Abstract
In plants, posttranscriptional gene silencing (PTGS) has been reported for cytoplasmic RNAs from endogenous nuclear genes, transgenes, viruses, and, recently, for a viroid with nuclear replication and accumulation. However, phenomena of this kind have not been described for mitochondrial or chloroplastic RNAs. Here we show that viroids that replicate and accumulate in the chloroplast are also targets of PTGS and this process may control viroid titer.
Posttranscriptional gene silencing (PTGS), a mechanism that regulates gene expression in eukaryotes, results in the sequence-specific degradation of single-stranded RNAs (ssRNAs) from genetic elements of internal or foreign origin (2). The present model proposes that PTGS is triggered by double-stranded RNAs, resulting in most cases from ssRNAs that reach anomalous cellular levels and serve as templates for an RNA-dependent RNA polymerase (18, 19), which are subsequently processed into 21- to 23-nucleotide (nt) fragments (7) called small interfering RNAs (siRNAs). These siRNAs presumably become associated with an RNase and guide it for the purpose of degrading their cognate ssRNA (8). Because siRNAs homologous and complementary to the targeted ssRNA have been detected in all systems exhibiting PTGS, the siRNAs are regarded as markers for this phenomenon.
In plants, PTGS has been reported for cytoplasmic ssRNAs from endogenous nuclear genes, transgenes, and RNA and DNA viruses (18). Recently, the presence of siRNAs homologous to the positive and negative strands of the Potato spindle tuber viroid (PSTVd) has been observed in plants infected by this pathogen and such an observation has been taken as evidence that PSTVd induces PTGS (11, 17). Like all viroids, PSTVd is a small circular ssRNA not coding for proteins and, therefore, has a strong dependence on host enzymes for completing its biological cycle. PSTVd is the type species of the family Pospiviroidae, comprising 25 species that have a central conserved region (CCR), lack hammerhead ribozymes, and replicate and accumulate in the nucleus (5). In contrast, the three members of the family Avsunviroidae, Avocado sunblotch viroid (ASBVd) (10), Peach latent mosaic viroid (PLMVd) (9), and Chrysanthemum chlorotic mottle viroid (CChMVd) (15) lack a CCR, self-cleave through hammerhead ribozymes that can be formed by both polarity strands, and, at least ASBVd and PLMVd (and most likely CChMVd), replicate and accumulate in the chloroplast (6). In this report, we have addressed the question of whether the viroids of this second family are also able to trigger a PTGS response in their natural hosts.
Nucleic acids from PLMVd-, CChMVd-, and ASBVd-infected material and from parallel healthy controls were extracted and fractionated on nonionic cellulose as reported previously (16). Samples corresponding to approximately 50 μg of healthy and viroid-enriched nucleic acid preparations were heat treated in buffered formamide and loaded onto 1× Tris-borate-EDTA polyacrylamide gels (15%) containing 8 M urea. DNA oligonucleotides ranging from 19 to 35 nt were also loaded onto the gels as size markers. After electrophoresis, the nucleic acids were electroblotted to positively charged nylon membranes (Hybond N+; Amersham Biosciences) and fixed by UV irradiation. Strand-specific riboprobes were generated by in vitro RNA transcription of appropriate cDNA clones in the presence of [α-32P]UTP. Prehybridization and hybridization were carried out in a mixture containing 50% formamide, 5× SSPE (1× SSPE is 0.15 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]), 0.1% Ficoll, 0.1% polyvinylpyrrolidone, 1% sodium dodecyl sulfate, and 0.1 mg of salmon sperm DNA/ml at 45 to 50°C for 2 and 16 h, respectively. Signals were visualized by autoradiography or with a bioimage analyzer (Fuji Bas 1500).
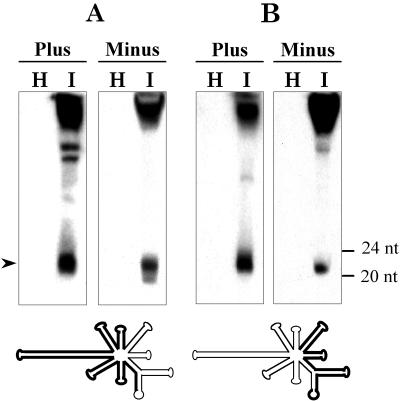
To test whether PLMVd could trigger a PTGS-like response in its natural host, PLMVd-infected peach material was analyzed for the presence of siRNAs with sequence specificity to this viroid. Figure 1 shows nucleic acid extracts from healthy and PLMVd-infected tissue hybridized with riboprobes specific for detecting positive and negative PLMVd strands. Small RNAs of approximately 21 to 23 nt were detected in PLMVd-infected plants but not in the healthy controls, thus indicating that PLMVd is an inducer of PTGS. The siRNAs could be detected with partial or full-length PLMVd-specific probes of either polarity (Fig. 1 and data not shown), showing that they form a population of sequences not restricted to specific viroid regions but likely representing most of the viroid molecule.
FIG. 1.
