Abstract
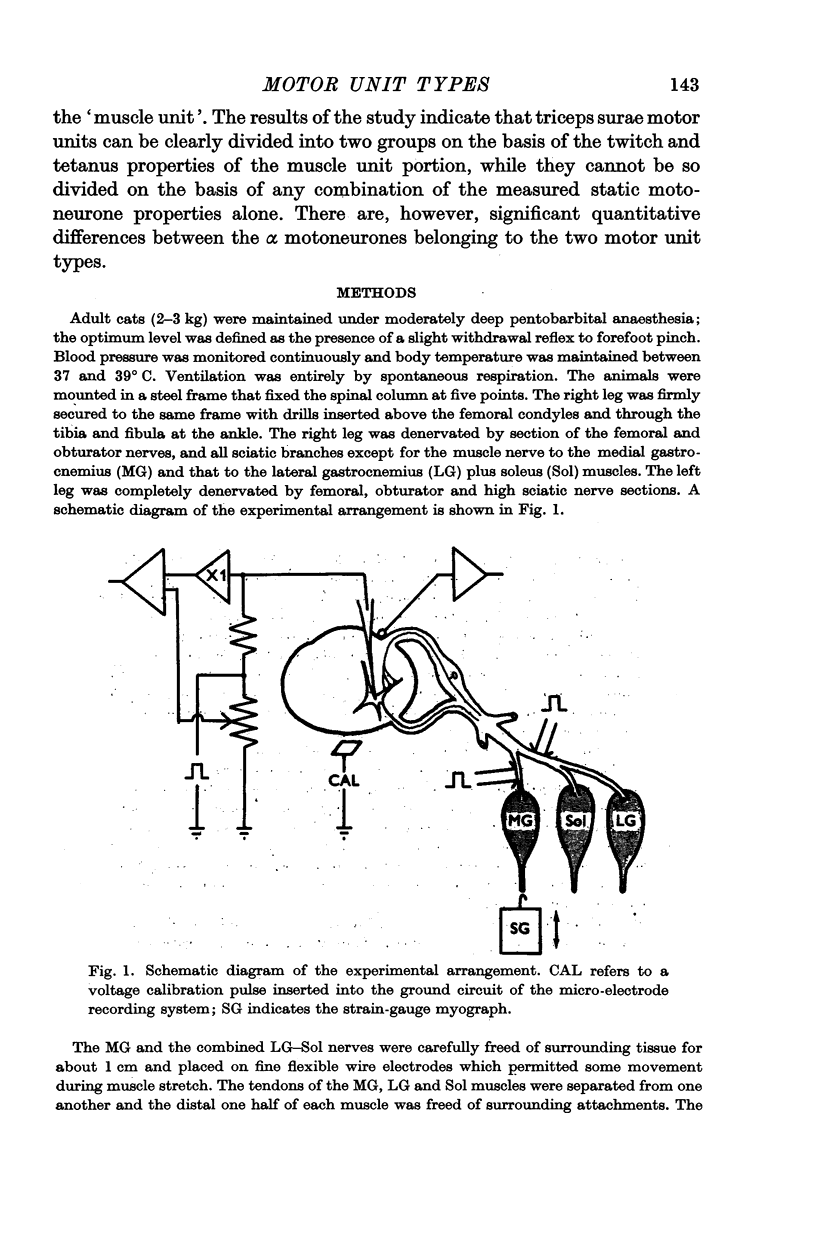
1. Motor units, defined as including a motoneurone (cell body, dendrites and axon) plus the muscle unit innervated, have been examined in the triceps surae motor pool of pentobarbital anaesthetized cats.
