Abstract
This work demonstrates cell swelling as a new regulatory mechanism for the cloned hyperpolarization-activated, cyclic nucleotide-gated channel 2 (HCN2). HCN2 channels were coexpressed with aquaporin1 in Xenopus laevis oocytes and currents were monitored using a two-electrode voltage-clamp. HCN2 channels were activated by hyperpolarization to −100 mV and the currents were measured before and during hypoosmotic cell swelling. Cell swelling increased HCN2 currents by 30% without changing the kinetics of the currents. Injection of 50 nl intracellular solution resulted in a current increase of 20%, indicating that an increase in cell volume also under isoosmotic conditions may lead to activation of HCN2. In the absence of aquaporin1 only negligible changes in oocyte cell volume occur during exposure to hypoosmotic media and no significant change in HCN2 channel activity was observed during perfusion with hypoosmotic media. This indicates that cell swelling and not a change in ionic strength of the media, caused the observed swelling-induced increase in current. The increase in HCN2 current induced by cell swelling could be abolished by cytochalasin D treatment, indicating that an intact F-actin cytoskeleton is a prerequisite for the swelling-induced current.
INTRODUCTION
HCN2 is a member of the family of hyperpolarization-activated, cyclic nucleotide-gated channels. In vertebrates, four different isoforms are known, HCN1–4 (1–6). The HCN channels are molecular correlates for the current abbreviated as Ih, for “hyperpolarization”, If for “funny”, or Iq for “queer” because of some peculiar features. The channels exhibit the classical voltage-gated potassium channel topology, including six transmembrane segments, a positively charged S4 segment involved in voltage gating, and a GYG motif in the central pore region. The GYG motif normally confers K+ selectivity to ion channels (7). However, HCN channels conduct K+, Na+, and Ca2+ ions. The residues outside the GYG triplet in HCN channels differ from other K+ channels, which may account for the low K+ selectivity. Unlike the classical voltage-gated potassium channels, the HCN channels are activated by hyperpolarizing steps to potentials more negative than −60 mV. Due to these unusual channel properties, HCN channels conduct a net inward current carried largely by Na+ at potentials near the resting membrane potential for most cells (8).
HCN channels have been detected in both heart and brain, where they have been proposed to be involved in pacing rhythmic activity as well as determination of the resting potential and membrane excitability (8). The expression of HCN varies between species. The dominant HCN isoform in the adult sinoatrial node of all species investigated (rabbit, mouse, dog) is HCN4, accounting for 80% of the total HCN mRNA (1). In the mouse, HCN2 constitutes the remaining transcripts (9). In contrast, in the rabbit sinoatrial node, a significant HCN1 transcript was observed (10). An open question is whether the expression pattern in the human sinoatrial node resembles that of mice or that of rabbits (11). In all species investigated, the HCN2 expression was high in the working myocardium (12).
The functional significance of HCN2 channels has been demonstrated in knock-out experiments. HCN2-deficient mice exhibited a cardiac arrhythmia characterized by varying RR intervals but normal PQ, QRS, and QT intervals and individual waveforms, indicating that the arrhythmia is due to dysfunction of the sinus node. In addition, these mice displayed neuronal defects such as reduced locomotor activity and spontaneous absence seizures (13,14).
The pacemaker channels are highly responsive to changes in cAMP and thereby involved in the adaptation of the heart to adrenergic stimulation leading to a faster heart rate (15–18). cAMP binds directly to the HCN channels in the C-terminal cyclic nucleotide-binding domain (16). In the absence of cAMP, the cyclic nucleotide-binding domain is thought to inhibit activation of the channels by hyperpolarization (19). Binding of cAMP shifts the voltage dependence of channels by relieving this inhibition (16), accelerating the rate of channel activation, and shifting the voltage dependence of HCN gating to more positive potentials (20). As a result, less hyperpolarization is necessary to activate the HCN channel, and the channel is thus more active at physiologically relevant potentials after cAMP binding.
Furthermore, the function of HCN channels is affected by associations with β-subunits of the KCNE type (21–24). KCNE2 is a member of the KCNE family of single transmembrane-spanning proteins and it has been demonstrated that association of HCN2 with KCNE2 increases expression levels and activation rate of HCN2 (23,24).
Other regulatory mechanisms for HCN channels are possible. HCN channels are present in cardiac tissue that is subject to morphological changes in shape and, during ischemia, also in volume (25). It is therefore tempting to speculate about a direct effect of cell volume changes on HCN channel activity. For other cardiac ion channels such as KCNQ1 such a regulatory mechanism has been demonstrated (26–28).
The aim of this study was therefore to examine the effect of volume changes on human HCN2 channels expressed in Xenopus laevis oocytes and to examine the mechanism(s) potentially linking channel activity and cell volume. Here we provide novel evidence that the HCN2 channels are modulated by cell swelling and this activation most likely involves interaction with the F-actin cytoskeleton.
MATERIALS AND METHODS
Molecular biology
cDNA encoding for human HCN2, a kind gift from Andreas Ludwig (Department of Pharmacology and Toxicology, the Technical University of Munich, Germany) and human KCNQ4, a kind gift from Thomas Jentsch (Centre for Molecular Neurobiology, Hamburg University, Germany), were subcloned into the oocyte expression vector pXOOM containing the 5′- and 3′-untranslated regions for X. laevis β-globin as well as a poly-A segment (29). Aquaporin 1 (AQP1) cDNA in the vector pMST1 was a kind gift from Peter Agre (Departments of Biological Chemistry and Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD). Human KCNE2 was subcloned into a custom-made vector adapted for oocyte expression. cRNA was synthesized by standard in vitro run-off transcription using the mCAP mRNA capping kit (Stratagene, La Jolla, CA). The plasmids were linearized downstream of the poly-A segment and mRNAs were synthesized from the T7 RNA polymerase promoter for the pXOOM and KCNE2 constructs, AQP1 mRNA was synthesized from the T3 RNA polymerase promoter (30). The RNA was resolved in TE buffer to a final concentration of 1 μg/μl and the integrity of the transcripts was confirmed by agarose gel electrophoresis.
Expression in Xenopus laevis oocytes
Oocytes were surgically removed from anaesthetized (2 g/l tricaine, Sigma, St. Louis, MO) X. laevis frogs in accordance with Danish National Committee for Animal Studies guidelines. The oocytes were defolliculated enzymatically as described earlier (31) before injection with 50.6 nl mRNA (∼50 ng) (Nanoject, Drummond, Broomell, PA). For coexpression of KCNQ4 or HCN2 with AQP1 and KCNE2, the mRNAs were mixed in equal molar ratios. The oocytes were kept in Kulori medium (90 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM HEPES, pH 7.4) at 19°C until electrophysiological measurements were performed.
Electrophysiology
All measurements were performed 3–5 days after mRNA injection using a standard two-electrode, voltage-clamp setup. Oocytes were impaled with a current electrode and a voltage-clamp electrode pulled from borosilicate glass on a DMZ-Universal Puller (Zeitz Instruments, Munich, Germany). Both the electrodes had a final tip resistance of 0.5–2 MΩ when filled with 1 M KCl and were connected to a two-electrode voltage-clamp amplifier (Clampator 1, Dagan, Chicago, IL). During the experiments the oocytes were placed in a small chamber (200 μl) and superfused with either isoosmotic (65 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 50 mM mannitol, 5 mM HEPES, pH 7.4 (188 mOsmol/l)), hypoosmotic (65 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM HEPES, pH 7.4 (137 mOsmol/l)), or hyperosmotic (65 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 100 mM mannitol, 5 mM HEPES, pH 7.4 (239 mOsmol/l)) medium. In some experiments, the oocytes were subjected to isoosmotic swelling by direct injection of ∼50 nl intracellular solution (81 mM KCl, 13 mM NaCl, 5 mM HEPES, pH 7.2 (193 mOsm/l)) or 50 nl of oil (light white, ICN Biomedicals, Aurora, OH) into the oocyte cytoplasm using a microinjector (Nanoject, Drummond, Broomell, PA).
cAMP experiments
Isoosmotic and hypoosmotic solutions containing a cAMP cocktail were prepared from stocks: 1 M IBMX (Calbiochem) in dimethylsulfoxide (DMSO), 0.1 M 8-bromoadenosine-3′-5′-cyclic monophosphate-cAMP (8-Br-cAMP, Sigma) in H2O, and 100 mM forskolin (Sigma) in DMSO to final concentrations of 1 mM IBMX, 400 μM 8-Br-cAMP, and 5 μM forskolin.
