Abstract
For use in humans, human immunodeficiency virus (HIV) DNA vaccines may need to include immunostimulatory adjuvant molecules. CD40 ligand (CD40L), a member of the tumor necrosis factor (TNF) superfamily (TNFSF), is one candidate adjuvant, but it has been difficult to use because it is normally expressed as a trimeric membrane molecule. Soluble trimeric forms of CD40L have been produced, but in vitro data indicate that multimeric, many-trimer forms of soluble CD40L are more active. This multimerization requirement was evaluated in mice using plasmids that encoded either 1-trimer, 2-trimer, or 4-trimer soluble forms of CD40L. Fusion with the body of Acrp30 was used to produce the 2-trimer form, and fusion with the body of surfactant protein D was used to produce the 4-trimer form. Using plasmids for secreted HIV-1 antigens Gag and Env, soluble CD40L was active as an adjuvant in direct proportion to the valence of the trimers (1 < 2 < 4). These CD40L-augmented DNA vaccines elicited strong CD8+ T-cell responses but did not elicit significant CD4+ T-cell or antibody responses. To test the applicability of the multimeric fusion protein approach to other TNFSFs, a 4-trimer construct for the ligand of glucocorticoid-induced TNF family-related receptor (GITR) was also prepared. Multimeric soluble GITR ligand (GITRL) augmented the CD8+ T-cell, CD4+ T-cell, and antibody responses to DNA vaccination. In summary, multimeric CD40L and GITRL are new adjuvants for DNA vaccines. Plasmids for expressing multimeric TNFSF fusion proteins permit the rapid testing of TNFSF molecules in vivo.
DNA vaccines directed against human immunodeficiency virus type 1 (HIV-1) and other viruses have been extensively studied in mice, macaques, and humans (17, 21, 26, 29, 74). However, there is a need to develop more effective DNA vaccines. Using a DNA vaccine for a secreted, codon-optimized form of HIV-1 Gag that was previously shown to be more immunogenic than nonsecreted Gag (69), the present study aimed at finding molecular adjuvants that could further increase the immunogenicity of this vaccine. CD40 ligand (CD40L; TNFSF5) has been proposed as a molecular adjuvant for DNA vaccines and was therefore studied in this context. DNA plasmids for four forms of CD40L were examined: the natural membrane form, a 1-trimer soluble form, a 2-trimer soluble form, and a 4-trimer soluble form. Consistent with other reports that studied secreted antigens, membrane CD40L had little adjuvant activity in this DNA vaccine. In contrast, soluble CD40L was an effective adjuvant for CD8+ cell responses in direct relationship to the valence of its trimers (1 < 2 < 4). To determine if the multimerization strategy could be applied to other ligands in the tumor necrosis factor (TNF) superfamily (TNFSF), plasmid DNA for 4-trimer soluble glucocorticoid-induced TNF family-related receptor ligand (GITRL) was also studied. While 4-trimer GITRL was somewhat less effective at adjuvanting CD8+ T-cell responses, it enhanced CD4+ T-cell proliferative responses and antibody responses to the Gag DNA vaccines. Taken together, these studies provide a novel platform for evaluating TNFSF ligands as molecular adjuvants for DNA vaccines.
MATERIALS AND METHODS
DNA plasmids for HIV-1 antigens.
pScGag is a secreted, codon-optimized form of the HIV-1 Gag protein cloned into the pcDNA3.1 expression vector (Invitrogen, San Diego, CA) as previously described (69). Additional experiments were performed using pSyngp140JR-FL (a codon-optimized secreted form of the HIV-1 JR-FL envelope) and pSynΔgp140 (an empty vector that serves as the control plasmid for pSyngp140JR-FL) (5).
Construction of CD40L and GITRL expression vectors.
pMemCD40L, encoding full-length murine CD40L (GenBank accession no. X65453.2), was previously described (48). 293 Cells transfected with this plasmid stained strongly for CD40L as judged by flow cytometry and were highly stimulatory to macrophages in culture (48).
pTr-CD40L, the plasmid for 1-trimer soluble CD40L, began with the tissue plasminogen activator signal sequence followed by an isoleucine zipper followed by the entire extracellular domain (ECD) of murine CD40L (both the membrane-proximal stalk and the TNF-like domain). This portion of CD40L is referred to as “full-length,” or “FL,” soluble CD40L in the biophysical studies of Morris et al. (63). However, the Flag purification tag present in the original soluble CD40L trimer (sCD40LT) sequence of Srinivasan and Spriggs (U.S. patent 5,716,805, February 1998) was not included in order to reduce the possibility that antibodies against this protein might develop, as has been reported during human trials of sCD40LT (95). The amino acid sequence of the mature, secreted protein was RMKQIEDKIEEILSKIYHIENEIARIKKLIGERTSS/DKVEEEV…, where the N-terminal portion is the isoleucine zipper and the C-terminal portion is the ECD of murine CD40L (amino acids 51 to 260 of GenBank protein sequence no. CAA46448.2), and the shill indicates the junction.