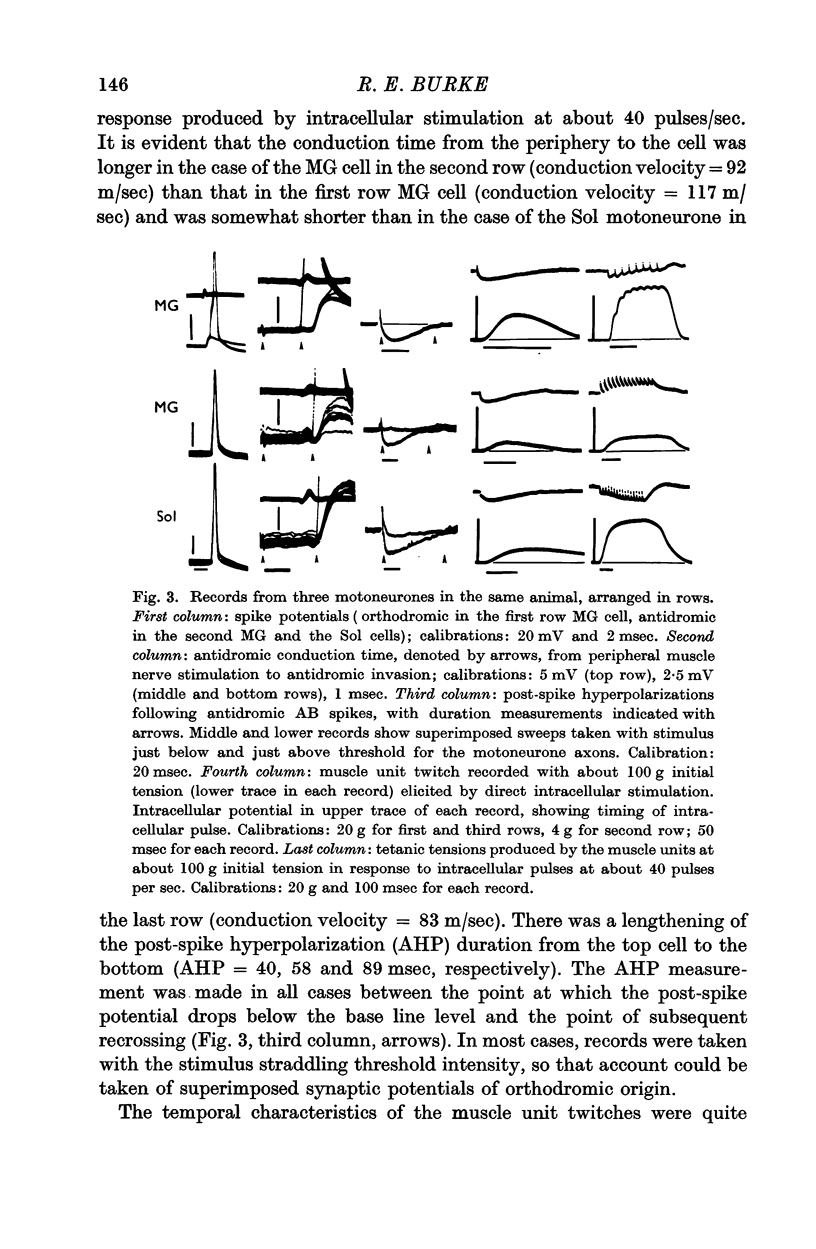
2. The technique of intracellular stimulation and recording which was used permitted measurement of the axonal conduction velocity, post-spike hyperpolarization duration and input resistance of individual motoneurones, and the correlation of these properties with the characteristics of the twitch and tetanus responses of the muscle unit innervated by the cell elicited by direct intracellular stimulation.
