Abstract
1. Insulin secretion from pieces of rabbit pancreas incubated in vitro was studied in media of different ionic composition and in response to different substances added to the media.
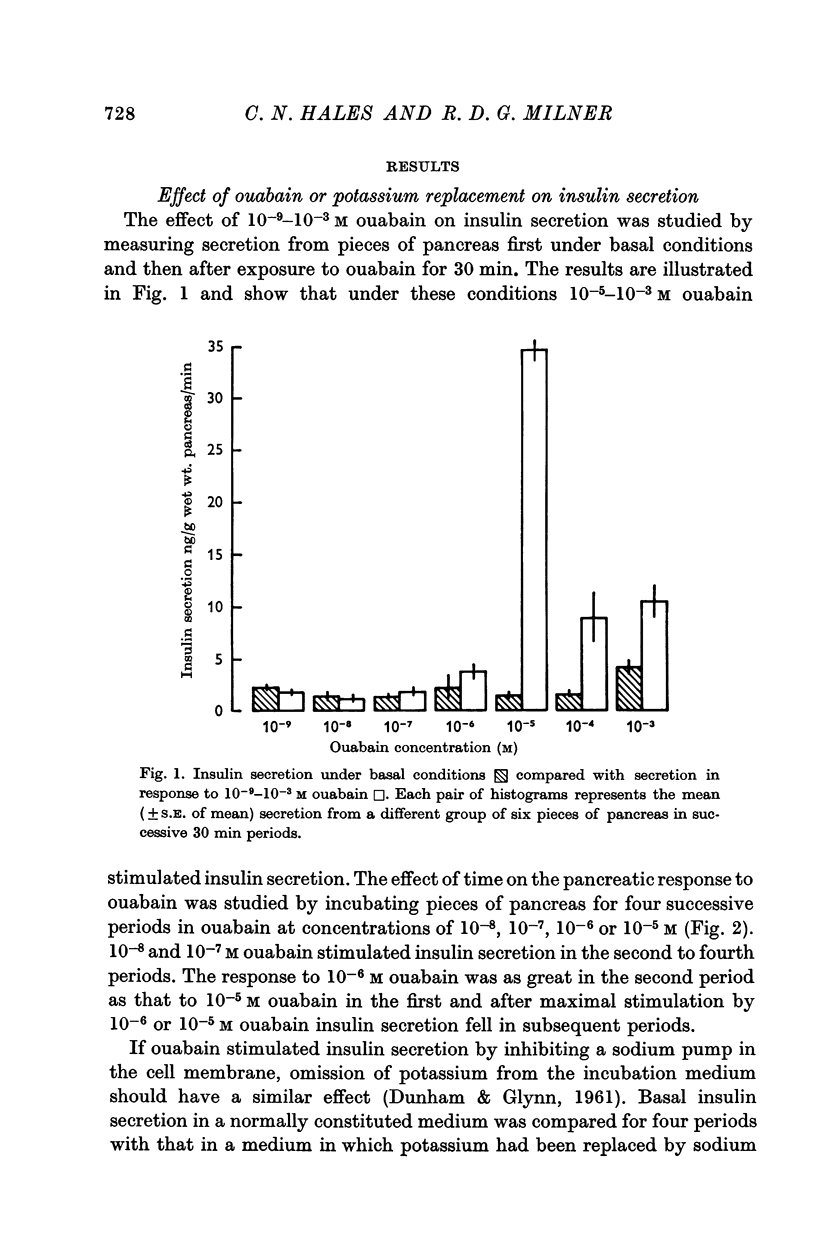
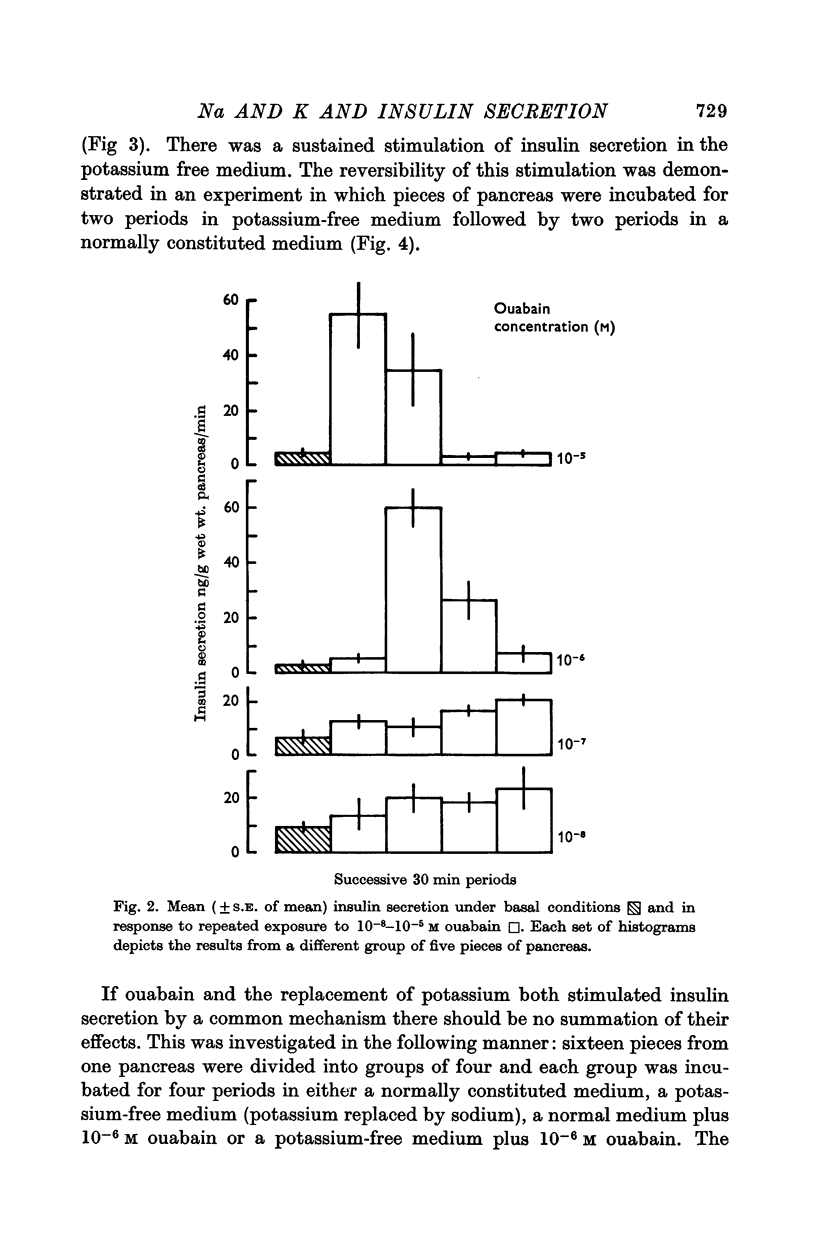
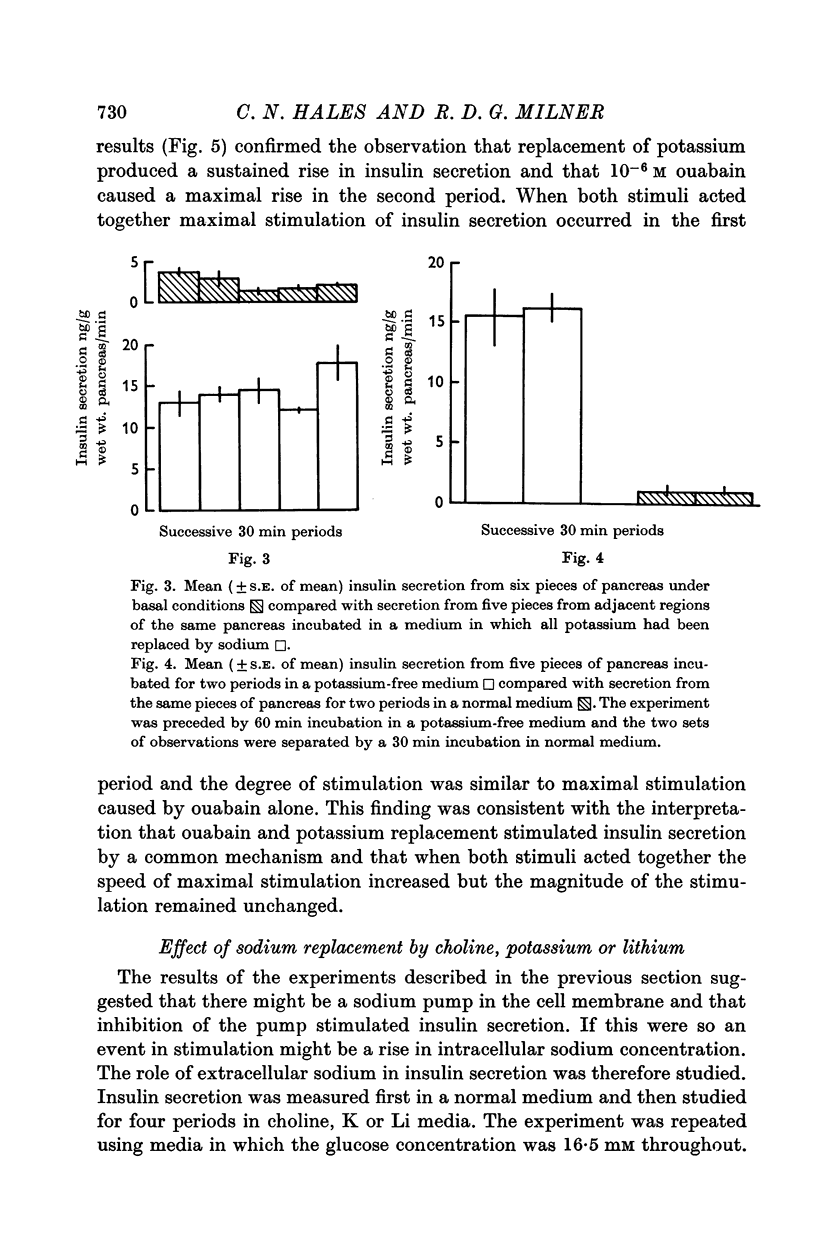
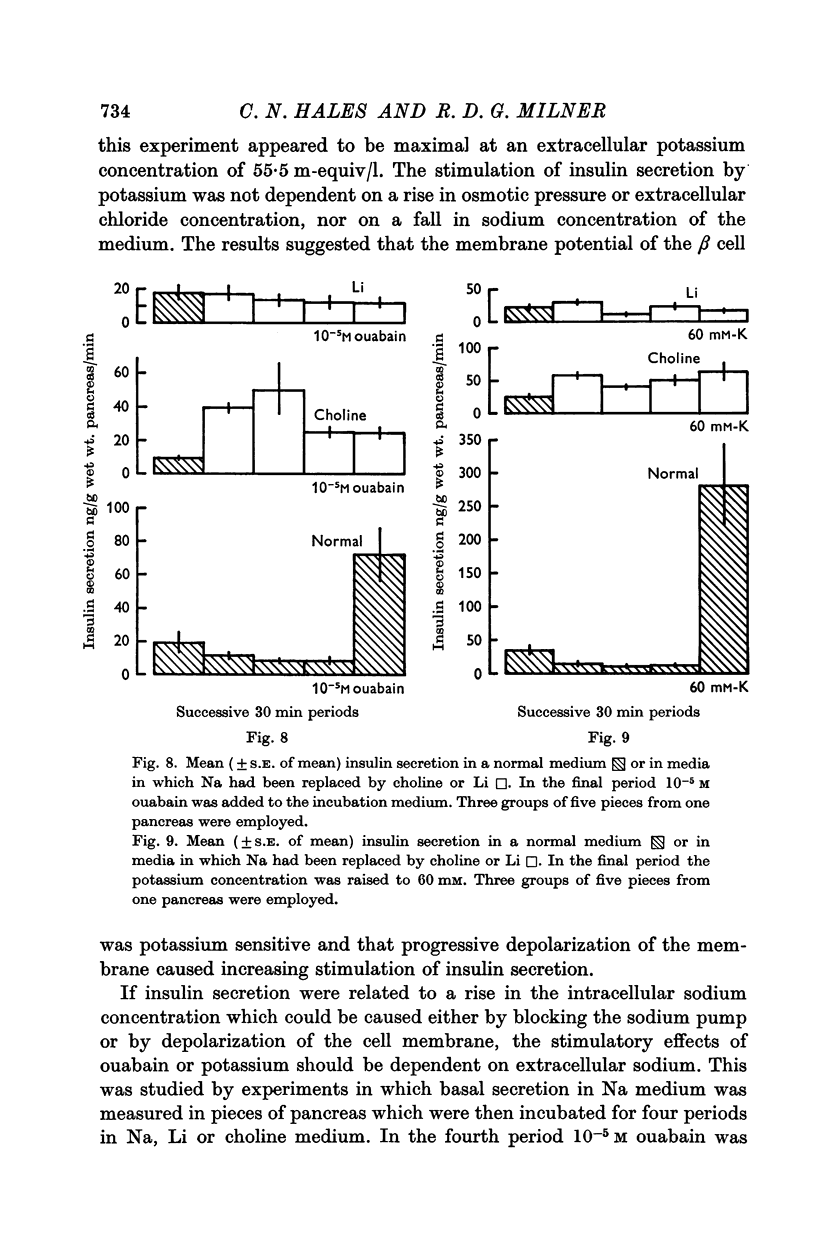
2. Experiments were performed which demonstrated that a sodium pump played a role in insulin secretion and that inhibition of the pump by ouabain, or by the omission of extracellular potassium, stimulated insulin secretion.