To construct the plasmid for a 2-trimer soluble form of murine CD40L (pAcrp30-CD40L), cDNA from mouse adipose tissue (BioChain Institute, Inc., Hayward, CA) was used to obtain a PCR product for the 5′ untranslated region and 5′ coding sequence of Acrp30. Using overlapping PCR primers, this sequence was fused to murine CD40L. The resulting sequence was identical to that referred to by Holler et al. (34) and Tschopp et al. (U.S. patent application 2003/0053984), with the exception that the N-terminal FLAG purification tag and linker were deleted. As in that construct, a two-amino-acid linker was placed at the fusion junction, and only the TNF-like portion of CD40L (without the membrane-proximal stalk) was included. The amino acid sequence around the fusion junction was …KGEPGELQGDEDPQIA…, where the N-terminal portion is from Acrp30 (amino acids 1 to 109 of GenBank protein sequence no. NP_033735), LQ (underlined) is the linker, and the C-terminal portion is the TNF-like domain of murine CD40L (amino acids 115 to 260 of GenBank protein sequence no. CAA46448.2, March 2003).
To construct the plasmid coding for a 4-trimer soluble form of murine CD40L (pSP-D-CD40L), mouse surfactant protein D (SP-D) was cloned by heminested PCR from reverse-transcribed murine lung mRNA (BD Clontech, Palo Alto, CA). Using overlapping PCR primers, this sequence was fused to the entire extracellular domain (ECD) of murine CD40L. The resulting sequence was similar to that referred to by Haswell et al. (33) and Al-Shamkhani and Glennie (U.S. patent application 2004/0047873, March 2004), except that the glycine linker (GGGNS) between SP-D and CD40L was deleted and three more amino acids of CD40L (HRR) were included. The amino acid sequence around the junction between SP-D and murine CD40L was …KAALFPDG/HRRLDKVE…, where the N-terminal portion is from SP-D (amino acids 1 to 256 of GenBank protein sequence no. NP_033186) and the C-terminal portion is the extracellular sequence of murine CD40L (amino acids 47 to 260 of GenBank protein sequence no. CAA46448.2).
To construct the plasmid coding for a 4-trimer soluble form of murine GITRL (pSP-D-GITRL), GITRL was cloned by reverse transcription-PCR using mRNA from anti-CD3/anti-CD28-activated spleen cells from BALB/c mice. This sequence contained the same T157 allele present in C57/BL6 (GenBank accession no. AAP70494) and 129/J (GenBank accession no. AAQ55265) mice, rather than the N157 allele reported for CBA/Ca mice (GenBank accession no. NP_899247). The amino acid sequence around the junction between SP-D and murine GITRL was …KAALFPDG/SLKPTAIE…, where the N-terminal portion is from SP-D (amino acids 1 to 256 of GenBank protein sequence no. NP_033186) and the C-terminal portion is the extracellular sequence of murine GITRL (amino acids 43 to 173 of GenBank protein sequence no. AAP70494).
Plasmid preparation.
Plasmids were propagated in Escherichia coli strain XL1 blue or TOP10. Two methods were used to purify the supercoiled plasmid DNA: two rounds of banding by CsCl-ethidium bromide density gradient ultracentrifugation, followed by butanol extraction to remove the ethidium bromide, or anion-exchange chromatography resin (EndoFree Plasmid MaxiKit; QIAgen, Inc., Valencia, CA). Differences were appreciated in the immunogenicity of plasmids prepared by these two methods, but they were too small to affect the conclusions of this report.
Transient transfections and Western blotting of fusion protein constructs.
293T cells were transiently transfected with the plasmid constructs using Lipofectamine 2000 (Invitrogen). Forty-eight hours later, supernatants were centrifuged and filtered. Biotinylated anti-CD40L (MR1; BD Pharmingen) or anti-GITRL (Alexis, San Diego, CA) antibody was bound to streptavidin-coated magnetic beads (Dynal Biotech, LLC, Brown Deer, WI) and used to pull down their respective proteins. The beads were then loaded onto sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis gels, electrophoresed, and blotted onto Immobilon-P membranes (Millipore, Inc., Billerica, MA). The membranes were blocked and then probed with goat anti-mouse CD40L (R&D Systems, Inc., Minneapolis, MN) or rabbit anti-mouse GITRL (Alexis Biochemicals, San Diego, CA), followed by horseradish peroxidase-conjugated anti-goat or anti-rabbit antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), respectively. The signal was developed onto X-ray film using chemiluminescence.