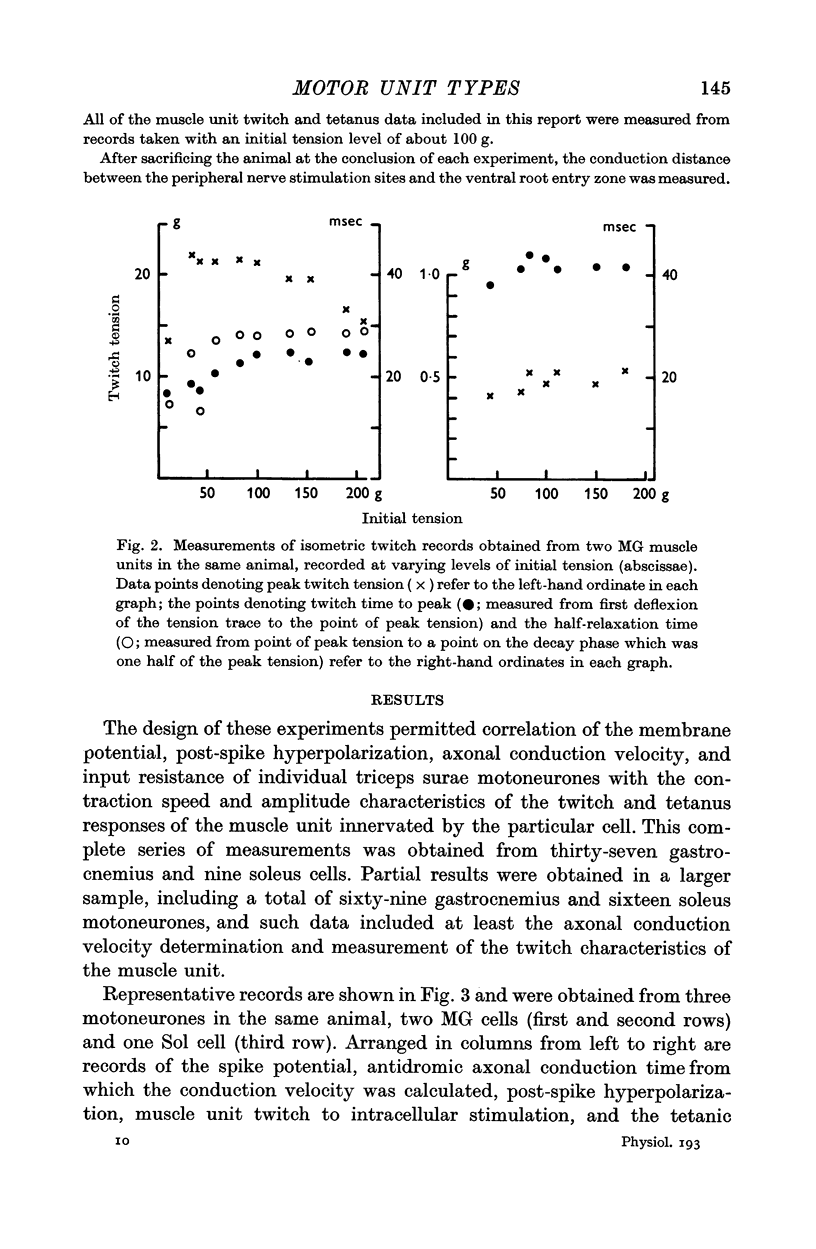
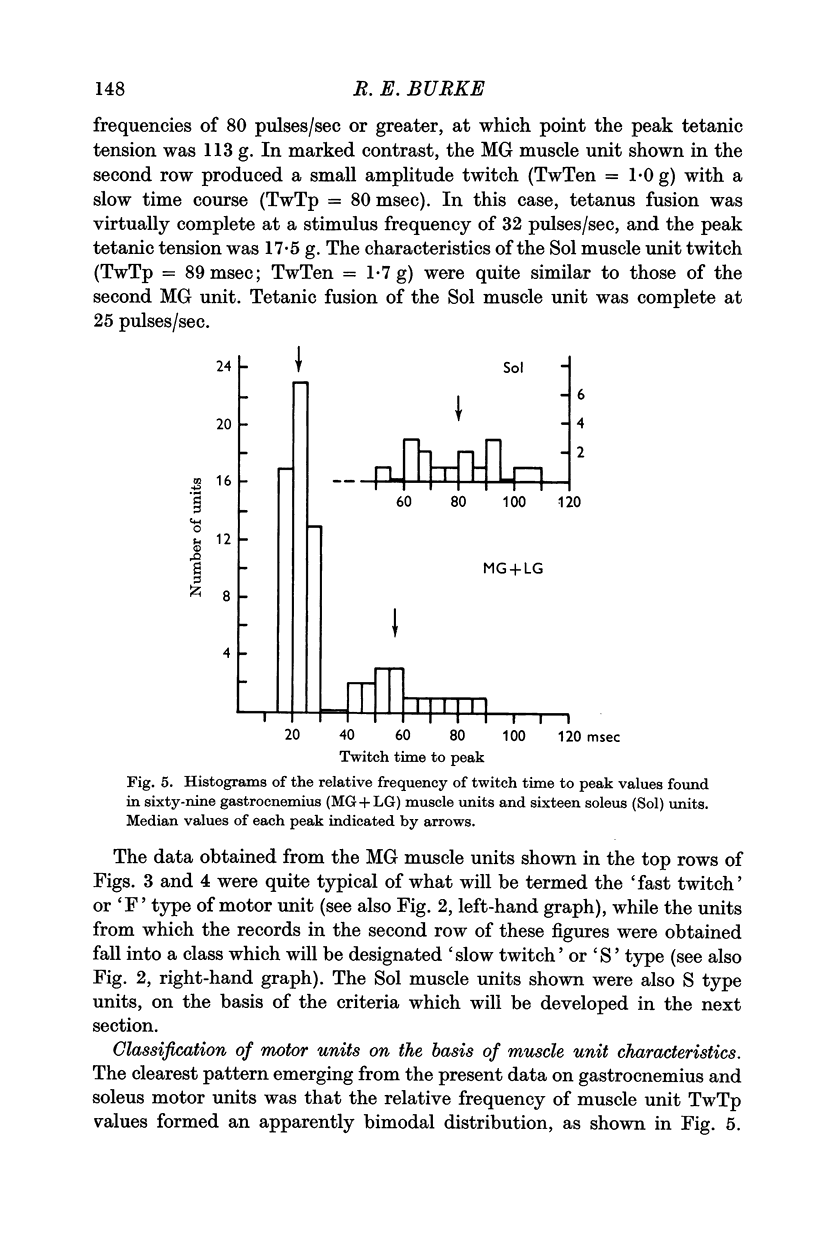
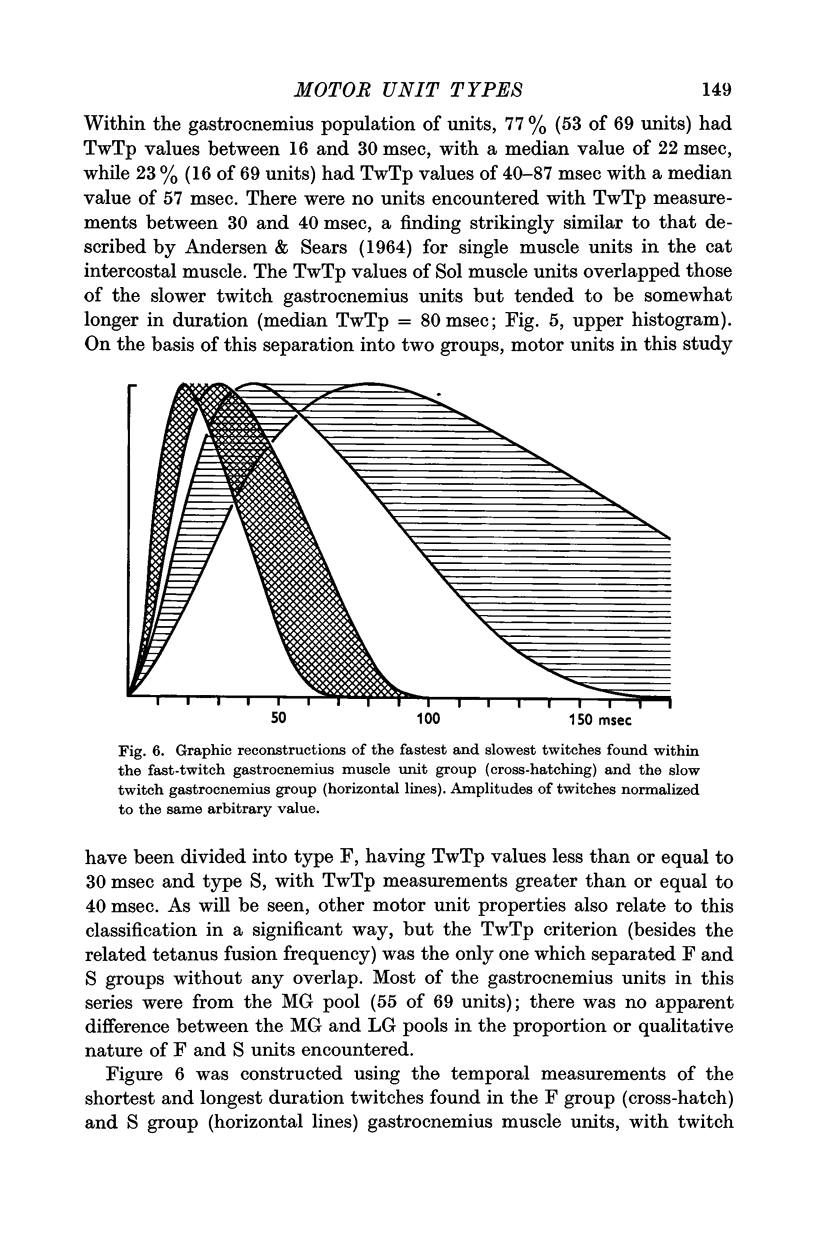
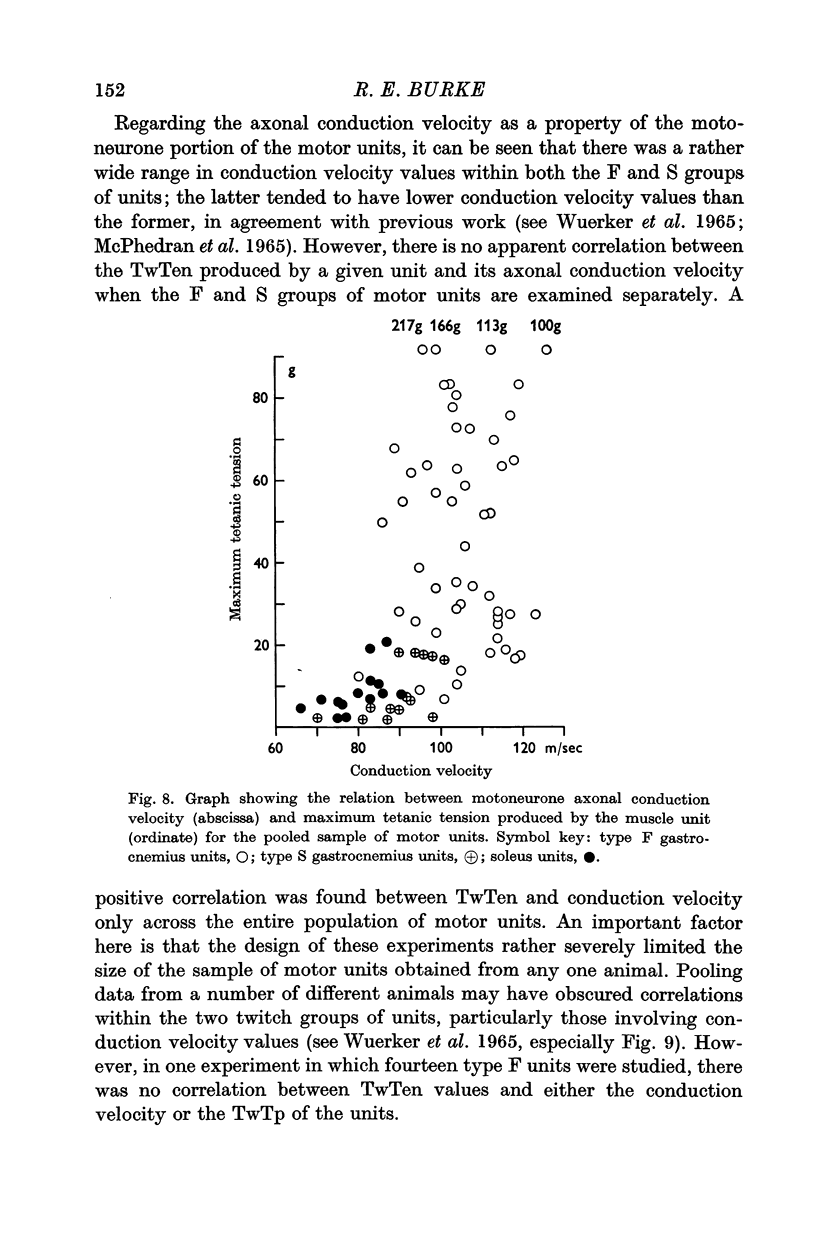
3. On the basis of muscle unit speed of contraction, motor units were divided into two groups: (a) fast twitch, or F, type with twitch time to peak (TwTp) less than or equal to 30 msec, and (b) slow twitch, or S, type with TwTp of 40 msec or greater. The twitch tensions (TwTen) produced by type F units were significantly larger (median value = 18 g) than the tensions generated by type S units (TwTen median value = 1·6 g). Type F muscle units had much higher tetanus fusion frequencies (median = 85 pulses/sec) than the S type (median 25 pulses/sec), and tended to have smaller tetanus to twitch tension ratios (Tet/Tw) (median = 2·6) than type S units (median = 5·4).
