Abstract
Mutations affecting either the N- or C-terminal regions of the Gag protein p12 block replication of Moloney murine leukemia virus (M-MuLV). Viruses carrying mutations in this portion of gag can mediate the assembly and release of virions but are unable to successfully carry out the early phase of the M-MuLV life cycle. Wild-type and mutant viruses were found to synthesize similar levels of linear viral DNA in both cytoplasmic and nuclear fractions, and there were no significant differences in either the density or sedimentation of the viral protein-nucleic acid complexes. Analysis of the termini of the linear viral DNAs showed that the 3′ ends of the mutant viral DNA were processed normally by the integrase. Further, the preintegration complexes extracted from the cytoplasm of cells infected with the mutant viruses were competent for integration into target DNA in vitro. Nevertheless, no circular viral DNAs were detected in cells infected by the mutants, and functional proviruses were not formed. These results suggest that p12 has an unexpected role in the early phase of the life cycle and is needed after viral DNA synthesis to deliver the incoming DNA to the correct location and in the appropriate state to permit either circularization or integration of the viral DNA in vivo.
The early phase of the retrovirus life cycle involves a complex series of steps: virus entry into the host cell, reverse transcription of the viral RNA into DNA, transport of the viral DNA into the nucleus, and integration of this DNA into the host cell genome (5). The viral nucleic acids remain associated with a number of viral proteins throughout these steps. Reverse transcription, occurring in the cytoplasm, results in the formation of a double-stranded linear viral DNA contained in a protein-DNA complex known as the preintegration complex (PIC) (2, 30). Soon after the synthesis of the linear DNA, two conserved nucleotides are removed from both 3′ ends by the endonuclease activity of the viral integrase protein (13, 35). The PIC then enters the nucleus by unknown mechanisms; for the simple retroviruses, nuclear localization of the PIC requires cell division (26, 29, 34), while for the lentiviruses, nuclear import can occur even in nondividing cells (25). Once inside the nucleus, a portion of the linear DNA gives rise to double-stranded circular forms containing either one or two copies of the long terminal repeat (LTR). These circular DNAs probably arise as off-pathway products of DNAs that fail to integrate (8, 28). However, they are useful indicators of viral DNA entry into the nucleus. The linear DNA in the nucleus also serves as the immediate precursor for the formation of the integrated provirus (3).
The overall protein composition and internal structure of the PIC are still poorly understood, though there is some information available (2, 30). The Moloney murine leukemia virus (M-MuLV) PIC includes at a minimum reverse transcriptase (RT) and integrase (IN), and the capsid (CA) and nucleocapsid (NC) proteins encoded by the gag gene are probably present (2, 11, 17). A variety of cellular proteins have also been identified in the PIC (9, 21, 23, 24, 30). These various proteins may facilitate reverse transcription, protect the viral nucleic acids from degradation, transport the PIC through the cytoplasm, target the PIC into the nucleus, or regulate proviral integration (see reference 14 for a review). The CA protein is certainly involved in these steps, because it acts as the target of the antiviral host gene Fv1, which can prevent viral infection after DNA synthesis (32; for a review, see reference 20). Very few viral mutants that are specifically blocked at these early stages of the life cycle, however, have been identified. Mutations in the Gag protein p12 are among the few that act after reverse transcription (42).
The p12 Gag protein of M-MuLV is a small polypeptide of uncertain function. It was previously shown that mutations affecting the PPPY motif in the central region of M-MuLV p12 led to late assembly and budding defects (41, 42). Mutations affecting the N- and C-terminal regions of p12 Gag protein, in contrast, did not affect virus particle production but caused severe defects in the early events of the viral life cycle (42). To further characterize the functions of p12, we have now examined the biochemical properties and activities of the PICs formed after infection with p12 mutant viruses. The mutant PICs are virtually indistinguishable from wild-type PICs in their density and sedimentation rates and in their distribution during fractionation of cell lysates. Furthermore, we found that the 3′ termini of the mutant DNAs are processed normally and that the isolated PICs have normal DNA integration activity as measured by in vitro integration assays. Nevertheless, the linear DNAs do not give rise to circular forms or to integrated proviruses. These data suggest that p12 is needed to deliver the PIC into the nucleus in a form that permits either the ligation of the termini or their insertion into the host genome.
MATERIALS AND METHODS
Cells and viruses.
NIH 3T3 fibroblasts and 293T cells were maintained in Dulbecco's modified Eagle's medium containing penicillin-streptomycin with 10% fetal calf serum. Both wild-type and mutant M-MuLVs were harvested from 293T cells after transient transfection with the wild-type plasmid pNCS (6) or its mutant derivatives (42). In some experiments, mutant virus was collected from stable producer NIH 3T3 cell lines. The virus-containing supernatants were collected 48 h later and filtered through a 0.45-μm-pore-size filter (Nalgene).
Cell fractionation and extraction.