Cytochalasin D/phalloidin treatment
Oocytes were in some experiments preincubated for 2–5 h in 10 μM cytochalasin D (CD, Sigma) and 25 μM phalloidin (Sigma) before being subjected to osmotic changes. CD was diluted from a 10 mM stock in DMSO and the phalloidin solution was prepared from a 50 mM stock in H2O.
Data analysis
Data was acquired using Pulse software (HEKA, Munich, Germany) and analyzed using Igor Pro 4.04 software (WaveMetrics, Lake Oswego, OR); t-tests were performed using GraphPad Prism version 3.00 (GraphPad Software, San Diego, CA). Statistics were calculated on nonnormalized currents unless otherwise indicated. The results are presented as mean ± SD and the significance levels are indicated by asterisks (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
RESULTS
Hypoosmotic cell swelling increases HCN2 currents
The purpose of this study was to examine the effect of changes in cell volume on cloned HCN2 channels expressed in X. laevis oocytes.
In previous work from our laboratory changes in cell volume in both native and AQP1 water channels expressing oocytes were determined using a two-electrode voltage-clamp setup equipped with a CCD camera to monitor changes in oocyte volume before and during various osmotic challenges (26,27). These studies showed that native oocytes respond with minor changes in cell volume after changes in extracellular osmolarity (<0.2% after 50 s in ±50 mOsm). In contrast, oocytes expressing AQP1 respond with significant changes in cell volume upon exposure to osmotic challenges (∼5% after 50 s and 8% after 100 s in ±50 mOsm, and the volume continued increasing for >300 s at a slower rate) independently of the applied voltage protocol (26). Thus, to ensure adequate response to changes in extracellular osmolarity, AQP1 channels were coexpressed with the HCN2 channels.
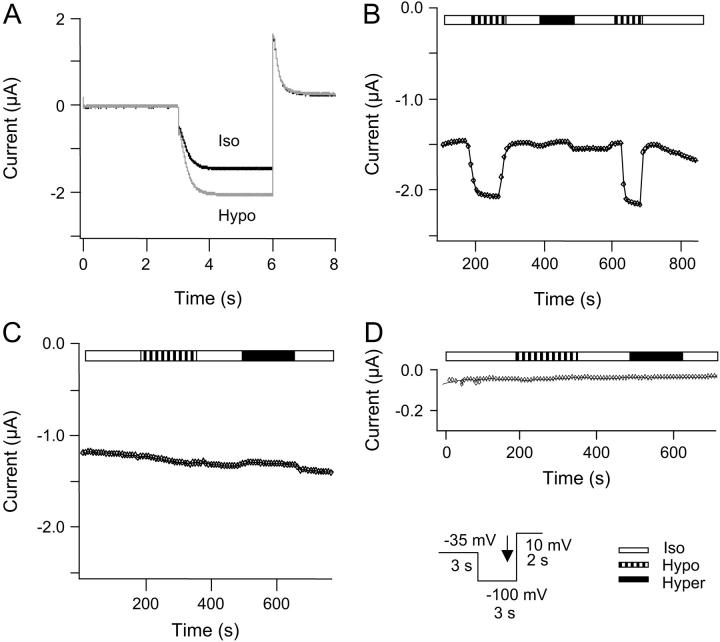
Fig. 1 A shows the results of an experiment where HCN2 channels were coexpressed with AQP1. The oocyte membrane was voltage-clamped and whole-cell currents were measured in oocytes superfused with isoosmotic extracellular solution. From a holding potential at −35 mV (3 s duration), oocytes were clamped to −100 mV for 3 s, followed by a step to 10 mV for 2 s. After obtaining stable current amplitudes, the oocytes were superfused with hypoosmotic extracellular solution, causing swelling of the oocyte (26). The increase in cell volume resulted in a significant increase in HCN2 currents of 30 ± 14% (n = 16, paired t-test, p = 0.0003, see also Fig. 7).
FIGURE 1.
Regulation of HCN2 channel current by changes in cell volume. HCN2 and AQP1 channels were expressed in X. laevis oocytes, and currents were activated by a step protocol, as indicated. Whole-cell currents were recorded at the end of the hyperpolarization step as indicated by the black arrow in the inset. The oocytes were superfused by isoosmotic extracellular solution followed by exposure to hypoosmotic extracellular solution and to hyperosmotic extracellular solution. (A) Current traces recorded in isoosomotic extracellular solution (black) and in hypoosmotic extracellular solution (shaded). For oocytes coexpressing HCN2 and AQP1 the mean increase in current after exposure to hypoosmotic extracellular solution was 30 ± 14% (n = 16). (B) The current depicted as a function of time after changes in extracellular osmolarity for the same experiment. (C) Time course of the whole-cell current in an oocyte expressing HCN2 channels without AQP1. The currents were recorded as described for B. The current was not significantly increased by exposure to hypoosmotic solution (1 ± 7%, n = 8, paired t-test, p = 0.9805) upon exposure to hypoosmotic extracellular solution. (D) Time course of whole-cell current in a native oocyte. The currents were recorded as described for A (n = 4).
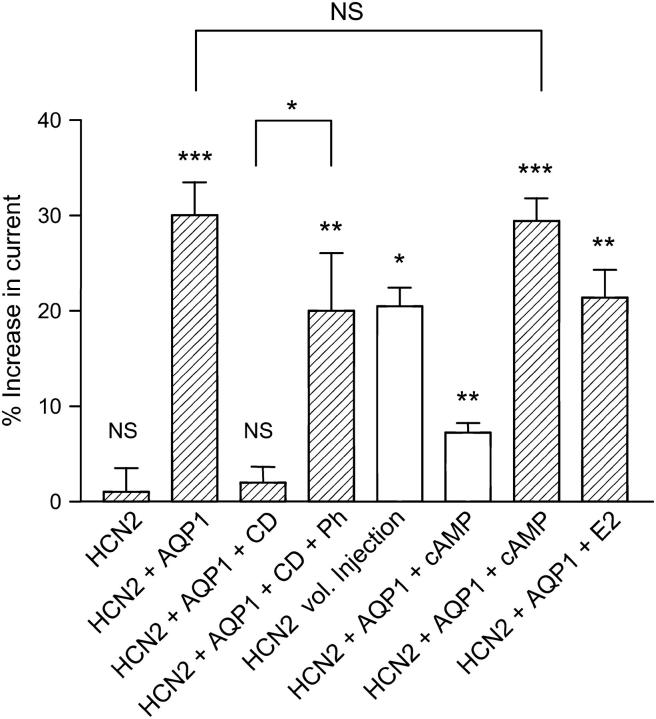
FIGURE 7.
Summary of the response of HCN2 channels to various stimuli. Relative responses as compared to controls are shown after different stimuli: (HCN2) For X. laevis oocytes expressing HCN2 and not AQP1, there was no significant increase in current after exposure to extracellular hypoosmotic solution (1 ± 7%, n = 8, paired t-test, p = 0.9805). (HCN2 + AQP1) For oocytes coexpressing HCN2 and AQP1, the current increased by 30 ± 14% after exposure to hypoosmotic extracellular solution. This increase is highly significant (n = 16, paired t-test, p = 0.0003). (HCN2 + AQP1 + CD) After exposure to 10 μM CD for t > 3 h before the experiments, there was no significant response to hypoosmotic volume changes (2 ± 7%, n = 9, paired t-test, p = 0.1920). (HCN2 + AQP1 + CD + Ph) The response could be at least partially rescued by adding 25 μM phalloidin to the 10 μM CD solution (20 ± 16%, n = 7, paired t-test, p = 0.0097). (HCN2 vol. Injection) Isoosmotic cell swelling of HCN2-expressing X. laevis oocytes was induced by injection of 50 nl isoosmotic solution. Injection resulted in a significant current increase by 21 ± 4% (n = 4, paired t-test, p = 0.0138). (HCN2 + AQP1 + cAMP) Increased cAMP resulted in an enhancement of HCN2 current of 7 ± 2% (n = 5, paired t-test, p = 0.001, open bar). Cell swelling in the presence of cAMP significantly increased HCN2 current by 29 ± 5% (n = 5, paired t-test, p < 0.0001) compared to isoosmotic media without cAMP. (HCN2 + AQP1 + E2) Coexpression of HCN2, AQP1, and KCNE2 resulted in a threefold current increase compared to oocytes expressing HCN2 and AQP1 alone (data not shown). The relative current increase upon exposure to hypoosmotic solution for HCN2, AQP1, KCNE2-expressing oocytes was 21 ± 6% (n = 4, paired t-test, p = 0.0069).