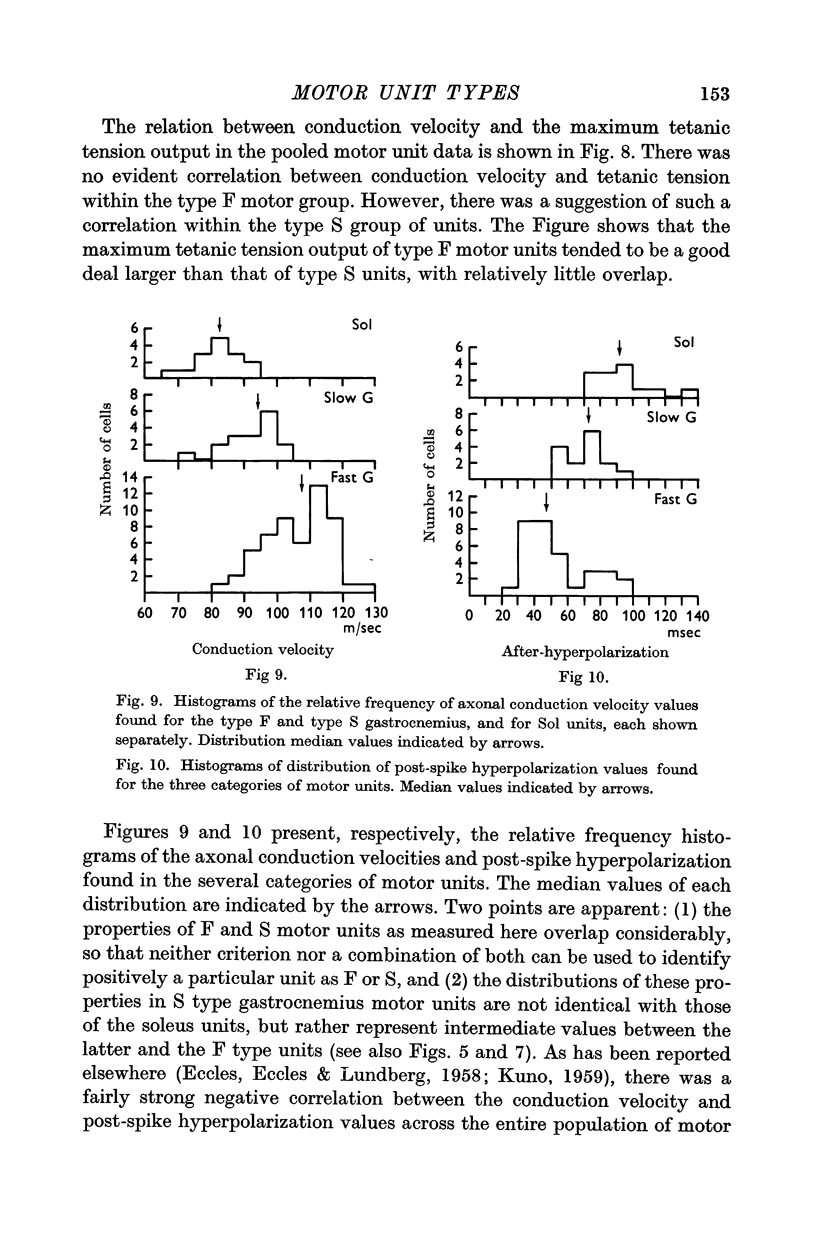
4. The gastrocnemius heads contained a mixture of F and S types of muscle units, the proportions found being about 3 to 1 respectively. Units encountered in the soleus muscle were uniformly of type S. The characteristics of gastrocnemius and soleus type S motor units were not identical but appeared to represent quantitative differences in units of the same qualitative type.