Approximately 107 NIH 3T3 cells in 175-cm2 flasks were infected with 30 ml of freshly collected virus-containing supernatant in the presence of 8 μg of Polybrene per ml. Viral titers were adjusted for equivalent RT activity in the supernatant. Infected cells were rapidly cooled to 4°C and incubated for 2 h to allow virus adhesion to the cell receptor but not virus internalization. Cells were then incubated at 37°C for 18 h, washed once in phosphate-buffered saline containing 0.5 mM EDTA, trypsinized, and washed once again with phosphate-buffered saline. All subsequent manipulations were carried out at 4°C. The pellet containing the infected cells was resuspended in 5 volumes of hypotonic buffer (10 mM HEPES, pH 7.9; 1.5 mM MgCl2; 10 mM KCl; 2 mM dithiothreitol [DTT]; 20 μg of aprotinin/ml; 2 μg of leupeptin/ml) and centrifuged for 5 min at 5,000 rpm in a Sorvall centrifuge. The pellet was resuspended in 3 volumes of hypotonic buffer and incubated for 10 min on ice. Cells were homogenized with 10 to 15 strokes in a Dounce homogenizer, and nuclei and unbroken cells were pelleted by centrifugation at 6,000 rpm for 15 min in an Eppendorf Microfuge. The supernatant (termed cytoplasmic extract) was clarified by centrifugation at 14,000 rpm for 20 min, and the pellet was discarded. The nuclear pellet from the previous centrifugation was resuspended in 600 μl of isotonic buffer (10 mM Tris-HCl, pH 7.4; 160 mM KCl; 5 mM MgCl2; 1 mM DTT; 20 μg of aprotinin/ml; 2 μg of leupeptin/ml) and homogenized in a ball-bearing homogenizer. The homogenate was then centrifuged at 14,000 rpm for 20 min, and the supernatant (termed nuclear extract) was collected. Nuclear and cytoplasmic extracts were immediately analyzed or were adjusted to 8% sucrose, snap-frozen in liquid N2, and stored at −70°C (10, 11).
Modified cell extraction method.
To rule out any possible viral DNA contamination of cytoplasmic components in the nuclear extract, a more stringent method was used to prepare nuclear extracts from infected cells. After the cell was broken and the cytoplasmic extract was removed, the pelleted nuclei and cell debris were washed with 3 volumes of hypotonic buffer containing 0.005% digitonin once and then washed with hypotonic buffer twice. The supernatant of each wash step was collected. The concentration of digitonin was selected so that the nuclei remained intact. The pellet was then homogenized in 600 μl of isotonic buffer and centrifuged in the Microfuge for 20 min, and the nuclear extract was collected. The remaining pellet was washed once with isotonic buffer plus 1% Triton and then extracted by Hirt's method (18). The pellet from the Hirt extraction was further extracted by phenol. The DNAs from each extraction step were precipitated with ethanol and analyzed by Southern blotting or PCR.
Equilibrium density gradients.
Continuous linear sucrose gradients (5 ml) were poured with a two-chamber Hoefer SG gradient maker with 20% sucrose solution in hypotonic buffer and 70% sucrose solution in D2O and kept on ice. The pH of the D2O was adjusted to 7.4 by dropwise addition of 10 mM NaOH. Gradients were overlaid with 0.5 ml of cytoplasmic or nuclear extracts and centrifuged at 35,000 rpm at 4°C for 20 h in a Beckman SW55 rotor. Gradients were fractionated by puncturing the bottom of the tube and collecting 12 fractions. The density was calculated by weighing 100 μl of each fraction.
Sedimentation velocity gradients.
Continuous gradients were poured as described above with 5 and 20% sucrose solutions in 50 mM sodium phosphate buffer (pH 7.4) containing 2 mM DTT, 20 μg of aprotinin/ml, and 2 μg of leupeptin/ml. Approximately 150 μl of the equilibrium density fraction containing the peak of viral DNA was diluted with 1.2 ml of hypotonic buffer, loaded onto a Centricon 50 concentrator (Amicon), and centrifuged at 4,000 × g for 30 min at 4°C in a Sorvall centrifuge. The concentrate (50 μl) was resuspended in 300 μl of 50 mM sodium phosphate buffer, loaded onto a 5 to 20% continuous sucrose gradient, and centrifuged at 23,000 rpm for 1 h at 4°C in a Beckman SW55 rotor. Fractions (0.4 ml each) were collected by puncturing the bottom of the tube. Calibration of the system was performed as described previously (11).
DNA analysis by PCR.
PCRs were performed in a final volume of 50 μl containing 1× PCR buffer, 100 mM (each) deoxynucleoside triphosphate, 2.5 mM MgCl2, 5 U of Taq polymerase (Perkin-Elmer), and 30 pmol of each primer. Primer sequences were as follows: strong-stop forward primer, 5′-GCGCCAGTCTTCCGATAGAC-3′, and strong-stop reverse complementary primer, 5′-AATGAAAGACCCCCGTCGTGG-3′. Five microliters of the equilibrium density fractions or 1.5 μl of the sedimentation velocity fractions was used as a template for PCR. Cycle parameters were 94°C for 3 min for the first cycle and 94°C for 1 min, 55°C for 30 s, and 68°C for 1 min for 35 to 45 cycles, followed by one final extension cycle at 68°C for 10 min. The PCR products were resolved on a 1% agarose-2% Nusieve gel and visualized by ethidium bromide staining.
The primers and PCR conditions to amplify the LTR-LTR junction to detect circular viral DNA were as previously described (42).
Viral DNA 3′-end processing.