Fig. 1 B shows the time course of whole-cell current as a function of time after changes in extracellular osmolarity. The HCN2 channels were activated by hyperpolarization as described above; the membrane potential was clamped to −100 mV for 3 s, and currents were recorded at the end of this hyperpolarization step. The response to cell swelling was a rapid increase in HCN2 current. This increase in HCN2 current was completely reversed by changing extracellular conditions from hypo- to isoosmotic solution. The response of the HCN channels to cell swelling could be repeated by subsequent exposures to hypoosmotic solution. In contrast, HCN2 currents did not change significantly when the oocytes were exposed to hyperosmotic extracellular solution (cell shrinkage). All further experiments were therefore devoted to investigate the increased activity of HCN channels after cell swelling. To verify that the observed increase in HCN2 currents was caused by an increase in cell volume and not by changes in osmolarity per se, currents in oocytes expressing HCN2 without AQP1 were measured under the same conditions as described above (Fig. 1 C). After exposure to hypoosmotic extracellular solution, no significant increase in current was observed (1 ± 7%, n = 8, paired t-test, p = 0.9805, see also Fig. 7). The HCN2 current could be abolished by application of 1 mM Cs+ (data not shown). Also, currents were studied in noninjected oocytes (n = 3) subjected to the same voltage protocol. The whole-cell current did not increase upon exposure to hypoosmotic extracellular solution (Fig. 1 D).
Endogenous currents do not contribute to the increase in current observed after changes in cell volume
In a previous study by our group, the voltage-dependent potassium channel KCNQ4 currents were measured simultaneously with oocyte volume (27). The increase in KCNQ4 current correlated with the increase in cell size as a function of time. The experiment was repeated several times (n > 10) and the increase in current upon swelling was very consistent. Hence, KCNQ4 currents were used to confirm cell volume changes in a control experiment to exclude contribution of endogenous swelling-induced currents and to confirm functional expression of AQP1 channels. X. laevis oocytes were coinjected with AQP1 and KCNQ4. The oocytes were subjected to the standard protocol used for HCN2 time course experiments: from a holding potential of −35 mV for 3 s, the voltage was clamped at −100 mV for 3 s, followed by a +10 mV step for 2 s. Whole-cell currents were measured upon exposure to isoosmotic, hypoosmotic, and hyperosmotic extracellular solution. As shown in Fig. 2 A, the KCNQ4 channels were closed at the −100 mV step and activated by the +10 mV step. Furthermore, a large increase in the KCNQ4 current at the +10 mV step upon hypoosmotic swelling of the oocyte was observed. Hyperosmotic induced shrinkage of the oocyte reduced KCNQ4 current. The time courses for this experiment are summarized in Fig. 2 B. These observations confirmed that AQP1 was expressed and functional, and that endogenous currents were not activated at hyperpolarized potentials by the standard protocol applied for HCN activation in either iso-, hypo-, or hyperosmotic solution. Oocytes expressing only AQP1 were also tested using the same voltage-clamp protocol and exhibited no change in current amplitude upon changes in external osmolarity (n = 3, data not shown).
FIGURE 2.
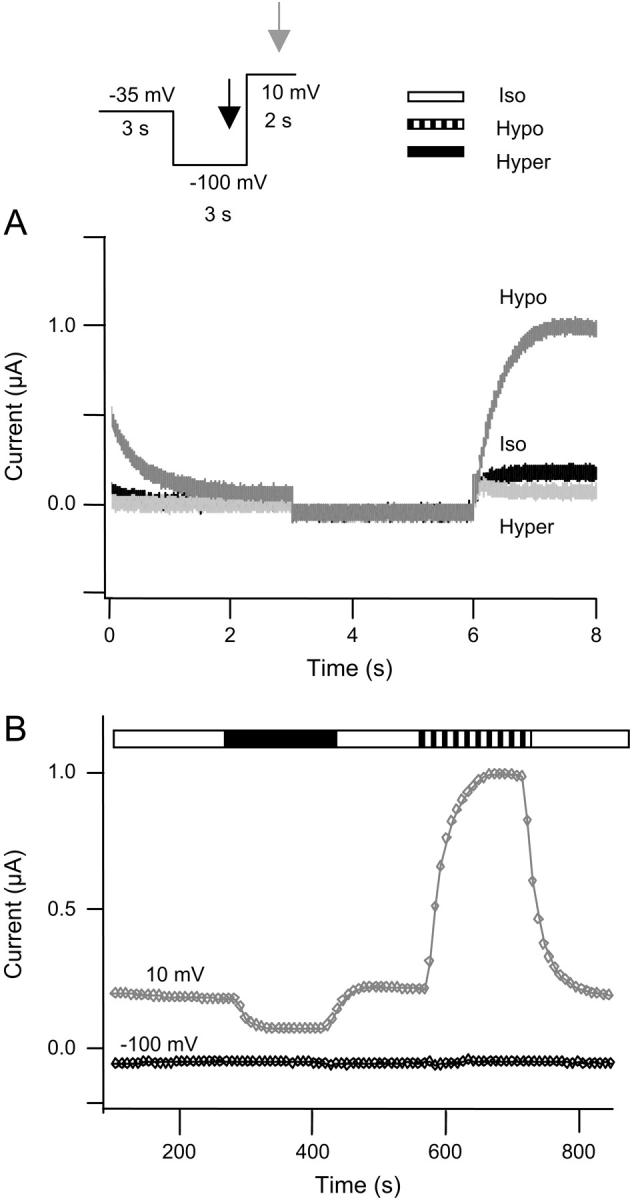
Control for endogenous currents KCNQ4 and AQP1 channels were expressed in X. laevis oocytes and currents were activated by a step protocol, as illustrated in the inset. (A) Current traces recorded in isoosmotic (black), hypoosmotic (dark shaded), and hyperosmotic (light shaded) extracellular solution. KCNQ4 currents were activated by a step to +10 mV. The KCNQ4 currents deactivated at −35 mV and at −100 mV no currents were observed. (B) Time course of whole-cell current in an oocyte coexpressing KCNQ4 and AQP1. Current as a function of time after changes in extracellular osmolarity is shown. Currents were simultaneously recorded at the end of the −100 mV step (indicated by black arrow in the inset) and at the end of the +10 mV step (indicated by shaded arrow in the inset). The current at −100 mV is shown in black symbols, whereas the current recorded at +10 mV is shown in shaded symbols. The oocytes were superfused by isoosmotic extracellular solution followed by exposure to hypoosmotic extracellular solution and to hyperosmotic extracellular solution.
Hypoosmotic cell swelling does not affect the HCN2 current kinetics
In the experiment shown in Fig. 3, HCN2 channels were coexpressed with AQP1 in X. laevis oocytes. To investigate the effects of hypoosmotic cell swelling on kinetics, currents were activated before and during hypoosmotic swelling by a step protocol; from a holding potential of −35 mV (2 s duration); currents were recorded at membrane potentials from −60 mV to −130 in (10 mV decrements) for 3 s, followed by a 10 mV step for 2 s. Fig. 3 A shows selected current traces before and during hypoosmotic cell swelling. Upon normalization of current traces no obvious changes in activation and deactivation kinetics were observed (Fig. 3 B). Furthermore, currents were measured at the end of each hyperpolarized step to establish whether the I-V relationship (Fig. 3 C) for HCN2 was changed during modulation by the change in cell volume. The apparent reversal potential and the overall voltage sensitivity for HCN2 channels were not affected by hypoosmotic cell swelling.
FIGURE 3.