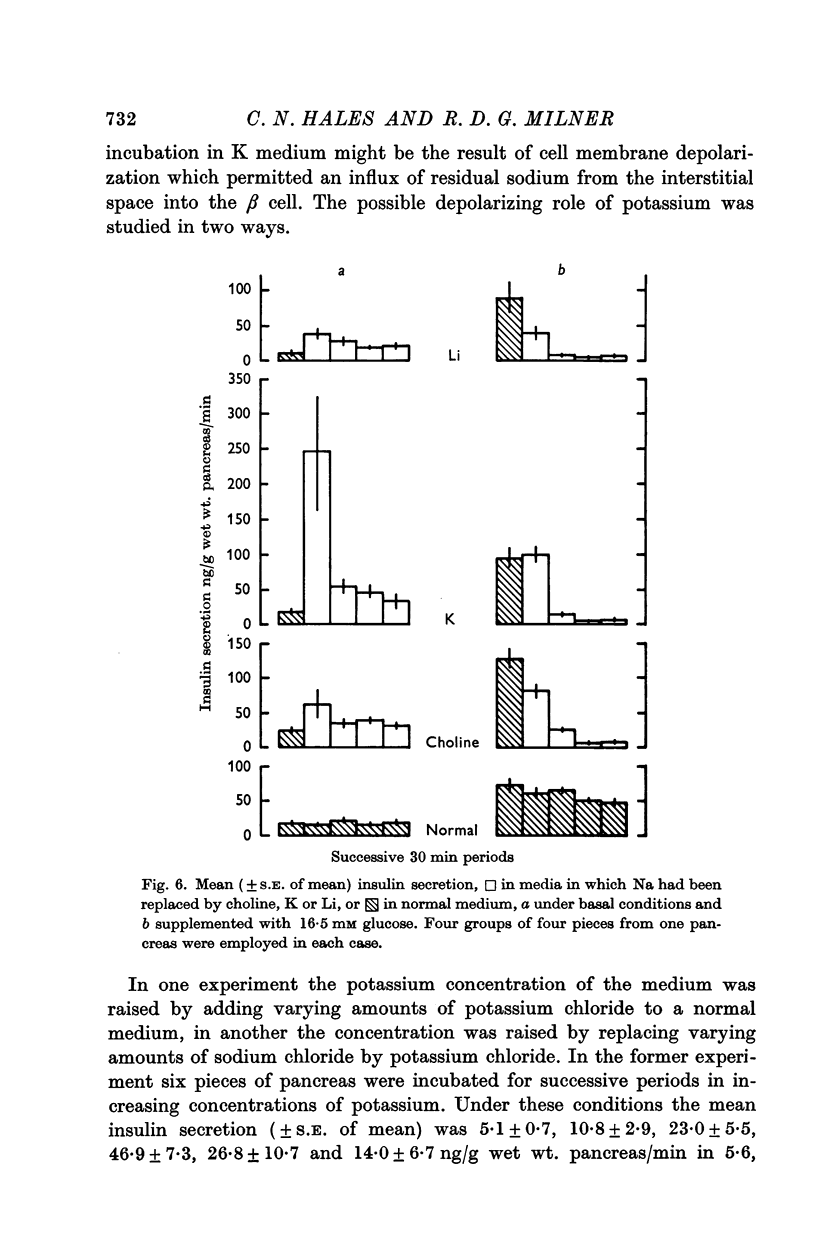
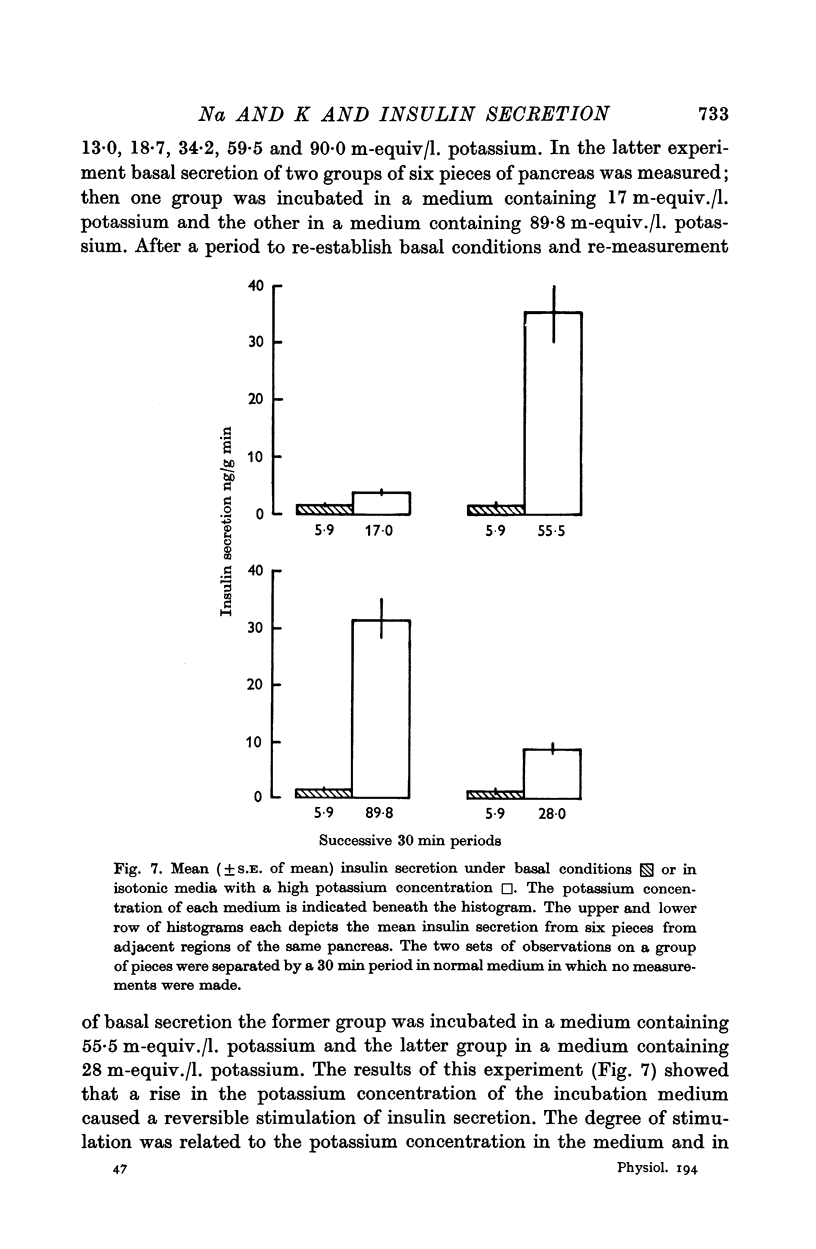
3. A rise in extracellular potassium concentration stimulated insulin secretion independently of changes in the osmolarity or sodium or chloride concentration of the incubation medium.
