007/BF02144828. [DOI] [PubMed] [Google Scholar]
- VERHEY B. A., VOORHOEVE P. E. ACTIVATION OF GROUP IA AND GROUP II MUSCLE SPINDLE AFFERENTS BY SUCCINYLCHOLINE AND OTHER CHOLINERGIC DRUGS. Acta Physiol Pharmacol Neerl. 1963 Apr;12:23–29. [PubMed] [Google Scholar]
- WILSON V. J., KATO M. EXCITATION OF EXTENSOR MOTONEURONS BY GROUP II AFFERENT FIBERS IN IPSILATERAL MUSCLE NERVES. J Neurophysiol. 1965 May;28:545–554. doi: 10.1152/jn.1965.28.3.545. [DOI] [PubMed] [Google Scholar]