Mice and vaccinations.
Prior to vaccination, the plasmids were resuspended in sterile phosphate-buffered saline (PBS) at 1 mg/ml, aliquoted, and stored at −20°C. Each immunization consisted of 80 μg of antigen plasmid (either pScGag or pSyngp140JR-FL) plus 20 μg of one of the CD40L or GITRL plasmids. As controls, some mice were either not immunized (“naive”) or immunized with 80 μg of antigen plasmid without an adjuvant plasmid (which was replaced in this instance with 20 μg of the empty expression vector, pcDNA3.1, as filler DNA). Female BALB/cByJ mice, 6 to 9 weeks old (The Jackson Laboratory, Bar Harbor, ME), were studied in groups of five under a protocol approved by the Institutional Animal Care and Use Committee of the VA San Diego Healthcare System. Three immunizations were performed under isofluorane anesthesia at 2-week intervals by injecting 50 μl of plasmid DNA solution into the quadriceps of each hind limb (100 μg DNA total) using an insulin syringe with a 28-gauge needle (69).
Antigen proteins and peptides.
The Gag antigen used to detect antibodies by enzyme-linked immunosorbent assay (ELISA) was an E. coli-produced glutathione S-transferase-Gag fusion protein purchased from Research Diagnostics, Inc. (Flanders, NJ), or Anogen, Inc. (Mississauga, Ontario). The Gag antigen used for splenocyte proliferation and cytokine production assays was baculovirus-produced, myristylated, particulate HIV-1IIIB p55 protein (catalog no. 3276; National Institutes of Health [NIH] AIDS Research and Reference Reagent Program). For detecting Gag-specific CD8+ T-cell responses, the H-2Kd immunodominant Gag199-207 peptide (AMQMLKETI) (69) was purchased from either Synpep Corp. (Dublin, CA) or Bachem Bioscience, Inc. (King of Prussia, PA).
The Env antigen used to detect antibodies by ELISA and for splenocyte proliferation and cytokine production assays was HIV-1SF162 gp120 protein (catalog no. 7363; NIH AIDS Research and Reference Reagent Program). For detecting Env-specific CD8+ T-cell responses, two 15-mer peptides spanning the tip of the B clade V3 loop consensus sequence (catalog nos. 8839 and 8840; NIH AIDS Research and Reference Reagent Program) were used as a 1:1 mix.
Splenocyte preparation.
Two weeks after the last immunization, mice were euthanized with pentobarbital. The spleens were removed and processed into single-cell suspensions in RPMI 1640 supplemented with 2 mM l-glutamine (Invitrogen), 10% fetal bovine serum (HyClone Systems, Inc., Logan, UT), 50 μM 2-mercaptoethanol, 100 U of penicillin per ml, and 100 μg/ml streptomycin. For each data point, splenocytes for the proliferation, cytokine, cytotoxic T lymphocyte (CTL), ELISPOT, and tetramer studies were typically obtained from the same mouse, providing direct comparison of the various techniques.
CTL assay.
As stimulator cells, splenocytes from unvaccinated naive mice were treated with mitomycin C and pulsed with major histocompatibility complex (MHC) class I-restricted peptide. After washing, these stimulator cells were then cocultured with responder spleen cells from vaccinated mice at a responder:stimulator cell ratio of 5:1 in the presence of 10 U/ml of interleukin-2 (IL-2). Six days later, viable splenocytes were isolated by centrifugation over Ficoll-Hypaque (Lympholyte M; Accurate Chemical and Scientific Corp., Westbury, NY), washed twice in RPMI 1640 without phenol-red or serum but supplemented with 2% bovine serum albumin, and recounted. A 4-h cytolytic assay was performed in the same media by incubating these effector cells with peptide-pulsed P815 target cells in 96-well round-bottom plates in triplicate using the release of lactate dehydrogenase to indicate cell killing (CytoTox 96; Promega Biosciences, Madison, WI). The percent specific release was calculated as 100 × (optical density at 490 nm [OD490] of experimental wells − OD490 of effector cell spontaneous lactate dehydrogenase [LDH] release − OD490 of target cell spontaneous LDH release)/(OD490 of maximum target cell LDH release − OD490 of target cell spontaneous LDH release).
IFN-γ ELISPOT assay.