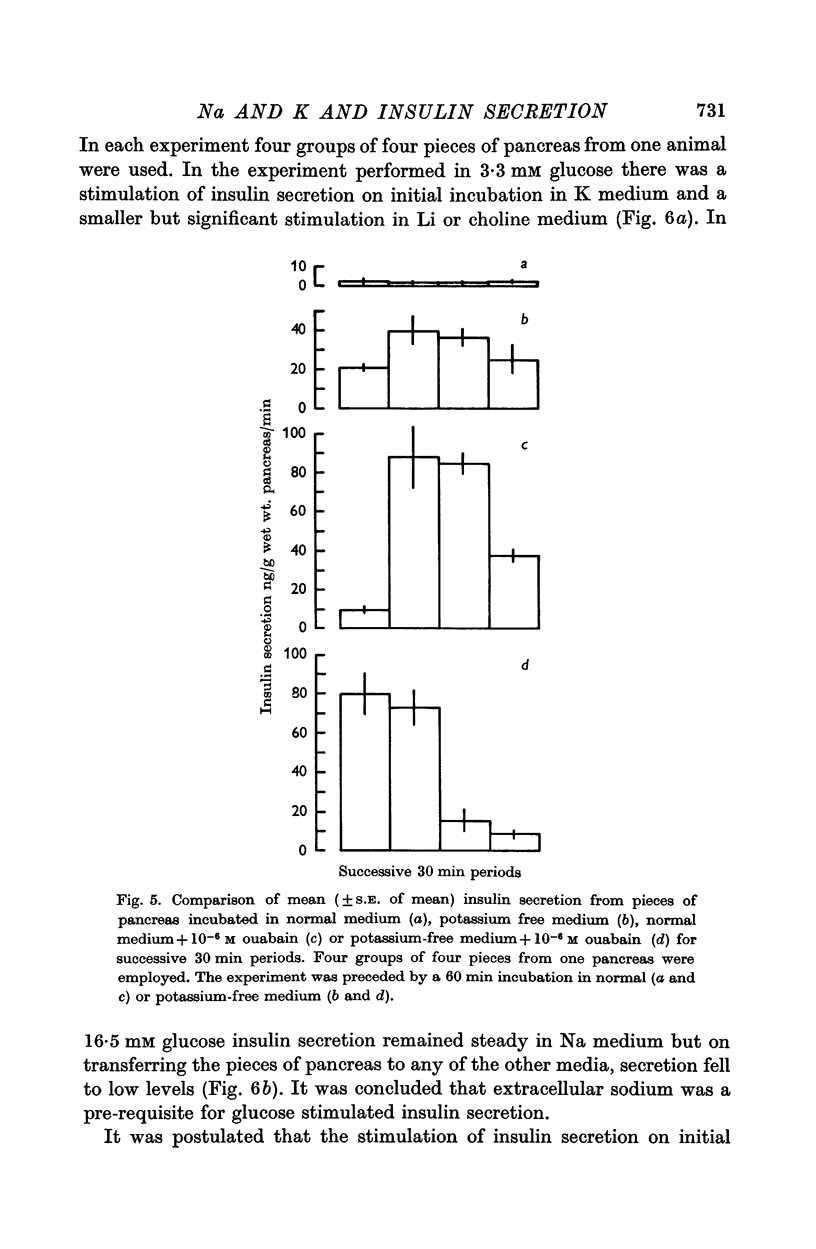
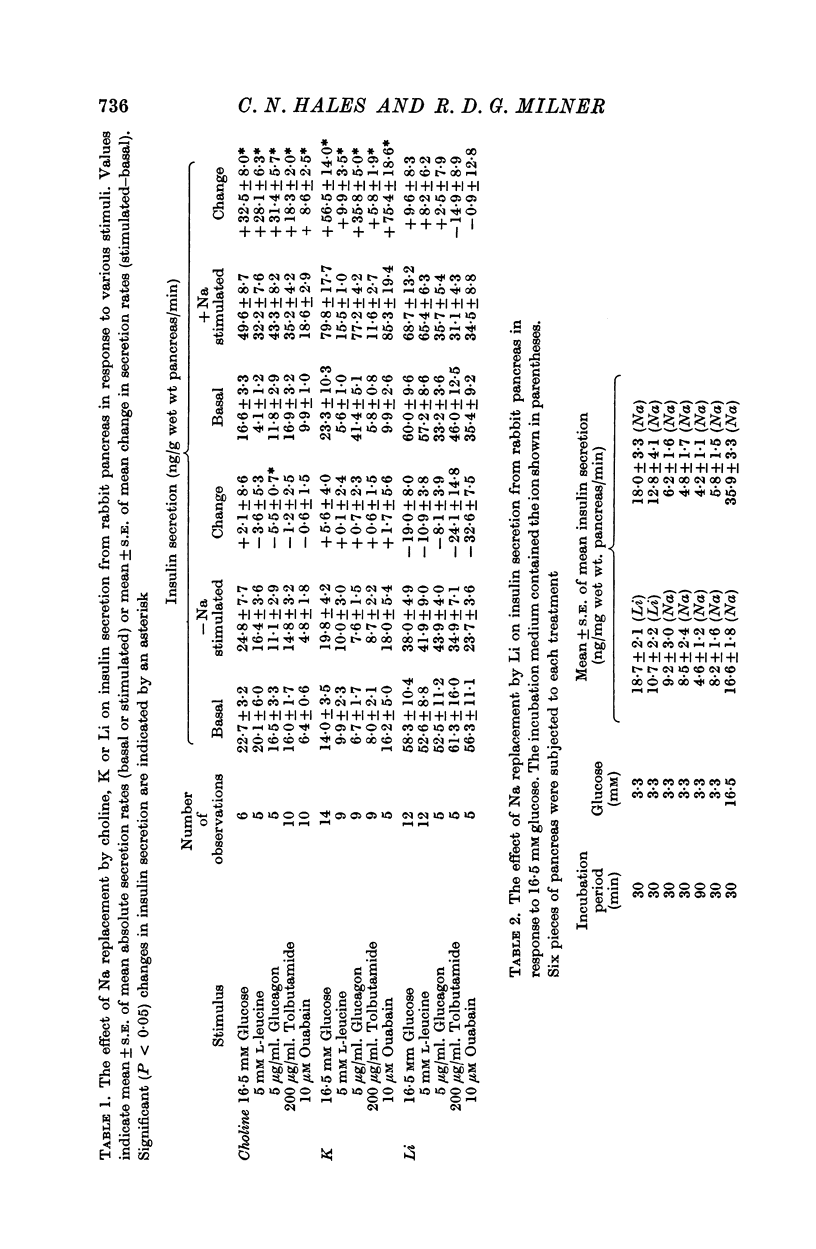
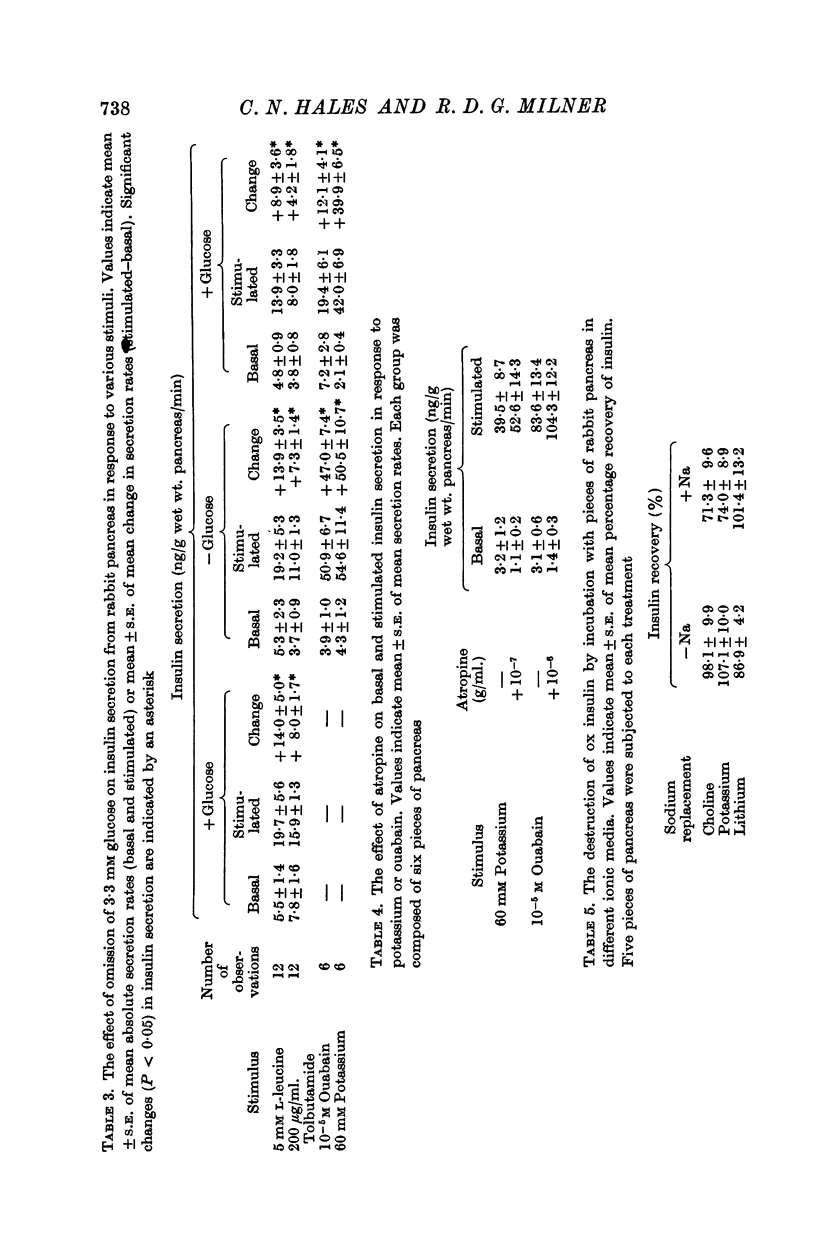
4. The role of extracellular sodium in insulin secretion was investigated. Extracellular sodium was a pre-requisite for insulin secretion stimulated by glucose, glucagon, L-leucine, tolbutamide, potassium or ouabain.
