Abstract
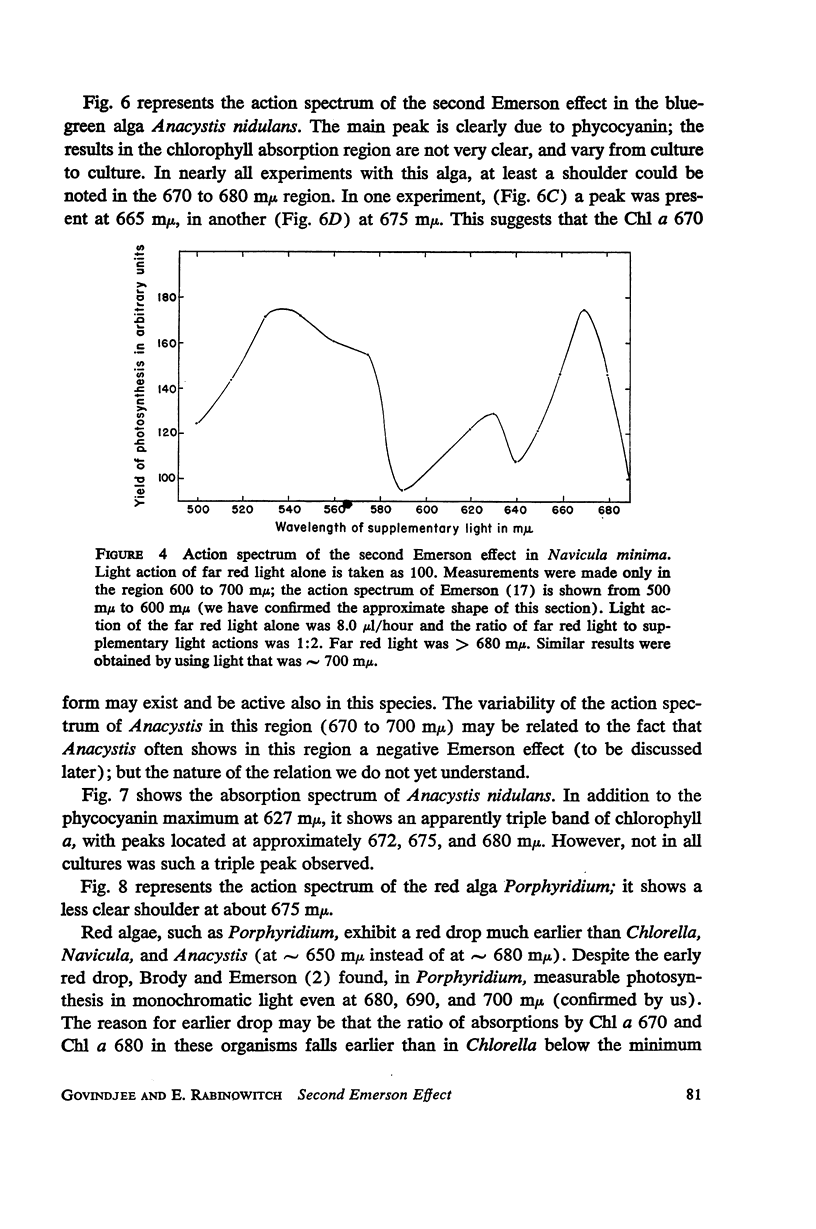
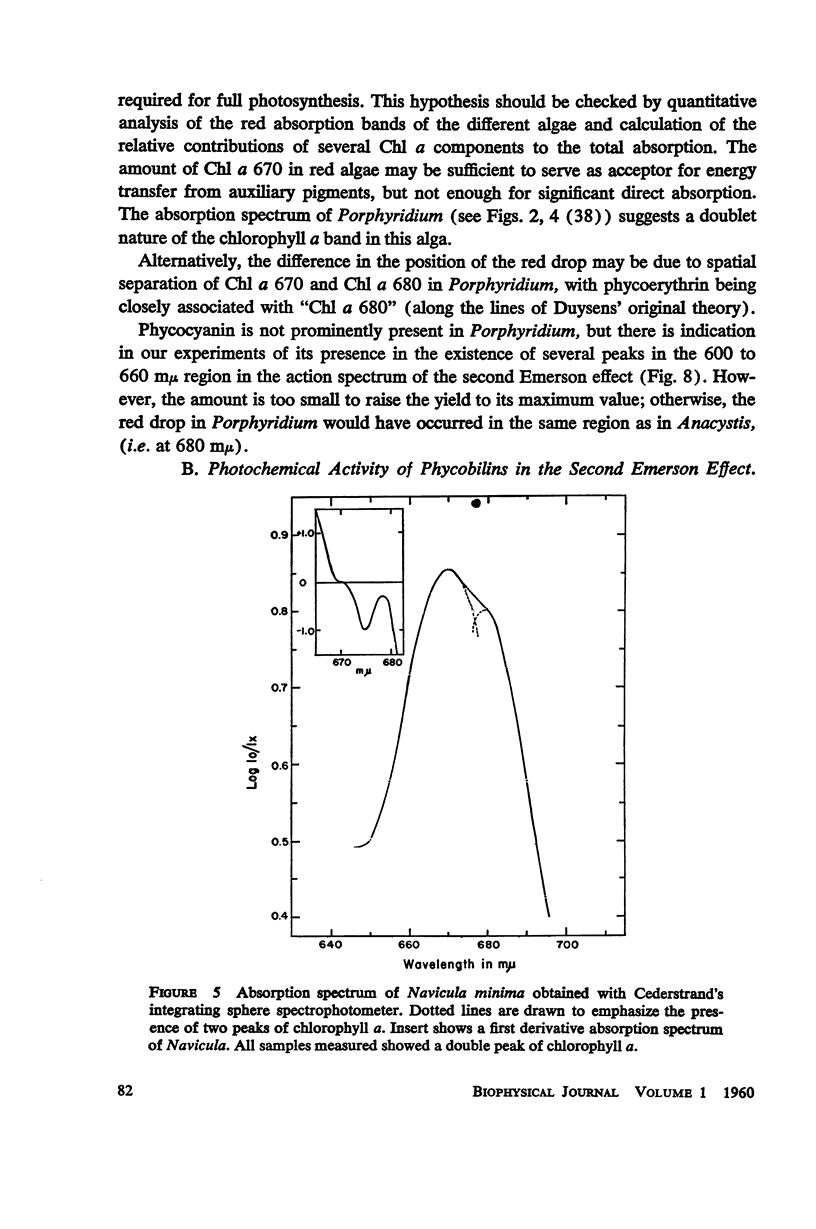
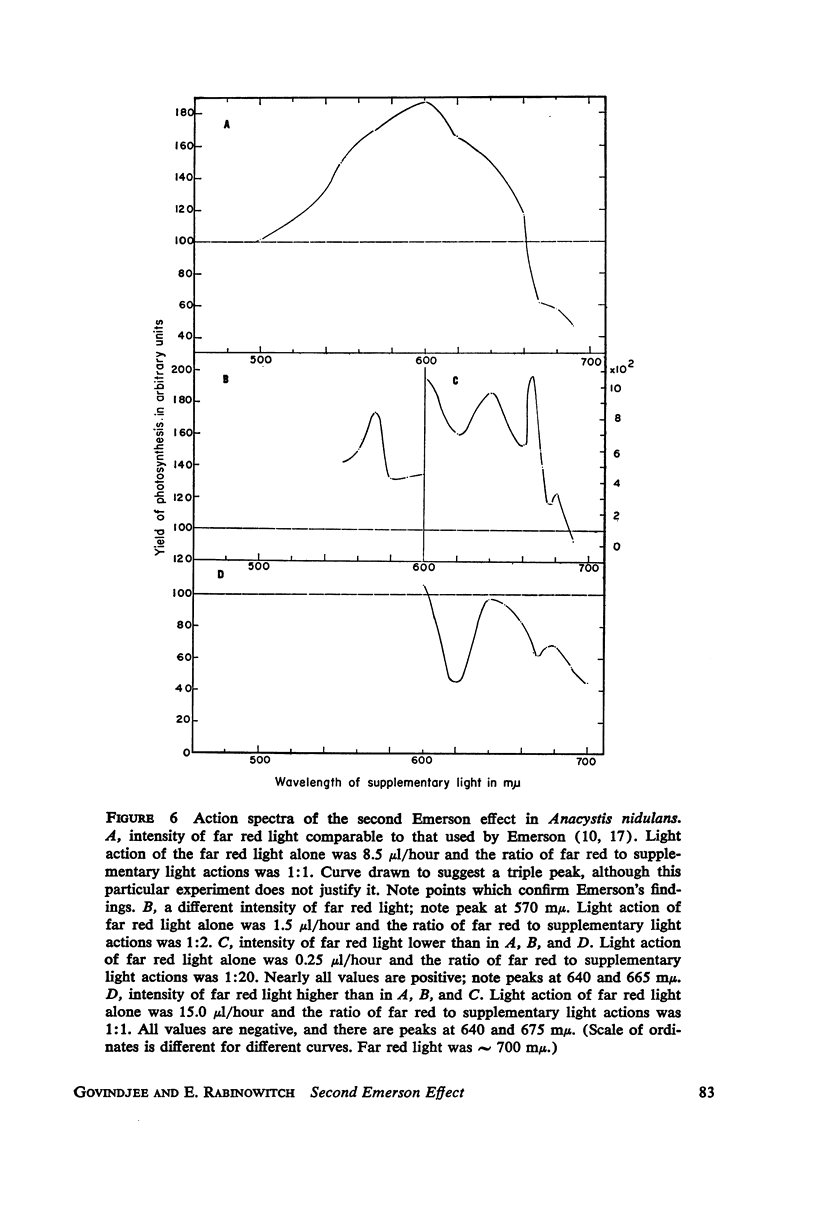
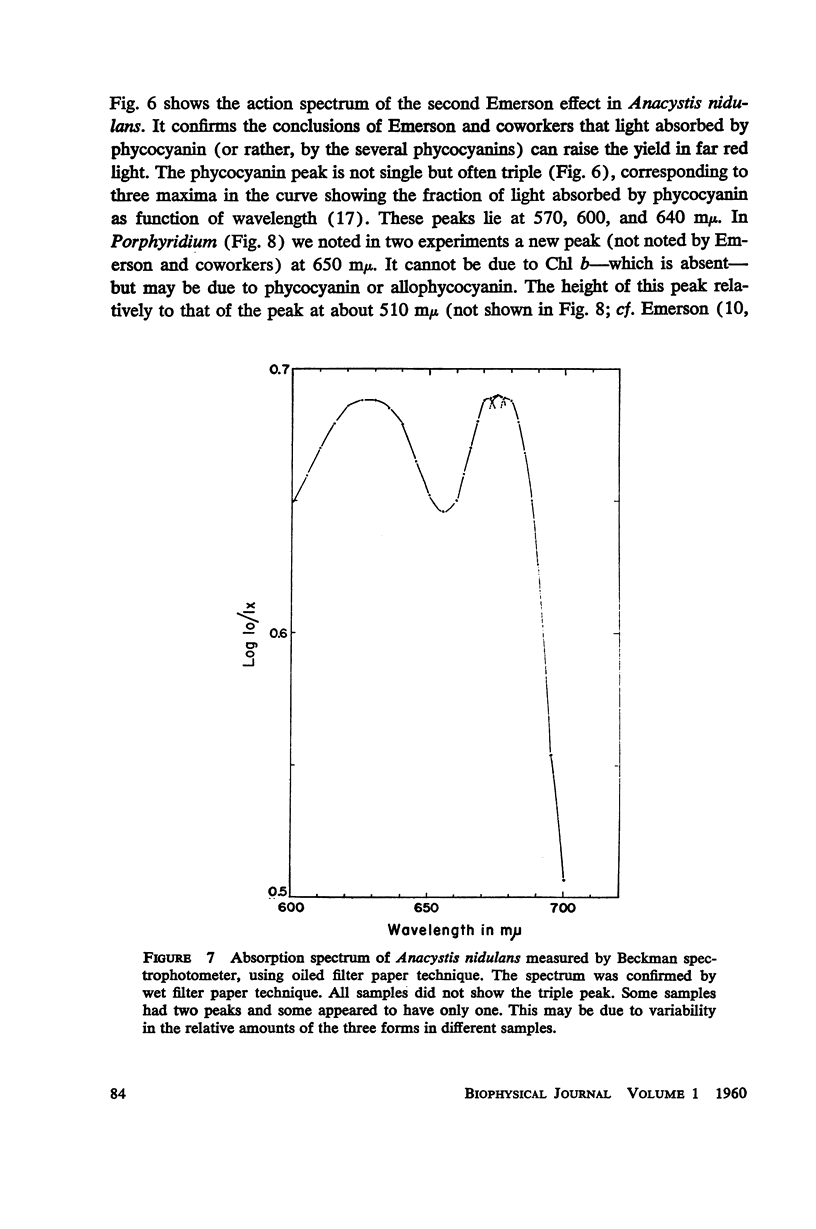
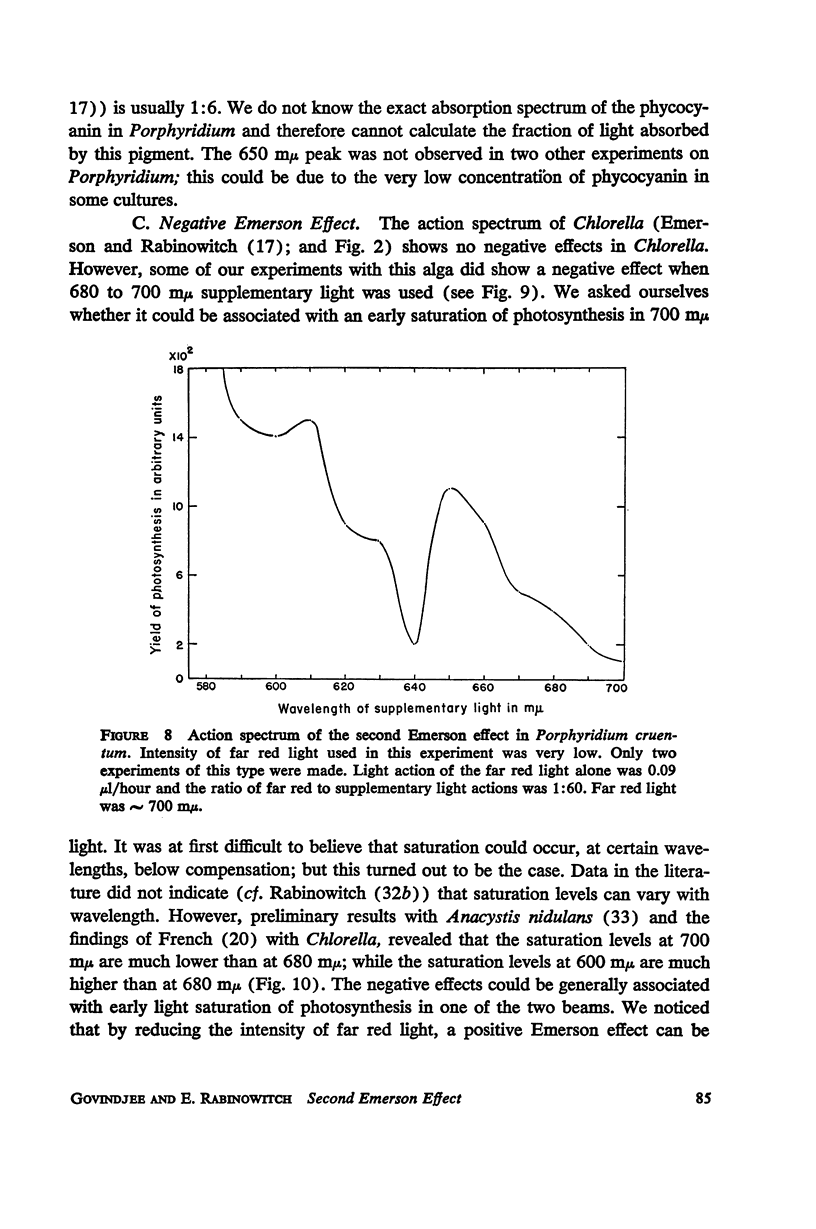
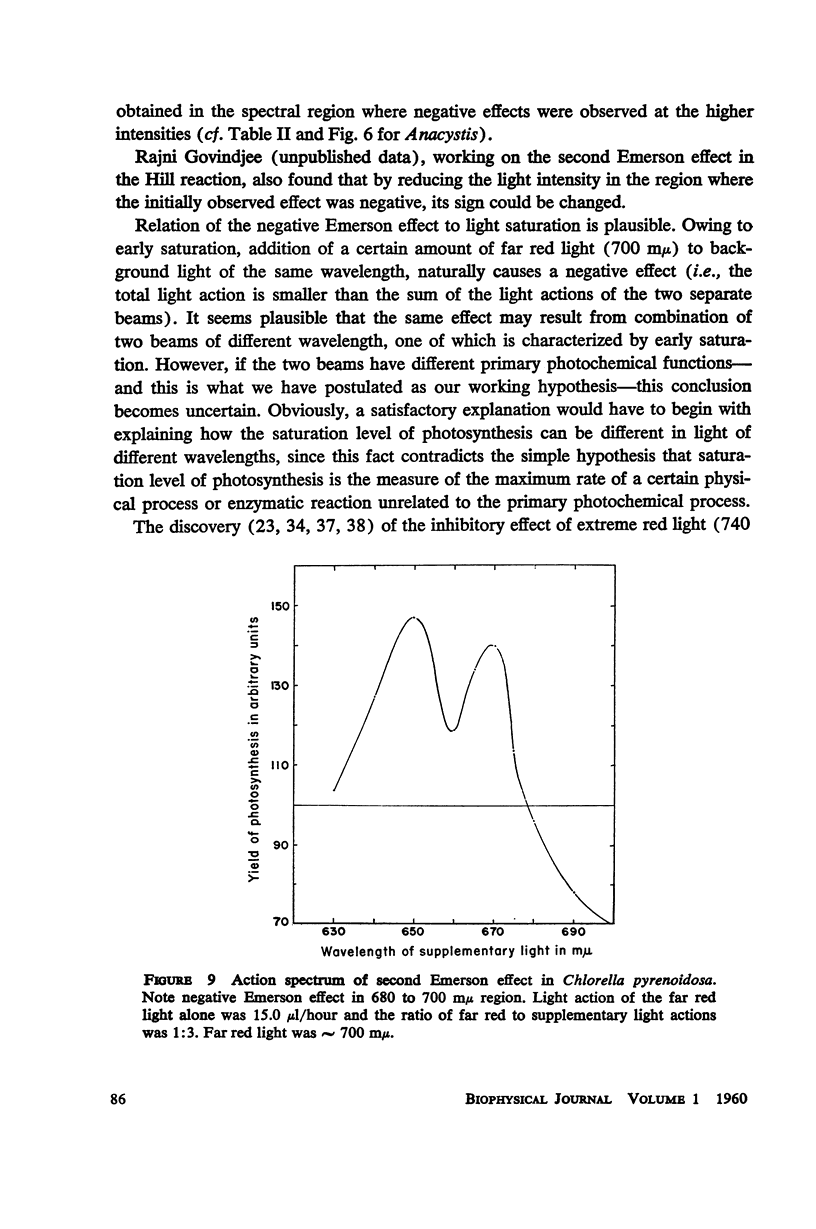
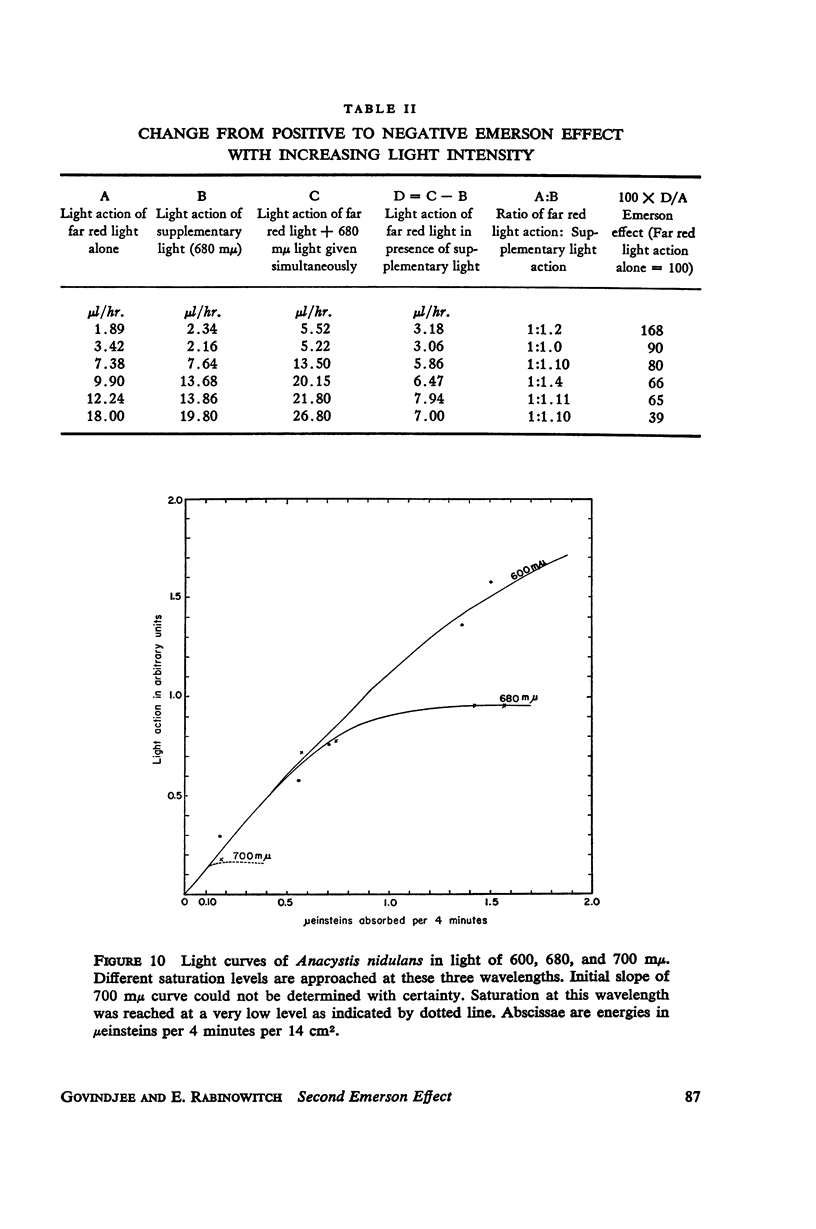
A peak is found at 670 mμ in the action spectrum of the “second Emerson effect”1 (22, 33), in the green alga Chlorella pyrenoidosa, and in the diatom Navicula minima; a shoulder appears in about the same location in the blue-green alga Anacystis nidulans and less clearly in the red alga Porphyridium cruentum. This peak (or shoulder) corresponds to an absorption band belonging to a form of chlorophyll a in vivo, which can be designated as “Chl a 670.” Light absorption by this