Dilutions of splenocytes were cocultured with P815 cells (1 × 105/well) that had been pulsed with or without MHC class I-restricted peptide and incubated at 37°C for 24 h on membrane filtration plates coated with anti-mouse gamma interferon (IFN-γ). These plates were processed as previously described (58), and the spots were counted using an automated reader (ImmunoSpot II; Cellular Technologies, Inc., Cleveland, OH).
Splenocyte proliferation and cytokine production assays.
Splenocytes were cultured in triplicate in 96-well round-bottom plates in 200 μl media with or without 10 μg/ml protein antigen in the absence of IL-2. After incubation at 37°C in 5% CO2 for 3 days, 100 μl of supernatant was removed for measurements of IFN-γ and IL-4 by ELISA (R&D Systems). This volume was replace with 20 μl media containing [3H]thymidine (1 μCi/well), and the plates were incubated for an additional 16 h, following which the cells were harvested onto glass fiber filters and counted in scintillant in a beta counter. Proliferation was expressed as a stimulation index (the ratio of the counts per minute obtained with antigen/counts per minute without antigen).
Flow cytometry and tetramer analysis.
Antibodies used for staining were peridinin chlorophyll α protein anti-CD8, fluorescein isothiocyanate anti-CD62L, and their corresponding isotype controls (BD Pharmingen). Staining with phycoerythrin AMQMLKETI/H-2Kd tetramers was performed as described previously (30).
Serum antibody measurements.
Mice were bled by saphenous vein puncture at the beginning of the studies and 1 week after each immunization. Antibody binding to protein-coated ELISA plates was detected using alkaline phosphatase-conjugated goat anti-mouse immunoglobulin (Ig) (Jackson ImmunoResearch Laboratories, Inc.), and the signal was developed using BluePhos substrate (KPL, Inc., Gaithersburg, MD).
ELISA for anti-SP-D-CD40L antibodies.
Sera from mice that had completed a vaccination series were assayed for antibodies against SP-D-CD40L protein using a specially constructed ELISA. Plates were coated with MR1 hamster anti-mouse CD40L at 4°C overnight, blocked with 3% bovine serum albumin in PBS for 2 h at room temperature, and then reacted with CHO-cell-produced SP-D-CD40L (12 μg/ml in Dulbecco's modified Eagle medium, 10% fetal bovine serum) for 2 h to provide a surface to which anti-SP-D-CD40L antibodies might bind. Following a wash, the wells were exposed to test sera for 2 h at room temperature with shaking, were washed, and then were reacted with goat anti-mouse IgG conjugated to alkaline phosphatase for 2 h at room temperature with shaking. Following a wash, color was developed with BluePhos substrate as described above.
Statistical analysis.
Results are expressed as the means ± standard errors of the means (SEM). Statistical comparisons were performed by a two-tailed paired t test (Prism 4.0; GraphPad Software, Inc., San Diego, CA). P < 0.05 was considered statistically significant.
RESULTS
Construction of expression plasmids for 1-, 2-, and 4-trimer versions of soluble CD40L.
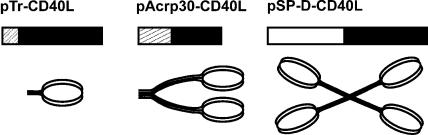
Previous in vitro studies of CD40L regulation of B-cell, macrophage, or dendritic cell (DC) activation have shown that multimerization of CD40L trimers can significantly increase their ability to activate these cells (33, 34, 47). To determine the effects of multimerization in vivo, we constructed plasmids encoding recombinant proteins that were previously shown to form 1-trimer (63), 2-trimer (34), and 4-trimer (33) structures of soluble CD40L in vitro. These constructs express the extracellular domain of CD40L fused with either an isoleucine zipper domain, the body of Acrp30 (adiponectin), or the body of SP-D, respectively (Fig. 1). Both Acrp30 and SP-D spontaneously assemble into multimeric structures with trimeric arms without the need for an added isoleucine zipper. Following transfection of plasmid DNAs into 293T cells, the secreted CD40L proteins were found to have the expected sizes by reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 2). When nonreducing conditions were used, additional high-molecular-weight bands were found for Acrp30-CD40L and SP-D-CD40L, consistent with the known disulfide bonds found in these spontaneously multimerizing molecules (data not shown). In parallel studies, pSP-D-GITRL, a plasmid for an SP-D-GITRL fusion protein (see below), was also shown to express soluble protein following transfection into 293T cells and Western blotting (Fig. 2).
FIG. 1.