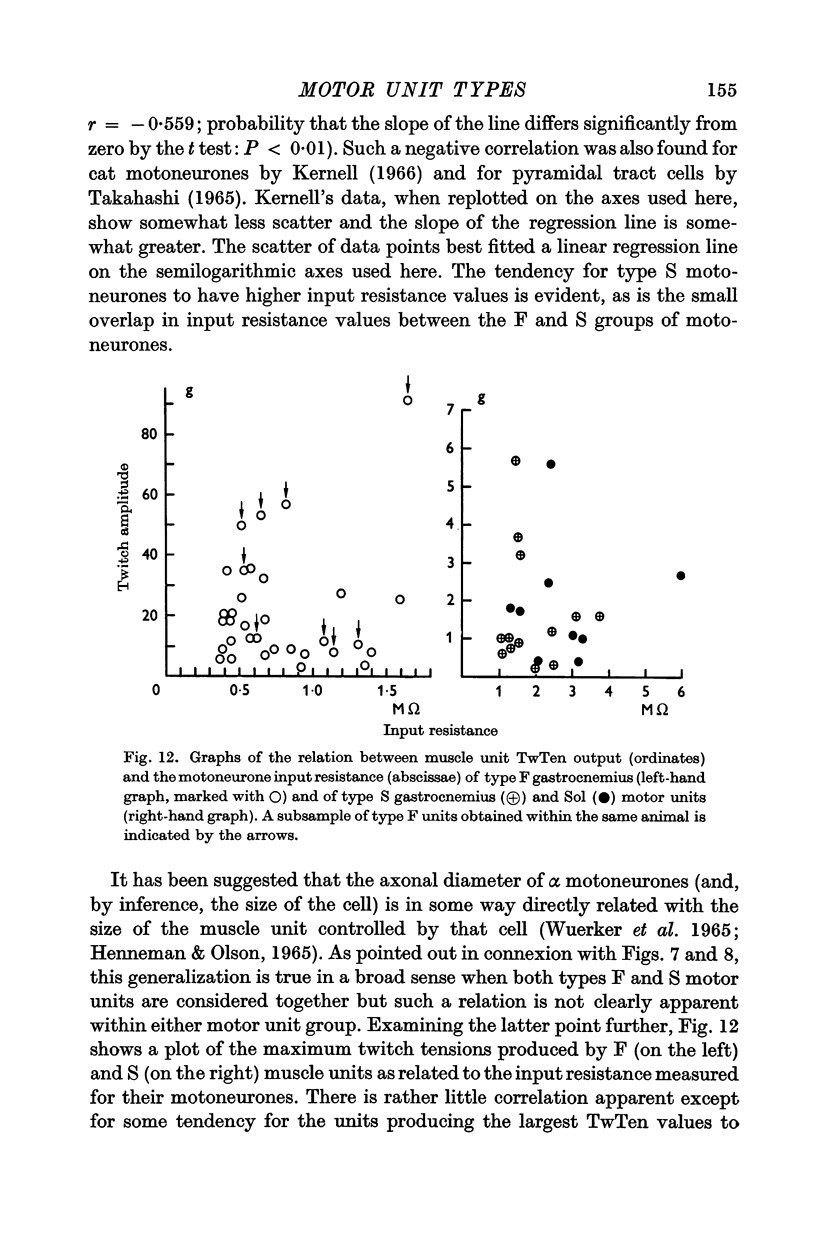
5. Motoneurones innervating type F muscle units had faster axonal conduction velocities, shorter post-spike hyperpolarizations and lower input resistances than those supplying type S units. However, no combination of motoneurone properties alone was sufficient to separate unambiguously types F and S motor units.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., SEARS T. A. THE MECHANICAL PROPERTIES AND INNERVATION OF FAST AND SLOW MOTOR UNITS IN THE INTERCOSTAL MUSCLES OF THE CAT. J Physiol. 1964 Sep;173:114–129. doi: 10.1113/jphysiol.1964.sp007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., ECCLES J. C., ECCLES R. M. Differentiation of fast and slow muscles in the cat hind limb. J Physiol. 1960 Feb;150:399–416. doi: 10.1113/jphysiol.1960.sp006394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., LEWIS D. M. FURTHER OBSERVATIONS ON MAMMALIAN CROSS-INNERVATED SKELETAL MUSCLE. J Physiol. 1965 May;178:343–358. doi: 10.1113/jphysiol.1965.sp007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The electrical constants of the motoneurone membrane. J Physiol. 1959 Mar 12;145(3):505–528. doi: 10.1113/jphysiol.1959.sp006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Effects of cross-union of motor nerves to fast and slow skeletal muscles. Nature. 1965 May 22;206(4986):831–832. doi: 10.1038/206831a0. [DOI] [PubMed] [Google Scholar]
- DEVANANDAN M. S., ECCLES R. M., WESTERMAN R. A. SINGLE MOTOR UNITS OF MAMMALIAN MUSCLE. J Physiol. 1965 May;178:359–367. doi: 10.1113/jphysiol.1965.sp007632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., HOLBOURN A. H. S. The mechanical activity of single motor units in reflex contractions of skeletal muscle. J Physiol. 1949 Dec 15;110(1-2):26–35. doi: 10.1113/jphysiol.1949.sp004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., PHILLIPS C. G. Slow and rapid components in a flexor muscle. Q J Exp Physiol Cogn Med Sci. 1953;38(1):35–45. doi: 10.1113/expphysiol.1953.sp001005. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D., STEG G. Tonic and phasic ventral horn cells differentiated by post-tetanic potentiation in cat extensors. Acta Physiol Scand. 1956 Sep 26;37(2-3):114–126. doi: 10.1111/j.1748-1716.1956.tb01347.x. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G., SKOGLUND S., STEG G. Differentiation of tonic from phasic alpha ventral horn cells by stretch, pinna and crossed extensor reflexes. J Neurophysiol. 1957 Sep;20(5):470–481. doi: 10.1152/jn.1957.20.5.470. [DOI] [PubMed] [Google Scholar]