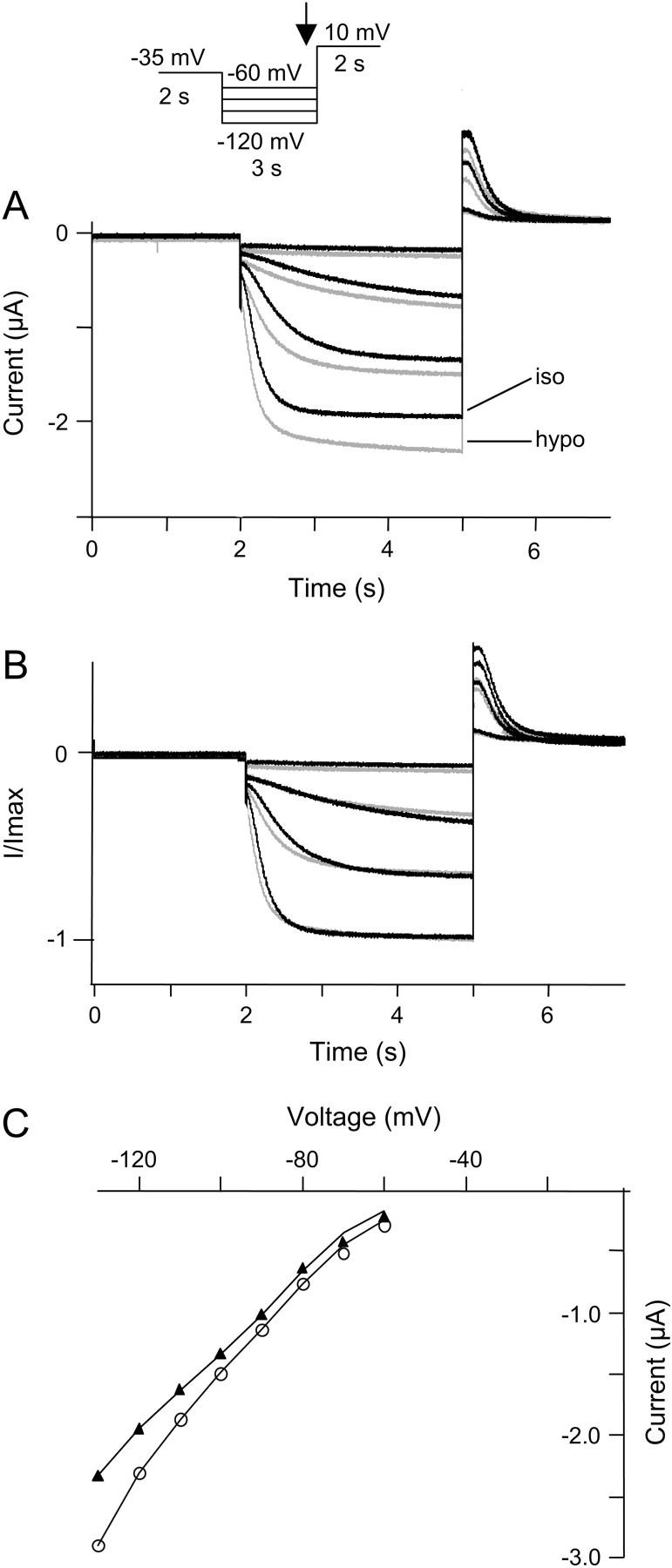
Kinetics of HCN2 current are unaffected by hypoosmotic cell swelling. HCN2 channels and AQP1 were expressed in X. laevis oocytes and currents were activated by a step protocol as indicated. (A) Initially, currents were recorded in oocytes superfused by an isoosmotic extracellular solution (black traces). The oocytes were then exposed to hypoosmotic extracellular solution for 4 min and currents were recorded (shaded traces). (B) Normalized current traces from A. (C) The corresponding I-V curves (plotted as the current measured at the end of the hyperpolarization versus voltage). (▴) Currents recorded in isoosmotic solution. (○) Currents measured in hypoosmotic solution.
Isoosmotic cell swelling increases HCN2 current amplitude without affecting channel kinetics
The changes in cell volume in the above experiments were induced by changes in the extracellular osmolarity. To establish whether isoosmotic swelling of the oocyte could increase the activity of HCN2, oocytes expressing HCN2 channels were injected with 50 nl isoosmotic intracellular solution. Assuming that the oocyte represented a sphere with a volume of 1 μl, injection of 50 nl would result in a volume increase of 5%. This 5% corresponds to the volume changes upon hypoosmotic swelling as measured previously in our laboratory. However, the oocyte membrane is strongly invaginated making it impossible to predict the effect of the injection. Currents were activated by a step protocol; from a holding potential at −35 mV (2 s duration), currents were recorded at potentials from −35 to −105 mV (3 s duration) in 10 mV decrements followed by a step to 10 mV for 2 s. Currents were measured in isoosmotic extracellular solution before and after injection, and selected traces are shown in Fig. 4 A. Injection of intracellular solution, leading to cell swelling, caused a significant current increase, on average 21 ± 4% (n = 4, p = 0.0138, paired t-test, see also Fig. 7).
FIGURE 4.
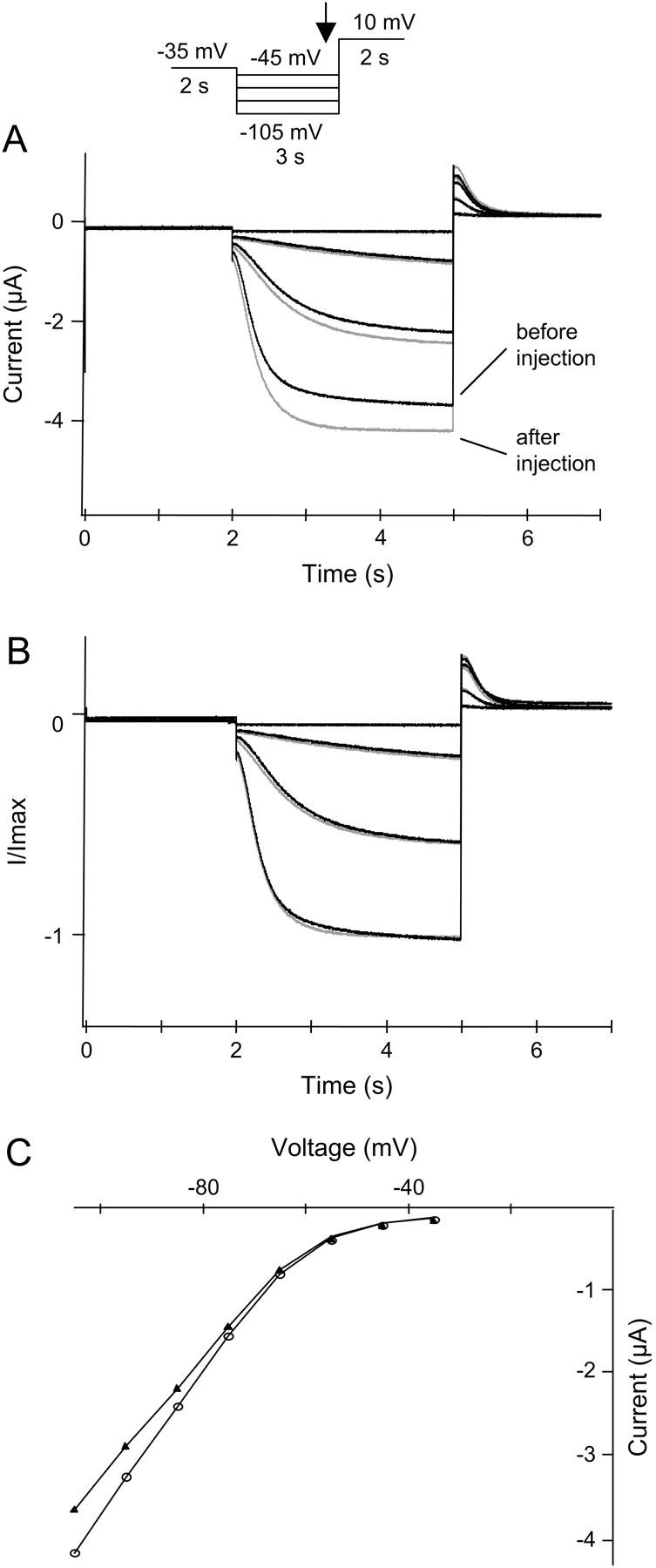
HCN2 currents are increased upon isoosmotic cell swelling. HCN2 channels were expressed in X. laevis oocytes and currents were activated by a step protocol as illustrated. Currents were measured in an isoosmotic extracellular solution before and during isoosmotic cell swelling. (A) HCN2 currents recorded before volume injection (black traces) and after injection of 50 nl intracellular solution (shaded traces), resulting in a current increase of 21 ± 4% (n = 4). (B) Normalized current traces from A. (C) The corresponding I-V curves (plotted as the current measured at the end of the hyperpolarization versus voltage). (▴) Currents recorded before injection. (○) Currents measured after injection of 50 nl intracellular solution.