form can enhance the yield of photosynthesis caused by far red light (680 to 720 mμ), as effectively as does light absorption by chlorophyll b, chlorophyll c, fucoxanthol, phycocyanin, or phycoerythrin. The action spectrum of the second Emerson effect in Anacystis nidulans shows three peaks attributable to phycocyanin, at 570, 600, and 640 mμ. These correspond well to peaks on the curve, calculated by Emerson (17), which shows the fraction of total absorbed light absorbed by phycocyanin as function of wavelength. Intensity relation between the two participating beams has an important bearing on the second Emerson effect. In Anacystis, the “negative effect,” described in (17), can be converted into a positive effect by change in this relation. In Anacystis, the saturation of photosynthesis occurs, at 700 mμ, in weaker light and on a lower level than at 680 mμ, and even more so, than at 600 mμ. This may explain, at least in part, the negative Emerson effect observed in this alga.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODY M., EMERSON R. The quantum yield of photosynthesis in Porphyridium cruentum, and the role of chlorophyll a in the photosynthesis of red algae. J Gen Physiol. 1959 Nov;43:251–264. doi: 10.1085/jgp.43.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. S. New Excited State of Chlorophyll. Science. 1958 Oct 10;128(3328):838–839. doi: 10.1126/science.128.3328.838. [DOI] [PubMed] [Google Scholar]