- HENATSCH H. D., SCHULTE F. J., BUSCH G. [Modification of the tonic-phasic reaction type of various extensor motor neurons by variation of their inflow]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1959;270:161–173. [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D. Input resistance, electrical excitability, and size of ventral horn cells in cat spinal cord. Science. 1966 Jun 17;152(3729):1637–1640. doi: 10.1126/science.152.3729.1637. [DOI] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HETEROGENEOUS PALE MUSCLE (M. GASTROCNEMIUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:85–99. doi: 10.1152/jn.1965.28.1.85. [DOI] [PubMed] [Google Scholar]
- MCPHEDRAN A. M., WUERKER R. B., HENNEMAN E. PROPERTIES OF MOTOR UNITS IN A HOMOGENEOUS RED MUSCLE (SOLEUS) OF THE CAT. J Neurophysiol. 1965 Jan;28:71–84. doi: 10.1152/jn.1965.28.1.71. [DOI] [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- SASAKI K., OTANI T. Accommodation in spinal motoneurons of the cat. Jpn J Physiol. 1961 Aug 15;11:443–456. doi: 10.2170/jjphysiol.11.443. [DOI] [PubMed] [Google Scholar]
- SASAKI K., TANAKA T. PHASIC AND TONIC INNERVATION OF SPINAL ALPHA MOTONEURONS FROM UPPER BRAIN CENTERS. Jpn J Physiol. 1964 Feb 15;14:56–66. doi: 10.2170/jjphysiol.14.56. [DOI] [PubMed] [Google Scholar]
- STEIN J. M., PADYKULA H. A. Histochemical classification of individual skeletal muscle fibers of the rat. Am J Anat. 1962 Mar;110:103–123. doi: 10.1002/aja.1001100203. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Slow and fast groups of pyramidal tract cells and their respective membrane properties. J Neurophysiol. 1965 Sep;28(5):908–924. doi: 10.1152/jn.1965.28.5.908. [DOI] [PubMed] [Google Scholar]
- Ushiyama J., Koizumi K., Brooks C. M. Accommodative reactions of neuronal elements in the spinal cord. J Neurophysiol. 1966 Nov;29(6):1028–1045. doi: 10.1152/jn.1966.29.6.1028. [DOI] [PubMed] [Google Scholar]