When normalizing the current traces before and after injection, no obvious changes in kinetics were observed (Fig. 4 B). Fig. 4 C shows the corresponding I-V relationship for currents measured at the end of each hyperpolarized step. The results indicated that the overall voltage sensitivity and the selectivity for HCN2 channels were not affected by changes in cell volume.
To test if the observed current increase was directly due to changes in cell volume and not an effect of dilution of some intracellular chemical signal, an additional experiment was performed. Instead of isoosmotic intracellular solution, the oocytes were injected with 50 nl oil. This resulted in a 21 ± 11% (n = 7) increase in HCN2 current. This experiment thereby supported the notion that the HCN2 current increase was actually caused by the changes in cell volume and not by dilution of any intracellular messengers.
The swelling-induced increase in HCN2 current is not enhanced by cAMP
To examine whether the observed increase in current could be enhanced by cAMP, X. laevis oocytes coexpressing HCN2 and AQP1 were superfused with isoosmotic or hypoosmotic extracellular solution in the absence or presence of increased cAMP (Fig. 5). Intracellular cAMP was increased by various combinations of IBMX (phosphodiesterase inhibitor), forskolin (adenylate cyclase activator), and 8-Br-cAMP (cell-permeable cAMP analog). Reproducible activation of the HCN2 currents was obtained by including 1 mM IBMX, 400 μM 8-Br-cAMP, and 5 μM forskolin in the extracellular solution. High concentrations of drugs are necessary in X. laevis oocyte experiments when applied to the extracellular surface of whole oocytes due to the viteline membrane and the yolk, which reduce the actual concentration of drugs at the cell membrane (32).
FIGURE 5.
Volume regulation of HCN2 channels in the presence of cAMP. HCN2 channels and AQP1 were coexpressed in X. laevis oocytes. The oocytes were superfused with isoosmotic and hypoosmotic extracellular solution with and without a compound mixture composed to increase cAMP l (1 mM IBMX, 400 μM 8-Br-cAMP, and 5 μM forskolin). Currents were activated by a step protocol as illustrated in the inset. (A) Current traces recorded in isoosmotic extracellular solution (black trace), hypoosmotic extracellular media (dark shaded trace), isoosmotic media containing cAMP (shaded trace), and hypoosmotic media solution (light shaded trace). (B) Time course of whole-cell current in an oocyte expressing HCN2 and AQP1. The figure shows the current as a function of time after changes in extracellular osmolarity and cAMP as indicated by the bars. The currents were recorded at the end of the hyperpolarization step (indicated by black arrow in inset). Increased cAMP resulted in an enhancement of HCN2 current of 7 ± 2% (n = 5, paired t-test, p = 0.001, open bar). Cell swelling in the presence of cAMP significantly increased HCN2 current by 29 ± 5% (n = 5, paired t-test, p < 0.0001) compared to isoosmotic media without cAMP.
HCN2 currents were activated by a step protocol; from a holding potential at −35 mV (3 s duration), the membrane potential was clamped to −100 mV for 3 s, followed by a step to 10 mV for 2 s, as illustrated in the inset. The corresponding current traces are shown in Fig. 5 A and the time course of whole-cell current in Fig. 5 B. The oocytes were first exposed to hypoosmotic cell swelling. The current was allowed to recover in isoosmotic solution and was then superfused by isoosmotic solution containing 1 mM IBMX, 400 μM 8-Br-cAMP and 5 μM forskolin to increase intracellular cAMP. This resulted in a significant current increase of 7 ± 2% on average (n = 5, paired t-test, p = 0.001). After obtaining a stable current level, the isoosmotic extracellular solution was replaced by hypoosmotic extracellular solution also containing 1 mM IBMX, 400 μM 8-Br-cAMP, and 5 μM forskolin resulting in a highly significant current increase by 29 ± 5% (n = 5, paired t-test, p < 0.0001) compared to isoosmotic solution without intracellular cAMP enhancement (see also Fig. 7). The 29% increase in current upon hypoosmotic cell swelling in the presence of cAMP was not significantly different from the 24 ± 6% increase in current observed in the initial hypoosmotic challenges without cAMP in the same experiment (n = 5, paired t-test, p = 0.259). These results indicated that cAMP did not influence the response of HCN2 current to hypoosmotic swelling. To control whether endogenous currents contributed to the change in current after exposure to cAMP and hypoosmotic solution, oocytes expressing AQP1 alone were subjected to the same treatment as the HCN2 + AQP1-expressing oocytes. No response to either volume changes or cAMP concentration was observed (n = 4, data not shown) for the AQP1-expressing oocytes.
The swelling-induced increase in HCN2 current is not affected by KCNE2
Association of HCN2 with KCNE2 has been reported to increase expression levels of HCN2 and its maximal conductance by more than fivefold in X. laevis oocytes (24). To investigate whether KCNE2 coexpression had any effect on HCN2 currents during hypoosmotic cell swelling, oocytes were injected with HCN2, KCNE2, and AQP1 in the ratio 1:4:1. In parallel, control oocytes were injected with equivalent amounts of HCN2 and AQP1. Currents were measured at −100 mV. In our hands, coexpression of HCN2 with KCNE2 resulted in a threefold increase in current compared to oocytes injected with HCN2 alone. The relative current increase of the KCNE2 coexpressing oocytes to hypoosmotic solution was 21 ± 6% (n = 4, paired t-test, p = 0.0069, see Fig. 7). This was not significantly different from the 30 ± 14% response observed in oocytes expressing HCN2 and AQP1 alone (unpaired t-test on normalized currents, p = 0.2393).
The swelling-induced increase in HCN2 current requires an intact actin cytoskeleton
In X. laevis stage VI oocytes the F-actin cytoskeleton is associated with the cortical layer of the oocyte and within the cytoplasm, surrounding the geminal vesicle and extending toward the vegetal cortex, forming a dense mesh of fibers (33). An intact actin cytoskeleton has previously been demonstrated to be a prerequisite for regulation of potassium channel activity by cell volume changes, e.g., for the Ca2+-activated potassium channels IK and SK3 (26,34) and for the voltage-gated potassium channel KCNQ1 (27). In general, cell swelling has been reported to cause a decrease in cellular F-actin (34) and this change in the structure of the F-actin cytoskeleton is thus a possible mechanism linking cell swelling to regulation of ion channel activity. In addition, it has been shown that treatment with CD, a drug commonly used to depolymerize F-actin, disrupts the volume modulation of IK, SK3 expressed in HEK-293 cells (34), and in X. laevis oocytes (27). In HEK-293 cells, CD significantly decreases net cellular F-actin content (34). Preincubation of oocytes with phalloidin, an actin filament stabilizing complex, eliminated the effect of CD, again indicating that the disruption of the volume response was due to CD's effect on the actin cytoskeleton (27).
To investigate whether the actin cytoskeleton was involved in mediating the response of HCN2 channels to cell volume changes, HCN2 channels and AQP1 were expressed in X. laevis oocytes incubated in Kulori (90 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM HEPES, pH 7.4) containing 10 μM CD for 2–5 h. In previous experiments, we have demonstrated that disruption of the F-actin cytoskeleton by CD treatment, in the concentrations and with the timescale applied in this study, does not influence the volume response of Xenopus oocytes (26). The currents were activated by a step protocol; from a holding potential at −35 mV (2 s duration), currents were recorded at potentials from −35 to −105 mV (3 s duration) in 10 mV decrements followed by a step to 10 mV for 2 s duration (Fig. 6 A). The corresponding time course of whole-cell current after changes in extracellular osmolarity is shown in Fig. 6 B. The currents were recorded at the end of a hyperpolarization step to only −70 mV to prevent damage to the oocytes. For oocytes coexpressing HCN2 and AQP1, the current increased by 30 ± 14% after exposure to hypoosmotic extracellular solution. After exposure to 10 μM CD for 2–5 h before the experiments, no significant response to hypoosmotic exposure (2 ± 7%, n = 9, paired t-test, p = 0.1920) was observed. This indicated a role for the F-actin cytoskeleton in linking cell volume increase to increased HCN2 current. The response to cell swelling could be at least partially rescued to 20 ± 16% by adding 25 μM phalloidin to the 10-μM CD solution, thereby inhibiting the CD-induced depolymerization of the cytoskeleton. Relative changes in HCN2 channel activity after changes in cell volume are summarized in Fig. 7.