The method to analyze viral DNA termini has been described previously (35). Four U3 probes that hybridize to different sequences of U3 3′ termini were synthesized. They were B1 (5′-CCACCTGTAGGTTTGGCAAGCTAGC-3′), B2 (5′-AAGTAACGCCATTTTGCAAGGCATG-3′), B3 (5′-AAAATACATAACTGAGAATAGAGAAGTTCA-3′), and B4 (5′-CAAGGTCAGGAACAGATGGAACAGCTG-3′). The oligonucleotides were labeled at the 5′ end with [γ-32P]ATP and polynucleotide kinase and purified through a G-25 spin column. Fresh NIH 3T3 cells were acutely infected with equal amounts of wild-type or mutant virus harvested from transfected 293T cells and normalized by RT activity in the culture medium. Low-molecular-weight DNAs were extracted from cells 24 h postinfection and digested with KpnI and PvuII (New England Biolabs). The digestion products were then resuspended in DNA sequencing gel loading buffer containing 70% formamide, denatured by heat, and fractionated by electrophoresis on a 10% polyacrylamide sequencing gel in TBE (90 mM Tris-HCl, 90 mM boric acid, 9 mM EDTA) containing 7 M urea. The gel was transferred to Whatman 3MM paper, and the DNAs were then electroblotted onto GeneScreen (NEN) paper. Filters were exposed to UV light to fix the DNA and hybridized with the mixture of labeled oligonucleotides. All four probes were used in the hybridization to enhance the signal.
PIC preparation and in vitro integration assay.
M-MuLV PICs were prepared essentially as described previously (4, 12, 27) with the following modifications. 293T cells were transfected with proviral DNAs of wild-type (pNCS) or the S78A mutant to produce virus, and these preparations were then used to infect Rat2-2 cells and to establish chronically infected Rat2-2 cell lines after extended passage. These virus-producing lines (2.4 × 106) were plated together with uninfected Rat2-2 cells (9.6 × 106) in a 140-mm-diameter dish to initiate the acute infection. Cells were trypsinized and harvested after 16 h of coculture. The pelleted cells were resuspended in 3 volumes of hypotonic buffer (10 mM HEPES, pH 7.4; 1.5 mM MgCl2; 10 mM KCl; 5 mM DTT; 20 μg of aprotinin/ml; 2 μg of leupeptin/ml) and incubated for 10 min at 0°C. Cells were homogenized with 10 to 15 strokes in a Dounce homogenizer, and nuclei and unbroken cells were pelleted by centrifugation at 3,300 × g for 15 min. The supernatant (called cytoplasmic extract) was clarified by centrifugation at 7,500 × g for 20 min. The cytoplasmic extracts were adjusted to buffer A (20 mM HEPES, pH 7.4; 5 mM MgCl2; 150 mM KCl; 5 mM DTT; 0.025% digitonin) with 7% sucrose and stored at −70°C.
The integration assay was carried out essentially as described previously (4, 12). The cytoplasmic extracts prepared from wild-type- or S78A-infected cells were incubated at 37°C for 30 min in buffer A (150 μl) containing linearized φX174 replicative form I (10 μg/ml). The reactions were stopped by the addition of 6 μl of 0.5 M EDTA, 15 μl of 1% proteinase K (Boehringer Mannheim), and 7 μl of 10% sodium dodecyl sulfate (SDS), and the reaction mixtures were further incubated at 55°C for 30 min. The DNA was purified by extraction with a 1:1 mix of phenol-chloroform, precipitated by ethyl alcohol, and analyzed by gel electrophoresis followed by Southern blotting with a 32P-labeled probe containing the LTR sequence.
RESULTS
Extraction and analysis of viral DNA in the cytoplasm of infected NIH 3T3 cells.
Previous work led to the identification of five mutants with alanine substitutions in the N- and C-terminal regions of p12 that were unable to carry out the early stages of the viral life cycle (42). The locations and the blocks of amino acid residues changed to alanine are shown in Fig. 1. Mutant PM14, containing a block substitution in the C-terminal region, was selected as having a canonical and strong phenotype. To characterize the PIC of this mutant, NIH 3T3 cells were acutely infected with equivalent concentrations of mutant or wild-type M-MuLV, and the cells were lysed in a hypotonic buffer by Dounce homogenization 18 h after infection (described in Materials and Methods). The lysates were separated into cytoplasmic and nuclear fractions (Fig. 2). The cytoplasmic extracts were subjected to equilibrium density centrifugation, and the fractions were analyzed by PCR to detect minus-strand strong-stop DNA (Fig. 3). For wild-type virus, the bulk of the viral DNA was recovered at high density, with a peak in fractions 2 and 3; a small amount of DNA was present at the top of the gradients (Fig. 3A). A virtually identical peak was found in the corresponding lanes for the mutant virus, and there was no significant difference in the total amount of DNA or its distribution (Fig. 3A, lanes 2 and 3). These data suggest that the mutant initiates DNA synthesis at normal efficiency and forms a PIC in the cytoplasm of infected cells with a similar density as that of the wild-type PIC.
FIG. 1.
p12 early-event mutants (42). The amino acid sequence of p12 is presented. The locations, names, and blocks of residues changed to alanines in each substitution mutant are indicated. S78A is a mutant that replaced serine 78 with alanine.
FIG. 2.
Schematic representation of the procedure for fractionation of lysates of infected cells. NIH 3T3 cells were infected with equal titers of either mutant PM14 or wild-type virus and lysed with hypotonic buffer, and cytoplasmic and nuclear fractions were prepared. The secondary pellet was extracted by the Hirt method (18). In addition, cytoplasmic and nuclear extracts were fractionated by equilibrium density centrifugation. The fractions of the nuclear extracts containing the peak of the viral DNA were further analyzed by velocity sedimentation.
FIG. 3.