Design of soluble CD40L constructs. pTr-CD40L is a 1-trimer protein consisting of an isoleucine zipper (boxed area with thick gray lines) followed by the CD40L extracellular domain (ECD) (▪), similar to the sCD40LT protein used in many studies (63). pAcrp30-CD40L is a 2-trimer protein produced as a fusion between the body of Acrp30 (boxed area with thin gray lines) and the CD40L ECD (▪). pSP-D-CD40L is a 4-trimer protein produced as a fusion between the body of surfactant protein D (□) and the CD40L ECD (▪).
FIG. 2.
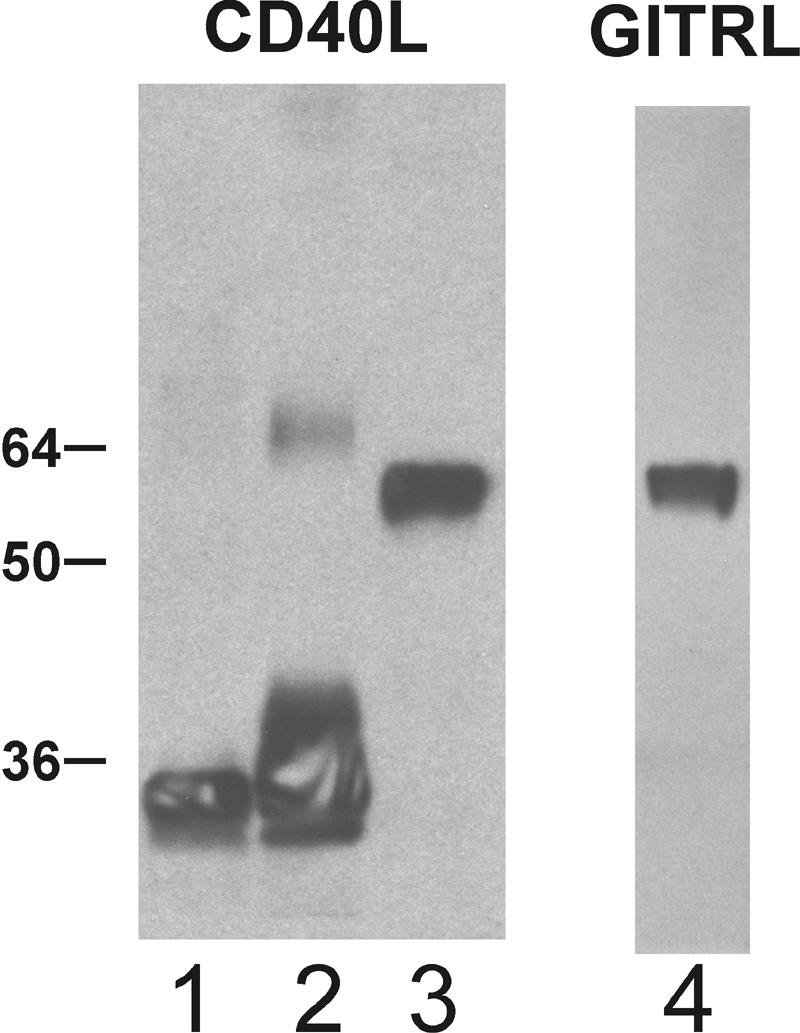
Protein expression by the plasmid constructs. The three plasmid constructs for soluble CD40L were transfected into 293T cells, and the resulting conditioned media were examined for secreted proteins by Western blotting with anti-CD40L antibodies. Lane 1, Tr-CD40L; lane 2, Acrp30-CD40L; lane 3, SP-D-CD40L. In lane 4, Western blotting for SP-D-GITRL was performed similarly. The heterodisperse mobility for Acrp30-CD40 and the less-than-predicted mobility for the two SP-D fusion proteins are consistent with the electrophoretic behaviors of their underlying multimeric scaffold moieties.
Multimeric soluble CD40L is more immunostimulatory than full-length CD40L in an HIV-1 DNA vaccine model.