FIGURE 6.
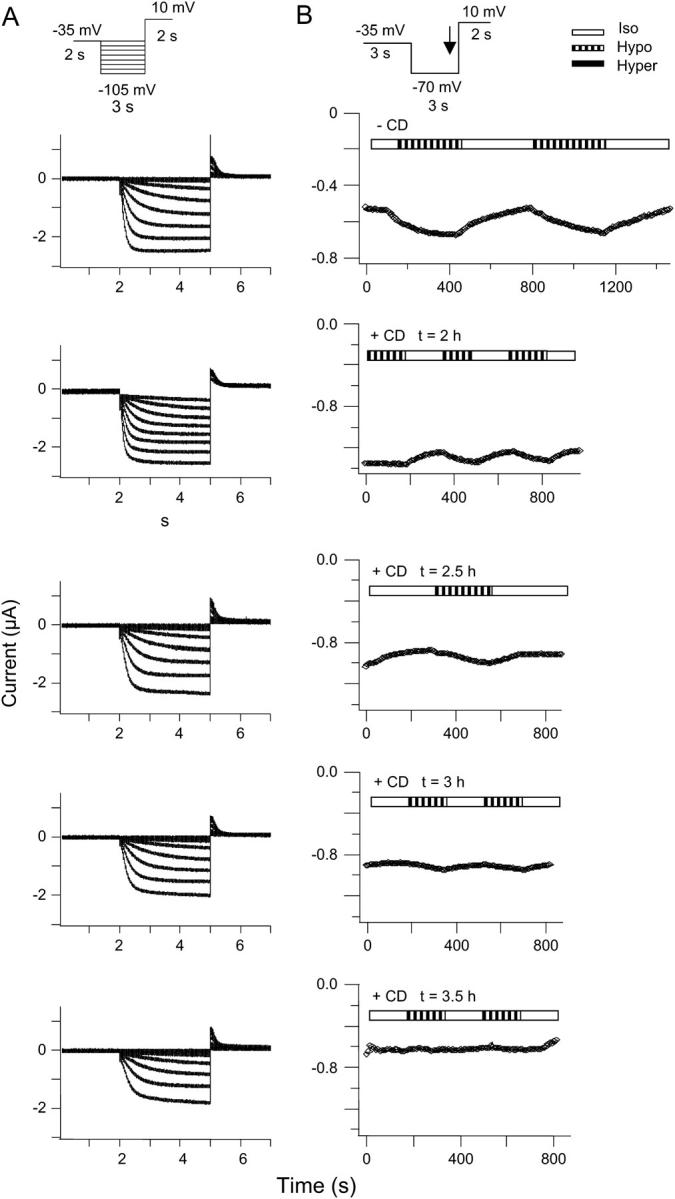
Modulation of HCN2 channels by cell swelling is dependent on an intact F-actin cytoskeleton. HCN2 channels and AQP1 were expressed in X. laevis oocytes. The oocytes were incubated in Kulori containing 10 μM CD for 2–5 h at 19°C. (A) The currents were activated by a step protocol,as indicated. The response to hypoosmotic volume changes was on average 2 ± 7% (n = 9) after 3 h in CD. (B) The corresponding time course of whole-cell current. Currents were activated by a step protocol as illustrated in the inset. The currents were recorded at the end of the hyperpolarization step (indicated by arrow in the inset). The figure shows the current as a function of time after changes in extracellular osmolarity, as indicated by the bars.
DISCUSSION
We examined the effects of changes in cell volume on cloned HCN2 channels expressed in X. laevis oocytes. HCN2 currents activated by hyperpolarization can be increased by 30% by hypoosmotic swelling of the oocyte. The response to hypoosmotic cell swelling was found to be dependent on an intact F-actin cytoskeleton. The increase in current was not influenced by interaction with the KCNE2 β-subunit and appeared to occur independently of cAMP levels.
The results presented in this study allow us to draw several conclusions concerning the mechanism for regulation of HCN2 channels by cell volume changes. Since potassium channels hydrate and dehydrate during conformational changes (35), it could be expected that extracellular osmotic changes influence the activity of these ion channels. However, in the absence of coexpressed AQP1, no measurable changes in HCN2 currents were found, indicating that the channels were sensitive to cell volume changes per se and not to changes in extracellular osmolarity.
When X. laevis oocytes are used to study the effect of cell volume changes on expressed proteins, the possible volume-dependent modulation of endogenous channels must be considered. Several types of endogenous volume-sensitive or mechanosensitive ion currents have been identified in X. laevis oocytes: a stretch-activated nonselective cation current ISA, a number of hypotonicity-activated Cl− channels, a swelling-activated Ca2+-independent Cl− current Icl swell, a Ca2+-activated Cl− current, and ClC-3- and ClC-5-like Cl− currents (36–41). Contribution of endogenous currents to the swelling-induced current increase was excluded by experiments where AQP1 was coexpressed with KCNQ4.
To investigate the effect of cell volume changes on HCN2 coexpressed with AQP1 in X. laevis oocytes, currents were measured by two-electrode voltage-clamp at −100 mV. Swelling of the oocyte resulted in a fast and reproducible increase in current by 30%. HCN2 currents were unaffected when the oocytes were exposed to hyperosmotic extracellular solution. The hypoosmotic-induced increase in HCN2 current could be mimicked by injection of 50 nl intracellular solution resulting in an isoosmotic volume. On injections, a current increase of 20% was observed. This current increase was not significantly different from the response seen after exposure to hypoosmotic solution. Neither hypoosmotic nor isoosmotic swelling of the oocyte changed the kinetics of the current or the apparent reversal potential.
In native tissue, KCNE1 (minK) has been shown to be a prerequisite for the response of KCNQ1 channels to cell-volume changes (42). This observation is not consistent with studies done on KCNQ1 channels expressed in the absence of KCNE1 in mammalian cells (43) or in X. laevis oocytes (27). The reason for this inconsistency is not clear. In our study, coexpression of human KCNE2 with human HCN2 resulted in a threefold larger conductance compared to oocytes expressing HCN2 alone. This is in the same range, but not identical to, previous findings in oocytes, where rat KCNE2 was reported to give a fivefold increase in mouse HCN2 currents (24). The discrepancy can possibly be explained by the different species investigated. The response of HCN2 to cell swelling is maintained upon coexpression with KCNE2 and the relative increase in current was similar to that of HCN2 alone.
Despite a large amount of experimental data demonstrating regulation of membrane proteins by cell volume, no firm evidence or conclusion exist as to how changes in cell volume are transmitted to changes in channel current (44).
To investigate the link between cell volume increase and the increase in current, a number of experiments were carried out.
cAMP is a well described and potent activator of HCN channels. HCN2 displays a shift in V½ of 12–14 mV in the positive direction upon cAMP stimulation, with K½ = 0.5 μM (2,3,19). Most groups investigate the effect of cAMP in inside-out patches; however, intact oocytes were a prerequisite for our investigation of the response to cell volume changes. Using this experimental setup, an increase in current of only 7% was found. This might have been due to the abnormally high and possibly variable levels of cAMP in the oocytes (45), which can also explain the observation that the HCN2 current started to activate at potentials less negative than in excised patches (19). To investigate if cAMP is involved in mediating the HCN2 current increase upon cell swelling, cAMP was added before and during hypoosmotic swelling of the oocyte. The relative current increase observed after intracellular cAMP enhancement was not significantly different from the current increase observed upon hypoosmotic cell swelling without cAMP enhancement, indicating that the cAMP level does not influence the current response of HCN2 to hypoosmotic swelling under these experimental conditions.