(A) PCR analysis of viral DNA in equilibrium density fractions of the cytoplasmic extracts. Cytoplasmic extracts of infected NIH 3T3 cells were collected 18 h postinfection, loaded on a 20 to 70% linear sucrose gradient in D2O, and centrifuged at 4°C for 20 h at 35,000 rpm in a Beckman SW55 rotor. Fractions from the gradient were analyzed by PCR with primers specific for strong-stop DNA (145 bp). An arrow indicates the direction of the gradient from the lowest to the highest density. (B) PCR analysis of viral DNA in equilibrium density fractions of the nuclear extracts. The nuclear extracts were fractionated on gradients, and fractions were collected and analyzed by PCR. The products derived from strong-stop DNA are indicated. The p12 mutant used here is PM14. M, marker; +, positive PCR control; −, negative PCR control; WT, wild type.
To rule out possible contamination by plasmid DNA from the transient transfection, plasmid-specific primers were used to amplify sequences of the ampicillin resistance gene. No detectable PCR products could be found in the different density fractions of the cytoplasmic extracts (data not shown), indicating that the viral DNAs detected in the above experiments were produced only by the infecting virus.
Analysis of viral DNA in the nuclear fraction of infected cells.
Since circular viral DNAs are generally regarded as hallmarks of viral DNA entry into the nucleus, the failure of most p12 mutants to produce circular viral DNAs suggested that nuclear import or intracellular transport of viral DNA might be affected. After removal of the cytoplasmic extract as described above, nuclear extracts of infected cells were obtained by breaking the nucleus by homogenization with a ball-bearing homogenizer (Materials and Methods) (Fig. 2). This nuclear extract was further fractionated by equilibrium density sedimentation, and fractions were assayed by PCR. Surprisingly, the analysis indicated that approximately equal amounts of viral DNAs were recovered in both mutant and wild-type nuclear extracts, and the peak of the viral DNA appeared in fractions of similar density (Fig. 3B, lanes 3 and 4). No contaminating plasmid DNA could be detected in any density fraction of nuclear extracts (data not shown). These data suggest that the mutant viral DNA becomes at least associated with the nucleus as efficiently as does the wild-type DNA.
To evaluate whether there were changes in the size of the mutant PIC, the density fractions containing the peak of the nuclear-associated viral DNA were further analyzed by velocity sedimentation through sucrose gradients. PCR analysis showed that mutant and wild-type virus had similar viral DNA distribution patterns in the velocity sedimentation fractions (Fig. 4, lanes 6 to 8). These data suggest that in the nuclear fraction the mutant PIC is of similar size and shape, and may contain similar components, as the wild-type PIC.
FIG. 4.
Velocity sedimentation analysis of viral PICs in nuclear extracts. Nuclear extracts collected 18 h postinfection were subjected to equilibrium density centrifugation. Fractions containing the peak of the viral DNA were pooled, and the samples were concentrated in a Centricon filter and sedimented through a 5 to 20% linear sucrose gradient for 1 h at 23,000 rpm in a Beckman SW55 rotor. The viral DNA in each fraction was analyzed by PCR with primers specific for the strong-stop DNA. +, positive PCR control; WT, wild type.
Further fractionation and analysis of viral DNA in infected cells.
To characterize the mutant PICs further, a more stringent washing method was developed to remove any possible cytoplasmic contamination of the nuclear extract (Materials and Methods) (Fig. 5). In addition, Southern blot assays were used to provide a more quantitative measure of the levels of viral DNA in each fraction than the PCR readouts. The infected cells were lysed in hypotonic buffer and fractionated by low-speed centrifugation into a cytoplasmic extract and a pellet that contained nuclei, membrane, cytoskeleton, and other associated proteins. The pellet was washed in hypotonic buffer containing digitonin and then washed twice more with the hypotonic buffer. These additional washing steps were designed to remove any remaining cytoplasmic viral DNA from the pellet without breaking the nuclei. The presence of both linear and circular viral DNAs in each wash and extraction step was detected by Southern blot assay. As shown in Fig. 6, the majority of linear viral DNA in the cytoplasm was extracted in the initial lysis in hypotonic buffer, and only a small amount of viral DNA could be detected in subsequent washing steps. The PM14 mutant virus exhibited a similar amount of viral DNA as did the wild type in all these fractions (Fig. 6, lanes 1 to 8), confirming that the p12 mutant completed viral DNA synthesis at normal efficiency and produced a PIC with normal behavior.
FIG. 5.
Schematic representation of modified procedure for fractionating lysates of infected cells. Infected NIH 3T3 cells were either extracted by the Hirt method (18) to obtain the total Hirt extract or lysed in hypotonic buffer to obtain the cytoplasmic extract. The nuclei were treated with three extra washing steps, once with the hypotonic buffer plus 0.005% digitonin and twice with the hypotonic buffer, to remove possible cytoplasmic viral DNA contamination in the nuclear extracts. The pellet was lysed in hypotonic buffer to obtain a nuclear extract. The remaining pellet was washed with isotonic buffer plus 1% Triton once and finally extracted by the Hirt procedure.
FIG. 6.
Southern blot analysis of linear and circular viral DNA in different cell fractions. Infected NIH 3T3 cells were fractionated as described in the Fig. 5 legend and analyzed by Southern blotting with a radiolabeled viral DNA probe. The positions of the linear and circular viral DNAs are indicated. WT, wild type.