Detection by Northern blot analysis of the PLMVd-specific small RNAs associated with PTGS. Nucleic acid preparations from healthy (H) and PLMVd-infected (I) peach leaves were separated in a 15% denaturing polyacrylamide gel and hybridized with positive- and negative-sense radioactive riboprobes derived from the “left” (A) and “right” (B) parts of the PLMVd molecule as indicated by thick lines on the schematic representation of the PLMVd secondary structure shown below the panels. Positions of the 24- and 20-mer DNA markers are at the right, and the signals corresponding to the small RNAs are indicated by an arrowhead at the left. The bands visible in the upper part of the gel are mainly generated by unit- and shorter-than-unit-length PLMVd RNAs.
Nucleic acid extracts from CChMVd-infected tissue were analyzed in the same way. In this case, nucleic acid preparations from chrysanthemum leaves infected either with the symptomatic or the nonsymptomatic CChMVd strains were included. As reported previously, both strains have a high homology and accumulate to similar levels but differ in the sequence of a tetraloop in the secondary structure of the viroid, which has been identified as the major pathogenicity determinant (4, 15). No significant difference in the PTGS response induced by both CChMVd strains was observed, since the accumulation levels of the siRNAs were essentially the same (Fig. 2). This lack of correlation between the virulence of the viroid strain and the in vivo concentration of the siRNAs was also observed for PSTVd (17), supporting the view that such a level is not directly involved in symptom development. As for PLMVd, siRNAs were also detected with partial-length probes of either polarity, indicating that different regions of the positive and negative CChMVd strands are represented in these RNAs (data not shown).
FIG. 2.
Detection by Northern blot analysis of the CChMVd-specific small RNAs associated with PTGS. Nucleic acid preparations from leaves of healthy (H) chrysanthemum plants and those infected with a nonsymptomatic (NS) or a symptomatic (S) strain of CChMVd were separated in a 15% denaturing polyacrylamide gel and hybridized with full-length radioactive riboprobes for the detection of positive and negative viroid strands. Positions of the 24- and 20-mer DNA markers are at the right, and the signals corresponding to the small RNAs are indicated by an arrowhead at the left. The bands visible in the upper part of the gel lanes containing the infected extracts are mainly generated by unit- and shorter-than-unit-length CChMVd RNAs. The weak signals observed in the upper part of the gel lanes containing the healthy extracts result from nonspecific hybridization.
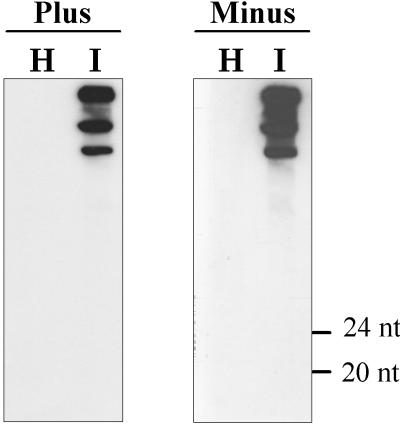
In contrast to the situation observed for PLMVd and CChMVd, no ASBVd-specific siRNAs were detected in nucleic acid preparations from ASBVd-infected avocado leaves, even when the hybridization temperature was reduced to 35°C in the presence or absence of formamide (Fig. 3). Interestingly, ASBVd accumulates at very high levels in this tissue, whereas PLMVd and CChMVd reach much lower concentrations (6). This inverse correlation between the viroid accumulation levels and the presence and/or the absence of the siRNAs is consistent with the involvement of the latter in a PTGS defense response of the host that would attenuate the detrimental effect of viroids by lowering their in vivo titer. In this context, it is worth noting that members of family Avsunviroidae are characterized by a restricted host range (6). The possibility exists that this narrow host range might be related to efficient PTGS processes induced in other plants by these viroids that would impede their ability to overcome the strong defense response.
FIG. 3.
Northern blot analysis to search for the viroid-specific small RNAs associated with PTGS in nucleic acid preparations from leaves of avocado plants. Preparations from healthy (H) and ASBVd-infected (I) samples were separated in a 15% denaturing polyacrylamide gel and hybridized with full-length radioactive riboprobes for the detection of positive and negative viroid strands. Positions of the 24- and 20-mer DNA markers are at the right. The bands visible in the upper part of the gel are generated by unit-, shorter-than-unit, and longer-than-unit-length ASBVd RNAs.
The reason that ASBVd infection did not lead to siRNA accumulation remains to be determined. ASBVd might not induce PTGS in avocado because of a particularly short transit time in the cytoplasm during intercellular movement (see below) or because once PTGS is induced the viroid might be able to suppress it. This latter possibility implies that some viroid RNAs could act as PTGS supressors (11), a function that has only been described for viral and cellular proteins so far (1, 19).