- Brown J. S., French C. S. Absorption Spectra and Relative Photostability of the Different Forms of Chlorophyll in Chlorella. Plant Physiol. 1959 May;34(3):305–309. doi: 10.1104/pp.34.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R., Chalmers R., Cederstrand C. SOME FACTORS INFLUENCING THE LONG-WAVE LIMIT OF PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1957 Jan 15;43(1):133–143. doi: 10.1073/pnas.43.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R., Chalmers R. Transient Changes in Cellular Gas Exchange and the Problem of Maximum Efficiency of Photosynthesis. Plant Physiol. 1955 Nov;30(6):504–529. doi: 10.1104/pp.30.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R., Rabinowitch E. Red Drop and Role of Auxiliary Pigments in Photosynthesis. Plant Physiol. 1960 Jul;35(4):477–485. doi: 10.1104/pp.35.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck J. REMARKS ON THE LONG-WAVE-LENGTH LIMITS OF PHOTOSYNTHESIS AND CHLOROPHYLL FLUORESCENCE. Proc Natl Acad Sci U S A. 1958 Sep 15;44(9):941–948. doi: 10.1073/pnas.44.9.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck J. REMARKS ON THE LONG-WAVE-LENGTH LIMITS OF PHOTOSYNTHESIS AND CHLOROPHYLL FLUORESCENCE. Proc Natl Acad Sci U S A. 1958 Sep 15;44(9):941–948. doi: 10.1073/pnas.44.9.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOVINDJEE, ICHIMURA S., CEDERSTRAND C., RABINOWITCH E. Effect of combining far-red light with shorter wave light on the excitation of fluorescence in Chlorella. Arch Biochem Biophys. 1960 Aug;89:322–323. doi: 10.1016/0003-9861(60)90063-1. [DOI] [PubMed] [Google Scholar]