The remaining pellet containing the nuclei was lysed by homogenization in isotonic buffer. Compared to the washing steps, an increased level of viral DNAs could be detected after breaking the nuclei, indicating that these viral DNAs were indeed obtained from the nucleus (Fig. 6, lanes 9 and 10). Mutant and wild-type viruses produced similar amounts of linear viral DNA in the nuclear extract (Fig. 6, lanes 9 and 10). Interestingly, almost no circular viral DNA could be detected in these extracts from either mutant or wild type (Fig. 6, lanes 9 and 10). The pellet was then washed in an isotonic buffer containing 1% Triton; no additional viral DNA appeared in the supernatants of the Triton washing step. The pellet was finally extracted by the Hirt procedure with SDS (18) (Fig. 6, lanes 13 and 14). A significant amount of linear viral DNA was recovered in this extraction, suggesting that these DNAs had been trapped in a form that required strong detergents to be solubilized. Again, the levels of linear DNA in this fraction were virtually identical for the mutant and the wild type. These results indicate that the mutant and wild-type PICs behaved similarly throughout the fractionation, with similar distribution in the cytoplasmic and nucleus-associated fractions.
The extraction of the wild-type nuclear fraction with SDS yielded not only some linear DNA but nearly all the circular DNAs; for the mutant virus, no such DNAs were detected (Fig. 6, lanes 13 and 14). These data indicate that about half of the linear and all of the circular DNA may be associated with an insoluble protein complex and could be extracted only with a strong detergent. Very little viral DNA could be detected in the final pellet of the Hirt fractionation, containing mainly high-molecular-weight DNA (Fig. 6, lanes 15 and 16). In control experiments, the infected cells were directly subjected to Hirt extraction at the first step. As expected, the PM14 mutant showed normal linear viral DNA synthesis but almost no circles (Fig. 6, lanes 17 to 19).
To confirm the Southern blot analysis, PCR methods were used to amplify either the strong-stop DNA or the LTR-LTR junction region in the above cell extracts. As shown in Fig. 7A, there were no significant differences in the fractionation of the minus-strand strong-stop DNA between wild-type and mutant extracts. The PCR-amplified LTR-LTR junction fragment could be detected in the wild-type nuclear Hirt extracts and in the total Hirt extract (Fig. 7B, lanes 7, 9, and 12). However, these PCR products could not be found in the corresponding fractions of the PM14 mutant (Fig. 7B, lanes 7 and 9). In addition, no circles could be detected in the wild-type nuclear extract lysed by the isotonic buffer or 1% Triton. These results are fully consistent with the data obtained by the Southern blot assay.
FIG. 7.
PCR analysis of linear and circular viral DNA in different cell fractions. Infected NIH 3T3 cells were fractionated as described in the Fig. 5 legend and analyzed by PCR with primers specific for the strong-stop DNA (A) or the LTR-LTR junction region (B). The PCR products of the strong-stop DNA and the LTR-LTR junction are indicated. WT, wild type.
Analysis of the 3′ end of linear unintegrated viral DNA.
Soon after synthesis of the full-length double-stranded viral DNA, two nucleotides at the 3′ ends of the two strands are removed by the endonuclease activity of the integrase protein. This cleavage event, occurring in the cytoplasm, creates the 3′ ends that are used as the nucleophiles in the subsequent strand transfer reaction. To determine whether the p12 mutants are defective in 3′-terminus processing, we analyzed the structure of a 3′ end of the mutant linear DNA. The preintegrative viral DNA was isolated from acutely infected cells and then cleaved with restriction enzymes KpnI and PvuII to release small terminal fragments. The DNA fragments were denatured, separated by gel electrophoresis in DNA sequencing gels, and electroblotted to nitrocellulose. The filters were then probed with labeled oligonucleotides specific for the 3′-terminal fragments of U3. For wild-type virus, two terminal fragments were detected (120 and 122 nucleotides, Fig. 8, lane 1) as expected (35). The longer fragment was derived from the full-length uncleaved viral 3′ end, and the shorter fragment was derived from the processed 3′ end, lacking two nucleotides. At the time of harvest, we estimate that approximately 80% of the 3′ ends were cleaved by integrase. The 3′ terminus of the p12 mutant (PM14) DNA showed exactly the same extent of processing as did the wild-type virus (Fig. 8, lane 2). This finding suggests that the processing of viral DNA 3′ termini by the integrase does occur normally for the p12 early-event mutants. The result further implies that the PIC must retain the integrase and that the protein has proper access to the termini.
FIG. 8.
Analysis of viral DNA 3′ termini. Viral DNA isolated 24 h after infection was digested, denatured, separated by electrophoresis in a DNA sequencing gel, blotted to nitrocellulose, and hybridized to 32P-labeled probes to detect the 3′ end of the U3 terminus. The positions and sizes of the viral DNA fragments are indicated. WT, wild type; nt, nucleotides.
Ability of the mutant PICs to mediate viral DNA integration in vitro.
To determine whether the PICs generated by the p12 early mutants were competent for integration, we examined their ability to insert their viral DNA into a target DNA in vitro. These experiments require the infection of large-scale cultures with virus at high multiplicity, and the preparations of virus derived from transient transfection of 293T cells were not found to provide sufficient virus. High-multiplicity infection could be best achieved by cocultivation of stable virus-producing cells with virus-sensitive cells. The cells producing the highest available levels of a p12 mutant were Rat2 cells expressing mutant S78A, containing a single Ser-to-Ala alteration within the region covered by mutant PM13 (Fig. 1) (A. Yueh, unpublished data). This mutant exhibited the same phenotype as did the earlier p12 mutants, with normal synthesis of PICs containing linear DNA but with no detectable synthesis of circular viral DNAs. To initiate infection, the wild-type- or mutant virus-producing Rat2 cells were plated together with naive Rat2 cells. The cocultures were lysed 16 h postinfection, and cytoplasmic extracts containing the PICs were prepared by standard procedures (see Materials and Methods).