The finding of siRNAs derived from PSTVd, a viroid with nuclear replication and accumulation, has suggested that PTGS may take place in the cell nucleus (17). Consistent with this notion, some observations point to a nuclear step in PTGS, although firm evidence in this respect is still lacking (3, 12, 14). The detection of siRNAs derived from RNAs that replicate and accumulate in the chloroplast raises the question of whether PTGS-like processes may occur in this organelle or, alternatively, in the cytoplasm in the course of viroid movement from cell to cell; the identification of the subcellular localization site of the siRNAs may provide a clue in this respect. The prokaryotic origin of chloroplasts (13) makes the answer to this question intriguing because, so far, PTGS has been exclusively described for eukaryotic systems.
Acknowledgments
This work was partially supported by grants PB98-0500 from the Dirección General de Enseñanza Superior and BMC2002-03694 from the Ministerio de Ciencia y Tecnología de España (to R.F.). A.E.M. was the recipient of a postdoctoral fellowship from the Basque Government.
We are grateful to A. Ahuir for taking care of the plants, to M. Bordás and V. Moncholí for technical assistance, and to J. W. Randles for reviewing the English text and for suggestions.
REFERENCES
- 1.Anandalakshmi, R., R. Marathe, X. Ge, J. M. Herr, Jr., C. Mau, A. Mallory, G. Pruss, L. Bowman, and V. B. Vance. 2000. A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science 290:142-144. [DOI] [PubMed] [Google Scholar]
- 2.Baulcombe, D. C. 2002. RNA silencing. Curr. Biol. 12:R82-R84. [DOI] [PubMed] [Google Scholar]
- 3.Cogoni, C., and G. Macino. 2000. Post-transcriptional gene silencing across kingdoms. Curr. Opin. Genet. Dev. 10:638-643. [DOI] [PubMed] [Google Scholar]
- 4.De la Peña, M., B. Navarro, and R. Flores. 1999. Mapping the molecular determinant of pathogenicity in a hammerhead viroid: a tetraloop within the in vivo branched RNA conformation. Proc. Natl. Acad. Sci. USA 96:9960-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flores, R., J. W. Randles, M. Bar-Joseph, and T. O. Diener. 2000. Subviral agents: viroids, p. 1009-1024. In M. H. V. van Regenmortel et al. (ed.), Virus taxonomy, 7th Report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 6.Flores, R., J. A. Daròs, and C. Hernández. 2000. The Avsunviroidae family: viroids containing hammerhead ribozymes. Adv. Virus Res. 55:271-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton, A. J., and D. C. Baulcombe. 1999. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286:950-952. [DOI] [PubMed] [Google Scholar]
- 8.Hammond, S. M., E. Bernstein, D. Beach, and G. J. Hannon. 2000. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293-296. [DOI] [PubMed] [Google Scholar]
- 9.Hernández, C., and R. Flores. 1992. Plus and minus RNAs of peach latent mosaic viroid self-cleave in vitro via hammerhead structures. Proc. Natl. Acad. Sci. USA 89:3711-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchins, C., P. D. Rathjen, A. C. Forster, and R. H. Symons. 1986. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 14:3627-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itaya, A., A. Folimonov, Y. Matsuda, R. S. Nelson, and B. Ding. 2001. Potato spindle tuber viroid as inducer of RNA silencing in infected tomato. Mol. Plant-Microbe Interact. 14:1332-1334. [DOI] [PubMed] [Google Scholar]
- 12.Lucy, A. P., H. S. Guo, W. X. Li, and S. W. Ding. 2000. Supression of post-transcriptional gene silencing by a plant viral protein localized in the nucleus. EMBO J. 19:1672-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margulis, L. 1993. Symbiosis in cell evolution, 2nd ed. W. H. Freeman and Co., New York, N.Y.
- 14.Morel, J. B., P. Mourrain, C. Beclin, and H. Vaucheret. 2000. DNA methylation and chromatin structure affect transcriptional and post-transcriptional transgene silencing in Arabidopsis. Curr. Biol. 10:1591-1594. [DOI] [PubMed] [Google Scholar]
- 15.Navarro, B., and R. Flores. 1997. Chrysanthemum chlorotic mottle viroid: unusual structural properties of a subgroup of self-cleaving viroids with hammerhead ribozymes. Proc. Natl. Acad. Sci. USA 94:11262-11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallás, V., A. Navarro, and R. Flores. 1987. Isolation of viroid-like RNA from hop different from hop stunt viroid. J. Gen. Virol. 68:3201-3205. [Google Scholar]
- 17.Papaefthimiou, I., A. J. Hamilton, M. A. Denti, D. C. Baulcombe, M. Tsagris, and M. Tabler. 2001. Replicating potato spindle tuber viroid is accompanied by short RNA fragments that are characteristic of post-transcriptional gene silencing. Nucleic Acids Res. 29:2395-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vance, V., and H. Vaucheret. 2001. RNA silencing in plant-defense and counterdefense. Science 292:2277-2280. [DOI] [PubMed] [Google Scholar]
- 19.Voinnet, O. 2001. RNA silencing as a plant immune system against viruses. Trends Genet. 17:449-459. [DOI] [PubMed] [Google Scholar]