Regulation of channel activity by changes in the structure of the cytoskeleton has been shown for a number of channels including Na+ channels, cardiac L-type and T-type Ca2+ channels, Cl− channels, and various K+ channels (44). In nearly all systems, volume changes cause alteration of the cytoskeletal organization. In the majority of the systems examined, hypotonic cell swelling evokes disorganization of the F-cytoskeleton (44). Changes in intracellular osmolarity modulate the actin polymer itself (44), and this may in turn modulate ion flux. Thus, it is possible that the cytoskeleton can act as a volume sensor or transducer of the response to hypoosmosis in some cell types. In HCN2, the response to volume changes was abolished when the actin cytoskeleton was depolymerized by CD. Whether HCN2 interacts directly with the cytoskeleton or whether intermediary proteins are involved, is currently unknown. In the brain, HCN2 is reported to form a protein assembly with tamalin, S-SCAM, and Mint2 scaffold proteins (46). Interestingly, S-SCAM has also been reported to interact with β1-adrenergic receptors (47) and HCN4 colocalizes with β1-adrenergic receptors in lipid rafts in the membranes of ventricular myocytes (48). HCN1 has been shown to interact with the cytoplasmic scaffold protein filamin-A. Filamin-A has actin-binding domains enabling the protein to link transmembrane proteins to the cytoskeleton (49). Further study is necessary to demonstrate whether any of these candidates form a link between HCN2 channels and the cytoskeleton and are involved in the response to volume changes.
Suggested physiological role
Cell swelling causes stretch and deformation of the cell membranes and the underlying cytoskeletal network, as well as diluting intracellular environments. Most cells respond to swelling by modulating transporters and or ion channels that permit efflux of intracellular osmolytes (and osmotically obliged water), which will restore cell volume to its original value. Under normal physiological conditions HCN2 channels will carry inward sodium current. Seen in this light the HCN2 current response to cell swelling is most puzzling. Since hypoosmotic cell swelling increases HCN2 currents by 30% this would lead to influx of sodium and further swelling of the cell. It is therefore most likely that HCN2 does not take part in restoration of cell volume upon swelling.
In the heart, osmotic stress is encountered during a period of myocardial ischemia when metabolites such as lactate accumulate intracellularly and to a certain degree extracellularly, and cause cell swelling. This swelling may be exacerbated further on reperfusion when the hypertonic extracellular fluid is washed out by normotonic blood (25). In cardiac tissue, ectopic foci may arise in the ischemic border zone, leading to cardiac arrhythmia, and it can be speculated that an increase in sodium influx via swelling-modulated HCN2 channels could contribute to the electrophysiological abnormalities causing arrhythmias.
We speculate that a more physiological role of the swelling-induced increase in HCN2 currents could instead be related to conductivity of the myocardium and heart rate control. Current-clamp recordings have shown that the membrane potential of HCN2-deficient sinoatrial cells is more hyperpolarized than that of wild-type cells (14). This suggests that HCN2 is activated in wild-type cells and sets the membrane potential to a more depolarized level. Hence, the predominant role of HCN2 in these cells may be stabilization of a normal diastolic membrane potential (11,13,14). If cardiac cells are challenged with a diminished oxygen supply as a consequence of increased work load, cell swelling could occur. This would increase HCN2 currents, and the membrane potential would be more depolarized and more easily excitable, thereby giving a faster heart rate and an increased conductivity (50), effects that would beneficially compensate the increase in work. However, cell-volume increase as a consequence of increased work load still needs further investigation.
In conclusion, our work demonstrates hypoosmotic cell swelling as a new regulatory mechanism for HCN2 channels.
An intact F-actin cytoskeleton is a prerequisite for the response to increased cell volume. However, elucidation of the exact molecular mechanisms behind HCN2 volume regulation awaits further study.
Acknowledgments
We thank Dr. A. Ludwig and Dr. F. Hofmann for cDNA encoding hHCN2, Dr. T. Jentsch for the KCNQ4 clone, and Dr. P. Agre for the AQP1 clone. We thank Ms. B. Lynderup, T. Soland, and I. Kjeldsen for expert technical assistance.
We acknowledge the financial support of The John and Birthe Meyer Foundation, The Velux Foundation, The NovoNordisk Foundation, The Danish Heart Foundation, The Danish Natural Sciences Research Council, The Danish Medical Research Council, and The Faculty of Health Sciences, University of Copenhagen, for a PhD scholarship to K.C.
References
- 1.Ishii, T. M., M. Takano, L. H. Xie, A. Noma, and H. Ohmori. 1999. Molecular characterization of the hyperpolarization-activated cation channel in rabbit heart sinoatrial node. J. Biol. Chem. 274:12835–12839. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig, A., X. G. Zong, M. Jeglitsch, F. Hofmann, and M. Biel. 1998. A family of hyperpolarization-activated mammalian cation channels. Nature. 393:587–591. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig, A., X. G. Zong, J. Stieber, R. Hullin, F. Hofmann, and M. Biel. 1999. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 18:2323–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santoro, B., S. G. Grant, D. Bartsch, and E. R. Kandel. 1997. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to eag and cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA. 94:14815–14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoro, B., D. T. Liu, H. Yao, D. Bartsch, E. R. Kandel, S. A. Siegelbaum, and G. R. Tibbs. 1998. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 93:717–729. [DOI] [PubMed] [Google Scholar]
- 6.Seifert, R., A. Scholten, R. Gauss, A. Mincheva, P. Lichter, and U. B. Kaupp. 1999. Molecular characterization of a slowly gating human hyperpolarization-activated channel predominantly expressed in thalamus, heart, and testis. Proc. Natl. Acad. Sci. USA. 96:9391–9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle, D. A., C. J. Morais, R. A. Pfuetzner, A. Kuo, J. M. Gulbis, S. L. Cohen, B. T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- 8.Robinson, R. B., and S. A. Siegelbaum. 2003. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65:453–480. [DOI] [PubMed] [Google Scholar]
- 9.Moosmang, S., J. Stieber, X. G. Zong, M. Biel, F. Hofmann, and A. Ludwig. 2001. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur. J. Biochem. 268:1646–1652. [DOI] [PubMed] [Google Scholar]
- 10.Shi, W. M., R. Wymore, H. G. Yu, J. Y. Wu, R. T. Wymore, Z. M. Pan, R. B. Robinson, J. E. Dixon, D. McKinnon, and I. S. Cohen. 1999. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ. Res. 85:E1–E6. [DOI] [PubMed] [Google Scholar]
- 11.Stieber, J., F. Hofmann, and A. Ludwig. 2004. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc. Med. 14:23–28. [DOI] [PubMed] [Google Scholar]
- 12.Biel, M., A. Schneider, and C. Wahl. 2002. Cardiac HCN channels: structure, function, and modulation. Trends Cardiovasc. Med. 12:206–213. [DOI] [PubMed] [Google Scholar]
- 13.Moosmang, S., J. Stieber, S. Kuhbandner, R. Feil, M. Biel, F. Hofmann, and A. Ludwig. 2002. Sinus arrhythmia in mice lacking the pacemaker channel HCN2. Naunyn Schmiedebergs Arch. Pharmacol. 365:R88. (Abstr.) [Google Scholar]
- 14.Ludwig, A., T. Budde, J. Stieber, S. Moosmang, C. Wahl, K. Holthoff, A. Langebartels, C. Wotjak, T. Munsch, X. Zong, S. Feil, R. Feil, M. Lancel, K. R. Chien, A. Konnerth, H. C. Pape, M. Biel, and F. Hofmann. 2003. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO. J. 22:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown, H. F., D. DiFrancesco, and S. J. Noble. 1979. How does adrenaline accelerate the heart? Nature. 280:235–236. [DOI] [PubMed] [Google Scholar]