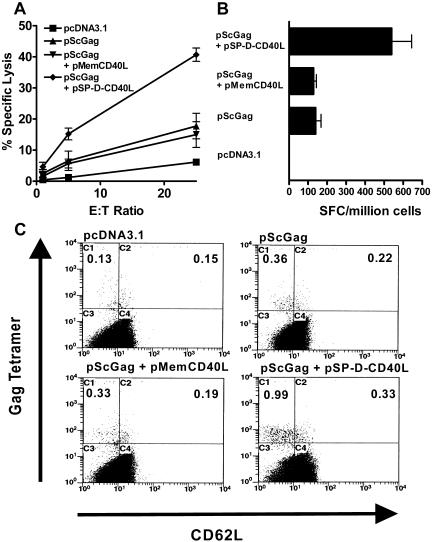
CD40L has significant immunostimulatory effects both in vitro and in vivo in a variety of model systems (70) and has been proposed as an adjuvant for vaccination (2). However, the challenges of purifying three different forms of soluble CD40L to sufficient purity while maintaining activity led us to study these proteins by using a DNA vaccination approach. Consequently, mice were immunized with pScGag, a plasmid expressing secreted HIV-1 Gag (69) either alone or with the test versions of CD40L. In comparison with an empty plasmid (pcDNA3.1), pScGag induced responses to an MHC class I-restricted peptide epitope of Gag as tested by CTL assay (Fig. 3A) or IFN-γ ELISPOT counts (Fig. 3B). The addition of a plasmid for membrane CD40L (pMemCD40L) to the pScGag vaccine had no effect on these elicited responses, in contrast to several reports (13, 31, 39, 57, 60, 80, 96) but consistent with two recent reports on DNA vaccination (61, 88). Remarkably, the plasmid for 4-trimer soluble CD40L (pSP-D-CD40L) significantly augmented the response to the pScGag DNA vaccine (Fig. 3A and B). Flow cytometry using antibodies to CD8 and H-2Kd/peptide tetramers confirmed that Gag-specific CD8+ T cells were elicited to significantly greater levels by the pSP-D-CD40L-containing DNA vaccine. Most of these Tet+ cells were also CD62Llo, consistent with an effector memory phenotype (Fig. 3C).
FIG. 3.
Effects of CD40L plasmids on Gag DNA vaccination. Plasmids were combined with the Gag antigen plasmid, pScGag, and used to immunize mice intramuscularly three times every 2 weeks. Two weeks following the last immunization, splenocytes were obtained for analysis. (A) CTL assay using peptide-pulsed target cells. pScGag alone was a weak immunogen but elicited more cytotoxic cells than the empty control plasmid, pcDNA3.1. Adding the plasmid for membrane CD40L did not augment the response to pScGag. However, adding the plasmid for 4-trimer soluble CD40L greatly augmented the generation of cytotoxic cells (P < 0.01). These results are representative of 10 experiments. (B) IFN-γ ELISPOT assay. Unlike the CTL assay which restimulated splenocytes for 6 days in vitro, ELISPOT-forming cells (SFC) were measured fresh in an overnight assay. This assay again demonstrated that pMemCD40L had no adjuvant activity in this system, but pSP-D-CD40L strongly enhanced responses to the MHC class I-restricted peptide (P < 0.001). (C) MHC class I tetramer analysis. Fresh splenocytes were stained with peridinin chlorophyll α protein anti-CD8, fluorescein isothiocyanate anti-CD62L, and phycoerythrin Gag peptide/H-2Kd tetramer. Using CD8 as the anchor gate, Tet+ cells were found to be significantly increased (P < 0.05) by the pSP-D-CD40L adjuvant plasmid (numbers are percentages of CD8+ cells). Most of the CD8+ Tet+ cells were CD62Llo, consistent with an effector memory phenotype. E:T, effector to target.
To determine if pSP-D-CD40L, the plasmid for multimeric soluble CD40L, could adjuvant other DNA vaccine antigens, mice were also vaccinated with pSyngp140JR-FL, a plasmid for a codon-optimized, secreted, trimeric version of HIV-1JR-FL Env. As a control, a homologous empty vector, pSynΔgp140, was used (5). In the CTL assay, the data for percent specific release at an effector:target ratio of 50:1 (n = 5, means ± SEM) were the following: pSynΔgp140, 26.5 ± 16.6; pSyngp140JR-FL alone, 16.7 ± 7.0; and pSyngp140JR-FL plus pSP-D-CD40L, 82.8 ± 15.2 (P < 0.05). Similarly, the IFN-γ ELISPOT data for spots/106 cells (n = 5, means ± SEM) were the following: pSynΔgp140, 2.2 ± 0.9; pSyngp140JR-FL alone, 49 ± 12.9; and pSyngp140JR-FL plus pSP-D-CD40L, 168 ± 15.3 (P < 0.001). Thus, pSP-D-CD40L also adjuvanted a DNA vaccine for a second HIV antigen, Env, suggesting that multimeric soluble CD40L could be used to adjuvant CD8+ T-cell responses to a wide range of antigens.
Increased multimerization of soluble CD40L trimers correlates with increased adjuvant activity for an HIV-1 DNA vaccine.