5. The presence of 3·3 mM glucose in the incubation medium was not essential for the stimulation of insulin secretion by L-leucine, tolbutamide or ouabain. Glucagon did not stimulate insulin secretion in the presence of 3·3 mM glucose but did so in the presence of 16·5 mM glucose.
6. The results obtained in these experiments suggested that a transmembrane sodium flux probably in the β cell was a fundamental event in the stimulation of insulin secretion by diverse stimuli.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COUPLAND R. E. The innervation of pan creas of the rat, cat and rabbit as revealed by the cholinesterase technique. J Anat. 1958 Jan;92(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Randle P. J. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J. 1964 Oct;93(1):66–78. doi: 10.1042/bj0930066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., STARK L. The effect of calcium ions on the motor end-plate potentials. J Physiol. 1952 Apr;116(4):507–515. doi: 10.1113/jphysiol.1952.sp004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., BATTS A. A., BENNETT L. L., VCELLA C., MCWILLIAMS N. B., SMITH D. F. EFFECTS OF CARBOHYDRATES ON SECRETION OF INSULIN FROM ISOLATED RAT PANCREAS. Am J Physiol. 1963 Oct;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. M., Macintosh F. C. Calcium and synaptic transmission in a sympathetic ganglion. J Physiol. 1940 Jan 15;97(3):408–416. doi: 10.1113/jphysiol.1940.sp003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E. The pancreatic beta cell. Structure and function. N Engl J Med. 1967 Jan 26;276(4):187–195. doi: 10.1056/NEJM196701262760401. [DOI] [PubMed] [Google Scholar]
- MEHNERT H., SCHAEFER G., KALIAMPETSOS G., STUHLFAUTH K., ENGELHARDT W. [Insulin secretion of the pancreas in extracorporeal perfusion. II. Perfusions of the pancreas with periston, glucose, carbutamide and biguanides]. Klin Wochenschr. 1962 Nov 15;40:1146–1151. doi: 10.1007/BF01484901. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H. A new method for the measurement in vitro of pancreatic insulin secretion. Endocrinology. 1967 Jan;80(1):99–108. doi: 10.1210/endo-80-1-99. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H., Ashmore J. Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology. 1967 May;80(5):975–978. doi: 10.1210/endo-80-5-975. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia. 1967 Mar;3(1):47–49. doi: 10.1007/BF01269910. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The sodium pump and insulin secretion. Biochim Biophys Acta. 1967 May 2;135(2):375–377. doi: 10.1016/0005-2736(67)90136-8. [DOI] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Turner D. S., McIntyre N. Stimulation by glucagon of insulin release from rabbit pancreas in vitro. Lancet. 1966 Feb 12;1(7433):351–352. doi: 10.1016/s0140-6736(66)91327-4. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin A. M., Wieneke A. A. The participation of calcium, adenosine triphosphate and adenosine triphosphatase in the extrusion of the granule proteins from the polymorphonuclear leucocyte. Biochem J. 1964 Mar;90(3):498–509. doi: 10.1042/bj0900498. [DOI] [PMC free article] [PubMed] [Google Scholar]