- 16.DiFrancesco, D., and P. Tortora. 1991. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 351:145–147. [DOI] [PubMed] [Google Scholar]
- 17.Ulens, C., and J. Tytgat. 2001. G(i)- and G(s)-coupled receptors up-regulate the cAMP cascade to modulate HCN2, but not HCN1 pacemaker channels. Pflugers Arch.. 442:928–942. [DOI] [PubMed] [Google Scholar]
- 18.DiFrancesco, D. 1995. The onset and autonomic regulation of cardiac pacemaker activity: relevance of the f current. Cardiovasc. Res. 29:449–456. [PubMed] [Google Scholar]
- 19.Wainger, B. J., M. DeGennaro, B. Santoro, S. A. Siegelbaum, and G. R. Tibbs. 2001. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 411:805–810. [DOI] [PubMed] [Google Scholar]
- 20.Kaupp, U. B., and R. Seifert. 2001. Molecular diversity of pacemaker ion channels. Annu. Rev. Physiol. 63:235–257. [DOI] [PubMed] [Google Scholar]
- 21.Decher, N., F. Bundis, R. Vajna, and K. Steinmeyer. 2003. KCNE2 modulates current amplitudes and activation kinetics of HCN4: influence of KCNE family members on HCN4 currents. Pflugers Arch. 446:633–640. [DOI] [PubMed] [Google Scholar]
- 22.Proenza, C., D. Angoli, E. Agranovich, V. Macri, and E. A. Accili. 2002. Pacemaker channels produce an instantaneous current. J. Biol. Chem. 277:5101–5109. [DOI] [PubMed] [Google Scholar]
- 23.Qu, J., Y. Kryukova, I. A. Potapova, S. V. Doronin, M. Larsen, G. Krishnamurthy, I. S. Cohen, and R. B. Robinson. 2004. MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. J. Biol. Chem. 279:43497–43502. [DOI] [PubMed] [Google Scholar]
- 24.Yu, H., J. Wu, I. Potapova, R. T. Wymore, B. Holmes, J. Zuckerman, Z. Pan, H. Wang, W. Shi, R. B. Robinson, M. R. El Maghrabi, W. Benjamin, J. Dixon, D. McKinnon, and I. S. Cohen. 2001. MinK-related peptide 1: a beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ. Res. 88:E84–E87. [DOI] [PubMed] [Google Scholar]
- 25.Tranum-Jensen, J., M. J. Janse, W. T. Fiolet, W. J. Krieger, C. N. D'Alnoncourt, and D. Durrer. 1981. Tissue osmolality, cell swelling, and reperfusion in acute regional myocardial ischemia in the isolated porcine heart. Circ. Res. 49:364–381. [DOI] [PubMed] [Google Scholar]
- 26.Grunnet, M., N. MacAulay, N. K. Jorgensen, S. Jensen, S. P. Olesen, and D. A. Klaerke. 2002. Regulation of cloned, Ca2+-activated K+ channels by cell volume changes. Pflugers Arch. 444:167–177. [DOI] [PubMed] [Google Scholar]
- 27.Grunnet, M., T. Jespersen, N. MacAulay, N. K. Jorgensen, N. Schmitt, O. Pongs, S. P. Olesen, and D. A. Klaerke. 2003. KCNQ1 channels sense small changes in cell volume. J. Physiol. 549:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hougaard, C., D. A. Klaerke, E. K. Hoffmann, S. P. Olesen, and N. K. Jorgensen. 2004. Modulation of KCNQ4 channel activity by changes in cell volume. Biochim. Biophys. Acta. 1660:1–6. [DOI] [PubMed] [Google Scholar]
- 29.Jespersen T., M. Grunnet, K. Angelo, D. A. Klaerke, and S. P. Olesen. 2002. Dual-function vector for protein expression in both mammalian cells and Xenopus laevis oocytes. Biotechniques. 32:536–540. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., and D. W. Russel. 2001. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Grunnet, M., B. S. Jensen, S. P. Olesen, and D. A. Klaerke. 2001. Apamin interacts with all subtypes of cloned small-conductance Ca2+-activated K+ channels. Pflugers Arch. 441:544–550. [DOI] [PubMed] [Google Scholar]
- 32.Kiehn, J., A. E. Lacerda, B. Wible, and A. M. Brown. 1996. Molecular physiology and pharmacology of HERG. Single-channel currents and block by dofetilide. Circulation. 94:2572–2579. [DOI] [PubMed] [Google Scholar]
- 33.Roeder, A. D., and D. L. Gard. 1994. Confocal microscopy of F-actin distribution in Xenopus oocytes. Zygote. 2:111–124. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen, N. K., S. F. Pedersen, H. B. Rasmussen, M. Grunnet, D. A. Klaerke, and S. P. Olesen. 2003. Cell swelling activates cloned Ca(2+)-activated K(+) channels: a role for the F-actin cytoskeleton. Biochim. Biophys. Acta. 1615:115–125. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerberg, J., and V. A. Parsegian. 1987. Water movement during channel opening and closing. J. Bioenerg. Biomembr. 19:351–358. [DOI] [PubMed] [Google Scholar]
- 36.Ackerman, M. J., K. D. Wickman, and D. E. Clapham. 1994. Hypotonicity activates a native chloride current in Xenopus oocytes. J. Gen. Physiol. 103:153–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, X. C., and F. Sachs. 1990. Characterization of stretch-activated ion channels in Xenopus oocytes. J. Physiol. 431:103–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryan-Sisneros, A. A., S. P. Fraser, and M. B. Djamgoz. 2003. Electrophysiological, mechanosensitive responses of Xenopus laevis oocytes to direct, isotonic increase in intracellular volume. J. Neurosci. Methods. 125:103–111. [DOI] [PubMed] [Google Scholar]
- 39.Duan, D., J. Zhong, M. Hermoso, C. M. Satterwhite, C. F. Rossow, W. J. Hatton, I. Yamboliev, B. Horowitz, and J. R. Hume. 2001. Functional inhibition of native volume-sensitive outwardly rectifying anion channels in muscle cells and Xenopus oocytes by anti-ClC-3 antibody. J. Physiol. 531:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voets, T., G. Buyse, J. Tytgat, G. Droogmans, J. Eggermont, and B. Nilius. 1996. The chloride current induced by expression of the protein pICln in Xenopus oocytes differs from the endogenous volume-sensitive chloride current. J. Physiol. 495:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes, J. P., C. Y. Hernandez-Carballo, P. Perez-Cornejo, U. Meza, R. Espinosa-Tanguma, and J. Arreola. 2004. Novel outwardly rectifying anion conductance in Xenopus oocytes. Pflugers Arch. 449:271–277. [DOI] [PubMed] [Google Scholar]
- 42.Lock, H., and M. A. Valverde. 2000. Contribution of the IsK (MinK) potassium channel subunit to regulatory volume decrease in murine tracheal epithelial cells. J. Biol. Chem. 275:34849–34852. [DOI] [PubMed] [Google Scholar]
- 43.Kubota, T., M. Horie, M. Takano, H. Yoshida, H. Otani, and S. Sasayama. 2002. Role of KCNQ1 in the cell swelling-induced enhancement of the slowly activating delayed rectifier K(+) current. Jpn. J. Physiol. 52:31–39. [DOI] [PubMed] [Google Scholar]
- 44.Wehner, F., H. Olsen, H. Tinel, E. Kinne-Saffran, and R. K. Kinne. 2003. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev. Physiol. Biochem. Pharmacol. 148:1–80. [DOI] [PubMed] [Google Scholar]
- 45.Perets, T., Y. Blumenstein, E. Shistik, I. Lotan, and N. Dascal. 1996. A potential site of functional modulation by protein kinase A in the cardiac Ca2+ channel alpha 1C subunit. FEBS Lett. 384:189–192. [DOI] [PubMed] [Google Scholar]
- 46.Kimura, K., J. Kitano, Y. Nakajima, and S. Nakanishi. 2004. Hyperpolarization-activated, cyclic nucleotide-gated HCN2 cation channel forms a protein assembly with multiple neuronal scaffold proteins in distinct modes of protein-protein interaction. Genes Cells. 9:631–640. [DOI] [PubMed] [Google Scholar]
- 47.Xu, J., M. Paquet, A. G. Lau, J. D. Wood, C. A. Ross, and R. A. Hall. 2001. β1-adrenergic receptor association with the synaptic scaffolding protein membrane-associated guanylate kinase inverted-2 (MAGI-2). Differential regulation of receptor internalization by MAGI-2 and PSD-95. J. Biol. Chem. 276:41310–41317. [DOI] [PubMed] [Google Scholar]
- 48.Barbuti, A., B. Gravante, M. Riolfo, R. Milanesi, B. Terragni, and D. DiFrancesco. 2004. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ. Res. 94:1325–1331. [DOI] [PubMed] [Google Scholar]
- 49.Gravante, B., A. Barbuti, R. Milanesi, I. Zappi, C. Viscomi, and D. DiFrancesco. 2004. Interaction of the pacemaker channel HCN1 with filamin A. J. Biol. Chem. 279:43847–43853. [DOI] [PubMed] [Google Scholar]
- 50.Wright, A. R., and S. A. Rees. 1998. Cardiac cell volume: crystal clear or murky waters? A comparison with other cell types. Pharmacol. Ther. 80:89–121. [DOI] [PubMed] [Google Scholar]