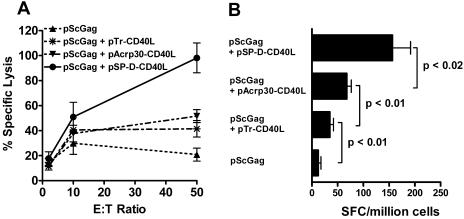
To determine the effects of multimerization on the activity of soluble CD40L, the pScGag antigen plasmid was tested alone or with three types of soluble CD40L plasmids: pTr-CD40L, encoding 1-trimer CD40L fused to an isoleucine zipper; pAcrp30-CD40L, encoding 2-trimer CD40L produced as a fusion protein with Acrp30 protein; or pSP-D-CD40L, encoding 4-trimer CD40L produced as a fusion protein with SP-D protein. In both CTL and IFN-γ ELISPOT assays (Fig. 4), pTr-CD40L (the plasmid for 1-trimer CD40L) had only modest adjuvant effects, consistent with reports that CD40L must be multimeric to stimulate cells in vitro (33, 34). Although pAcrp30-CD40L (the plasmid for 2-trimer CD40L) did augment the response to DNA vaccination, pSP-D-CD40L (the 4-trimer CD40L plasmid) was consistently superior. Thus, the valence of the multimeric soluble CD40L constructs appeared to correlate with the vaccine-induced responses (1 < 2 < 4). This was also true when an HIV-1 envelope plasmid was used (pSyngp140JR-FL), indicating that these results are independent of the antigen used for immunization.
FIG. 4.
Effect of valence on soluble CD40L as an adjuvant. (A) CTL assay and (B) IFN-γ ELISPOT assay. Multimeric soluble CD40L plasmids augmented the response to pScGag in direct proportion to their number of trimers, 1 < 2 < 4 (P < 0.01 for pScGag plus pSP-D-CD40L versus pScGag plus pAcrp30-CD40L). These results are representative of three experiments, including one using a different antigen plasmid encoding HIV envelope (pSyngp140JR-FL). E:T, effector to target. SFC, ELISPOT-forming cells.
Multimeric soluble CD40L constructs do not significantly augment proliferative, cytokine, or antibody responses to DNA vaccination.
While CD40 stimulation is sufficient to activate DCs to generate CD8+ T-cell responses (70), experiments with transgenic knock-out mice have shown that CD40-CD40L interactions are not strictly necessary for CD4+ cell responses (66). In addition, vaccination using an agonistic anti-CD40 antibody led to only modestly augmented CD4+ T-cell responses (25). As a screen for CD4+ T-cell responses, a standard proliferation assay was used, where protein antigen is expected to be taken up by DCs and presented by MHC class II in the absence of a stimulus for cross-presentation. Accordingly, splenocytes from immunized mice were cultured with protein antigen and proliferation was measured by [3H]thymidine incorporation. Somewhat predictably, none of the soluble CD40L constructs significantly increased the proliferative response of splenocytes to Gag protein (Fig. 5C and data not shown). Correspondingly, measurements of IFN-γ and IL-4 released by the antigen-stimulated splenocytes from the CD40L-immunized mice were only modestly increased above background (data not shown). Surprisingly, however, the addition of various forms of CD40L to the Gag DNA vaccine did not increase antibody production in 21 out of 24 experiments, even though CD40 was originally discovered as an activation receptor on B cells and CD40L is recognized as the T-cell molecule mediating contact-dependent B-cell activation (70). In some experiments, pSP-D-CD40L even appeared to suppress the antibody responses induced by pScGag alone (Fig. 5D). These findings suggest that the CD40L constructs used in this study bypass normal CD4+ T-cell-driven immunological pathways yet still induce strong CD8+ T-cell responses.
FIG. 5.
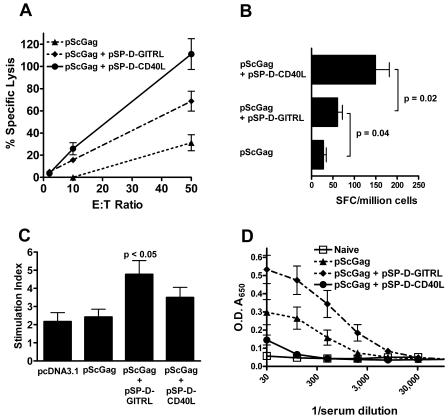
Adjuvant effects of a plasmid for soluble GITRL. (A) CTL assay and (B) IFN-γ ELISPOT assay. Compared to pScGag alone, pSP-D-GITRL significantly augmented CTL and cellular IFN-γ production (P < 0.01 for pScGag plus pSP-D-GITRL versus pScGag alone). However, pSP-D-CD40L was a more effective CD8+ T-cell stimulant than pSP-D-GITRL (P < 0.05 for pScGag plus pSP-D-CD40L versus pScGag plus pSP-D-GITRL). SFC, ELISPOT-forming cells. (C) Splenocyte proliferation assay. Spleen cells were incubated with Gag protein, and [3H]thymidine incorporation was measured. Results are expressed as the stimulation index. pScGag did not induce proliferation responses, nor were responses significantly augmented when pSP-D-CD40L was added to the immunization. This was a consistent result in 10 experiments. However, pSP-D-GITRL significantly augmented the proliferative response to antigen. (D) Anti-Gag antibody responses. Immunization with pScGag elicited modest levels of anti-Gag antibodies. The addition of pSP-D-CD40L did not significantly increase antibody titer and even appeared to suppress it in some experiments. In contrast, pSP-D-GITRL significantly augmented antibody production (P < 0.05). O.D., optical density.