- GOVINDJEE, RABINOWITCH E. Two forms of chlorophyll a in vivo with distinct photochemical functions. Science. 1960 Aug 5;132(3423):355–356. doi: 10.1126/science.132.3423.355. [DOI] [PubMed] [Google Scholar]
- Govindjee R., Thomas J. B., Rabinowitch E. "Second Emerson Effect" in the Hill Reaction of Chlorella Cells with Quinone as Oxidant. Science. 1960 Aug 12;132(3424):421–421. doi: 10.1126/science.132.3424.421. [DOI] [PubMed] [Google Scholar]
- HAXO F. T., BLINKS L. R. Photosynthetic action spectra of marine algae. J Gen Physiol. 1950 Mar;33(4):389–422. doi: 10.1085/jgp.33.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS J., FRENCH C. S. Evidences from action spectra for a specific participation of chlorophyll b in photosynthesis. J Gen Physiol. 1960 Mar;43:723–736. doi: 10.1085/jgp.43.4.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy of Sciences. Science. 1957 Apr 19;125(3251):746–752. doi: 10.1126/science.125.3251.746. [DOI] [PubMed] [Google Scholar]
- Rabinowitch E., Govindjee, Thomas J. B. Inhibition of Photosynthesis in Some Algae by Extreme-Red Light. Science. 1960 Aug 12;132(3424):422–422. doi: 10.1126/science.132.3424.422. [DOI] [PubMed] [Google Scholar]
- SHIBATA K., BENSON A. A., CALVIN M. The absorption spectra of suspensions of living micro-organisms. Biochim Biophys Acta. 1954 Dec;15(4):461–470. doi: 10.1016/0006-3002(54)90002-5. [DOI] [PubMed] [Google Scholar]
- THOMAS J. B., MARSMAN J. W. On the pigment system of the red alga Porphyra lacineata. Biochim Biophys Acta. 1959 Oct;35:316–323. doi: 10.1016/0006-3002(59)90380-4. [DOI] [PubMed] [Google Scholar]