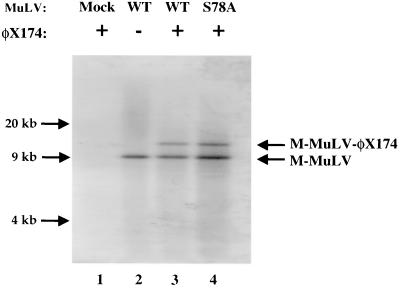
The PICs were incubated in vitro with double-stranded DNA of phage φX174 as a target, and the reaction products were then displayed by gel electrophoresis and blotted to nitrocellulose. The structures of the retroviral DNAs were then examined by probing of the filters with a radioactive viral DNA probe (Fig. 9). Incubating the PICs in the absence of the target DNA showed the presence of only the full-length 8.8-kb viral DNA, with no other discrete DNAs detected. Incubating wild-type PICs in the presence of the φX174 target DNA resulted in the integration of a substantial fraction of the viral DNA into the target, forming a slower-migrating band. The formation of this product requires concerted integration of both ends of the viral DNA into the target. Reactions with the p12 mutant PICs showed that almost exactly the same fraction of the viral DNA was able to integrate into the target DNA as seen for the wild-type PICs (Fig. 9). The product with the mutant PICs was of the same size as that for the wild-type PICs, consistent with concerted integration, and there were no novel species indicative of half-reactions with only one end of the viral DNA inserted. These results suggest that the p12 mutant PICs after isolation from the cell are fully competent for concerted integration of the viral DNA when assayed in vitro. All the components required for carrying out the reaction in trans must be present in the PIC. Thus, the failure of the PICs to integrate in vivo must be attributable either to an inhibitor that is very labile and lost during extraction or to a failure of the PIC to enter the correct intracellular location or compartment in the nucleus and find its target DNA.
FIG. 9.
In vitro integration analysis. The PICs of wild type (WT) or the S78A mutant virus were extracted after acute infection initiated by coculture and were used to carry out an integration assay in vitro. φX174 DNA was used as the target DNA. The locations of the linear M-MuLV preintegrative viral DNA and the integrated M-MuLV-φX174 DNA are indicated by arrows.
DISCUSSION
In this study, we have performed further characterization of p12gag mutants of M-MuLV that are defective in the early events of the viral life cycle. These mutations are almost certain to mediate their effects through changes in the p12 protein itself, rather than through changes in the virion DNA, since many retroviral vector DNAs that are transmitted efficiently do not retain the region. The block to replication is profound and suggests that p12 is critical for this phase of replication. These p12 mutants are unique among all Gag mutants in the timing of the block to infection: the mutants arrest after synthesis of the linear viral DNA but before formation of circular DNAs or the integrated provirus. These DNA forms are usually considered to be hallmarks of nuclear entry of the PIC and important markers for successful retroviral infection. The findings suggest that p12 must play an important role in specifying the composition, conformation, or intracellular localization of the incoming PIC and so promoting its normal DNA integration activities.
The two circular DNAs present in a normal infection are thought to arise from several pathways (see references 16 and 38 for reviews). The smaller circles, with one copy of the LTR, can be formed during reverse transcription from a circular intermediate in the process of plus-strand strong-stop DNA translocation; they can also arise by homologous recombination between the two LTRs at the termini of the linear DNA. The relative proportions of the circles that are generated by each of these two pathways are not known. Many of the larger circles, with two LTRs, arise by ligation of the termini of the linear DNA, but a significant portion of DNAs of the same size arise by intramolecular autointegration, forming an inverted segment (37). The nearly complete absence of both circles in the p12 mutants suggests that all these pathways that contribute to the two DNAs are blocked. Since reverse transcription is apparently normal in the p12 mutants, the results indicate that the strong-stop translocation intermediate may not contribute significantly to the one-LTR circles. Homologous recombination, DNA end joining, and intramolecular integration all seem to be blocked in the mutants.
The analysis of the PICs from mutant virus-infected cells shows that their overall properties are remarkably similar to those of the wild-type virus. The levels of linear viral DNA were normal, showing that reverse transcription was unaffected by the mutation. The isopycnic densities of the mutant PICs from both cytoplasmic and nuclear fractions were indistinguishable from those of the wild type, and the sedimentation rates of the complexes from the nuclear fractions were also unchanged. Thus, there were likely to be no gross alterations in the overall ratios of protein to nucleic acid, or in the overall size or conformation of the PICs, though subtle changes cannot be ruled out. Furthermore, the location of the PICs in infected cells, as judged by the distribution of the PICs into various fractions, was remarkably similar for the mutant and wild-type viruses. In simple separations into cytoplasmic and nuclear fractions, the viral DNAs were distributed in very similar proportions. In more elaborate fractionation protocols, including steps in which nuclei were subjected to various washing steps, the mutant and wild-type DNAs were recovered at virtually identical levels in every fraction (Fig. 6). Similar experiments with slight variations in the salts and detergent levels in the various washes always gave analogous results, with no significant abnormalities in the behavior of the p12 mutant DNAs (data not shown). The only sharp difference was the consistent absence of circular DNAs in the mutant virus-infected cells.
These results of the fractionation experiments would suggest that the mutant PICs may enter the nucleus. The nuclei could be washed extensively, and then after disruption of the nuclei the PICs were still recovered at wild-type levels. However, we cannot rule out the possibility that the mutant PICs are not fully imported into the interior of the nuclei but rather are only bound tightly to the outside of the nuclear envelope. In this model, p12 would help promote late stages of nuclear entry. Furthermore, it is possible that the mutant PICs may have entered the nucleus but were not correctly localized or released into an intranuclear compartment where integration and circularization could occur. The trafficking of the PICs toward and inside the nucleus and their ultimate localization within the nucleus are not well characterized. The results obtained here raise the possibility of a role for the p12 protein in controlling these steps early in retrovirus infection. An interesting observation about the nuclear PICs is that a significant fraction of the viral DNA, of both the wild type and the mutant, was not extracted from the nuclei with buffers containing 1% Triton but only with buffer containing SDS (Fig. 6, lanes 11 to 14). Virtually all of the circular DNAs of the wild-type virus were present in this fraction (lane 14). Thus, the intranuclear PICs are not likely to be free but may be tightly bound to components of the nuclear matrix.
It is known that cell division and mitosis are required for infection by the simple retroviruses, including M-MuLV (26, 34). The simplest explanation for this requirement is that there is no true nuclear import of the PICs through an intact nuclear pore but rather only a targeted inclusion of the PIC to chromatin or the nuclear matrix within the nucleus as it re-forms from dispersed vesicles after mitosis. The cells in all the experiments reported here were rapidly dividing at the time of infection, and the cell numbers increased at similar rates after infection with either wild-type or mutant viruses (data not shown). Thus, there was no indication of a novel inhibition of mitosis by the mutants. It is therefore possible that the p12 mutants are defective in their ability to target the re-forming nucleus, to be retained in the nucleus, or to localize correctly to a specific compartment within the nucleus.
The lack of circular DNAs initially suggested to us that the termini of the linear DNA of the p12 mutants might be blocked or otherwise inaccessible to the host ligases that are thought to be responsible for formation of the two-LTR circles. Analysis of the termini of the linear DNA, however, showed that the 3′ ends in fact were properly processed by the nuclease activity of the integrase (Fig. 8). Thus, the termini were at least available to the integrase and apparently were not fully blocked from all access. Another possibility was that the termini might not be properly assembled into the large, stable complex of proteins that can be detected by footprint analysis of the viral DNA (39, 40). However, the PICs extracted from the infected cells were fully competent at integration in vitro and performed concerted end joining to the target, suggesting that a normal complex of all those proteins required for integration of both termini into a target in trans had been properly formed (Fig. 9). The failure of the viral DNA to circularize or integrate in vivo must therefore be attributed either to a labile inhibitor that is lost upon extraction or to the mislocalization of the PIC within the infected cell. We note that the behavior of the p12 mutants is quite similar to that of sensitive viruses in the infection of cells carrying the Fv1 resistance gene. Fv1 encodes a Gag-related protein (1) that inhibits incoming virus as a dominant-acting function (32, 36; for reviews, see references 15 and 20). This gene, like the p12 mutations, blocks virus infection largely after reverse transcription and before formation of circular DNAs and the integrated provirus. In this situation, the PICs are also integration competent when extracted from infected cells (33). Thus, the Fv1 gene product may act at a similar time and through a similar mechanism to prevent the progression of the PICs on their normal pathway of infection. Fv1 restriction is known to target the CA protein, and its activity depends on a specific residue in CA (7, 19, 22, 31). These results further suggest that both p12 and CA may be present in the PIC and play a role in these early events.
The early events of retrovirus infection have been difficult to study and are only now being uncovered through both genetic and biochemical approaches. It is not surprising that we do not know all the cellular proteins that are involved, but it is somewhat surprising that we do not even know all the viral proteins that act in these steps. The p12 protein seems unlikely to play a direct role in viral DNA integration, since the mutant PICs have properties similar to those of the wild type and are fully functional for integration in vitro. It seems more likely that p12 acts to direct the PIC to the nucleus, or into the correct location in the nucleus, to allow its normal integration in vivo. As such, p12 may provide an important entree into the cellular machinery that is responsible for these events.
Acknowledgments
We thank Eran Bacharach and Guangxia Gao for helpful discussions and Theodora Hatziioannou and Sharon Boast for reading early drafts.
This work was partially supported by Public Health Service grant CA 30488 from the National Cancer Institute. A.F. is a Wellcome Trust International Prize Research Fellow. A.Y. is an Associate and S.P.G is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382:826-829. [DOI] [PubMed] [Google Scholar]
- 2.Bowerman, B., P. O. Brown, J. M. Bishop, and H. E. Varmus. 1989. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 3:469-478. [DOI] [PubMed] [Google Scholar]
- 3.Brown, P. O. 1997. Integration, p. 161-203. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 4.Brown, P. O., B. Bowerman, H. E. Varmus, and J. M. Bishop. 1987. Correct integration of retroviral DNA in vitro. Cell 49:347-356. [DOI] [PubMed] [Google Scholar]
- 5.Coffin, J. M., S. H. Hughes, and H. E. Varmus. 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 6.Colicelli, J., and S. P. Goff. 1988. Sequence and spacing requirements of a retrovirus integration site. J. Mol. Biol. 199:47-59. [DOI] [PubMed] [Google Scholar]
- 7.DesGroseillers, L., and P. Jolicoeur. 1983. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J. Virol. 48:685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis, J., and A. Bernstein. 1989. Retrovirus vectors containing an internal attachment site: evidence that circles are not intermediates to murine retrovirus integration. J. Virol. 63:2844-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farnet, C. M., and F. D. Bushman. 1997. HIV-1 cDNA integration: requirement of HMG I(Y) protein for function of preintegration complexes in vitro. Cell 88:483-492. [DOI] [PubMed] [Google Scholar]
- 10.Fassati, A., and S. P. Goff. 2001. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J. Virol. 75:3626-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fassati, A., and S. P. Goff. 1999. Characterization of intracellular reverse transcription complexes of Moloney murine leukemia virus. J. Virol. 73:8919-8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiwara, T., and K. Mizuuchi. 1988. Retroviral DNA integration: structure of an integration intermediate. Cell 54:497-504. [DOI] [PubMed] [Google Scholar]
- 13.Goff, S. P. 1992. Genetics of retroviral integration. Annu. Rev. Genet. 26:527-544. [DOI] [PubMed] [Google Scholar]
- 14.Goff, S. P. 2001. Intracellular trafficking of retroviral genomes during the early phase of infection: viral exploitation of cellular pathways. J. Gene Med. 3:517-528. [DOI] [PubMed] [Google Scholar]
- 15.Goff, S. P. 1996. Operating under a Gag order: a block against incoming virus by the Fv1 gene. Cell 86:691-693. [DOI] [PubMed] [Google Scholar]
- 16.Goff, S. P. 2001. Retroviridae: the retroviruses and their replication, p. 1871-1939. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 17.Gorelick, R. J., W. Fu, T. D. Gagliardi, W. J. Bosche, A. Rein, L. E. Henderson, and L. O. Arthur. 1999. Characterization of the block in replication of nucleocapsid protein zinc finger mutants from Moloney murine leukemia virus. J. Virol. 73:8185-8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins, N., J. Schindler, and R. Hynes. 1977. Six NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J. Virol. 21:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolicoeur, P. 1979. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr. Top. Microbiol. Immunol. 86:67-122. [DOI] [PubMed] [Google Scholar]
- 21.Kalpana, G. V., S. Marmon, W. Wang, G. R. Crabtree, and S. P. Goff. 1994. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science 266:2002-2006. [DOI] [PubMed] [Google Scholar]
- 22.Kozak, C. A., and A. Chakraborti. 1996. Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology 225:300-305. [DOI] [PubMed] [Google Scholar]
- 23.Lee, M. S., and R. Craigie. 1998. A previously unidentified host protein protects retroviral DNA from autointegration. Proc. Natl. Acad. Sci. USA 95:1528-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, M. S., and R. Craigie. 1994. Protection of retroviral DNA from autointegration: involvement of a cellular factor. Proc. Natl. Acad. Sci. USA 91:9823-9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis, P., M. Hensel, and M. Emerman. 1992. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 11:3053-3058. (Erratum, 11:4249.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, L., C. M. Farnet, W. F. Anderson, and F. D. Bushman. 1998. Modulation of activity of Moloney murine leukemia virus preintegration complexes by host factors in vitro. J. Virol. 72:2125-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobel, L. I., J. E. Murphy, and S. P. Goff. 1989. The palindromic LTR-LTR junction of Moloney murine leukemia virus is not an efficient substrate for proviral integration. J. Virol. 63:2629-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, D. G., M. A. Adam, and A. D. Miller. 1990. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 10:4239-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, M. D., C. M. Farnet, and F. D. Bushman. 1997. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J. Virol. 71:5382-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou, C.-Y., L. R. Boone, C. K. Koh, R. W. Tennant, and W. K. Yang. 1983. Nucleotide sequences of gag-pol regions that determine the Fv-1 host range property of BALB/c N-tropic and B-tropic murine leukemia viruses. J. Virol. 48:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pincus, T., W. P. Rowe, and F. Lilly. 1971. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to Friend murine leukemia virus. J. Exp. Med. 133:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pryciak, P. M., and H. E. Varmus. 1992. Fv-1 restriction and its effects on murine leukemia virus integration in vivo and in vitro. J. Virol. 66:5959-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roe, T., T. C. Reynolds, G. Yu, and P. O. Brown. 1993. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 12:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth, M. J., P. L. Schwartzberg, and S. P. Goff. 1989. Structure of the termini of DNA intermediates in the integration of retroviral DNA: dependence on IN function and terminal DNA sequence. Cell 58:47-54. [DOI] [PubMed] [Google Scholar]
- 36.Rowe, W. P., J. B. Humphrey, and F. Lilly. 1973. A major genetic locus affecting resistance to infection with murine leukemia viruses. 3. Assignment of the Fv-1 locus to linkage group 8 of the mouse. J. Exp. Med. 137:850-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoemaker, C., J. Hoffman, S. P. Goff, and D. Baltimore. 1981. Intramolecular integration within Moloney murine leukemia virus DNA. J. Virol. 40:164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Telesnitsky, A., and S. P. Goff. 1997. Reverse transcription. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Wei, S. Q., K. Mizuuchi, and R. Craigie. 1998. Footprints on the viral DNA ends in Moloney murine leukemia virus preintegration complexes reflect a specific association with integrase. Proc. Natl. Acad. Sci. USA 95:10535-10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei, S. Q., K. Mizuuchi, and R. Craigie. 1997. A large nucleoprotein assembly at the ends of the viral DNA mediates retroviral DNA integration. EMBO J. 16:7511-7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan, B., S. Campbell, E. Bacharach, A. Rein, and S. P. Goff. 2000. Infectivity of Moloney murine leukemia virus defective in late assembly events is restored by late assembly domains of other retroviruses. J. Virol. 74:7250-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan, B., X. Li, and S. P. Goff. 1999. Mutations altering the Moloney murine leukemia virus p12 gag protein affect virion production and early events of the virus life cycle. EMBO J. 18:4700-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]