pSP-D-CD40L does not elicit systemic immune activation or local inflammation.
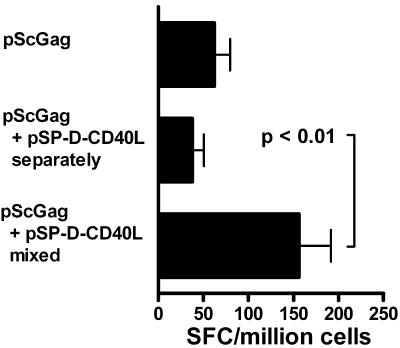
Plasmids encoding secreted cytokines have been reported to cause systemic immune activation (72), which could potentially lead to toxic side effects. However, in the present studies, the immunized mice showed no signs of distress, and spleen size and cell counts were not increased by the immunizations (data not shown). This contrasts with the splenomegaly, septic shock-like effects and occasional mortality observed when agonistic anti-CD40 antibodies were given systemically (8, 94). The most revealing experiment, however, was functional and involved separating the pScGag antigen plasmid from the pSP-D-CD40L adjuvant plasmid. Rather than mixing these two plasmids in a single syringe for the immunizations as before, they were delivered in separate syringes into opposite quadriceps (left for pScGag and right for pSP-D-CD40L). As shown in Fig. 6, the adjuvant effect of pSP-D-CD40L was lost when it was injected separately from the antigen plasmid. From these data, we infer that the pScGag and pSP-D-CD40L plasmids release sufficient antigen and adjuvant proteins, respectively, to initiate immune responses in the lymph nodes draining the vaccination site yet produce too little of these proteins to induce systemic immune responses or toxic effects.
FIG. 6.
pSP-D-CD40L must be coinjected with the antigen plasmid. To show that pSP-D-CD40L does not systemically activate immune responses, immunizations either were conducted with a mixture of pScGag and pSP-D-CD40L as before or the two plasmids were injected with separate syringes into opposite legs. Control mice were immunized with pScGag (80 μg pScGag and 20 μg pcDNA3.1 mixed), and half was injected into each quadriceps. A second series of mice was immunized with 80 μg of pScGag in the right quadriceps and 20 μg of pSP-D-CD40L in the left quadriceps. As before, a third series of mice was immunized with 80 μg pScGag and 20 μg of pSP-D-CD40L mixed together, and half was injected into each quadriceps. As shown by IFN-γ ELISPOT assay, only the mixture showed an augmented response. This indicated that the adjuvant activity of pSP-D-CD40L is not systemic but instead was localized to the side into which it was injected. SFC, ELISPOT-forming cells.
Also, since inflammation can initiate systemic immune activation, paraffin sections of the injected muscles were evaluated 48 h after immunization. While necrotic myocytes were seen along the track of the vaccination needle, no cellular infiltrates were present following the injection of either pScGag, pSP-D-CD40L, or the combination of these plasmids. Other studies on DNA vaccination have similarly shown an absence of muscle inflammation when DNA vaccines were tested without chemoattractant cytokines (52, 88). This made it unlikely that secondary inflammatory mediators were contributing to the immunization effects.
As a screen for autoimmune responses, lungs from SP-D-CD40L- and SP-D-GITRL-vaccinated mice were examined by histology at the conclusion of a vaccination series (day 42), but no inflammatory infiltrates or other pathology was seen. Additionally, sera collected at the end of a vaccination series were tested by ELISA for the formation of antibodies against SP-D-CD40L protein. Using sera from naive mice as a negative control, the pSP-D-CD40L-vaccinated mice did not produce any detectable antibodies to this protein. While further studies are desirable, these data failed to reveal the induction of autoimmunity by these molecular adjuvants.
Plasmid DNA for multimeric soluble GITRL is an adjuvant for an HIV-1 DNA vaccine.
Other multimeric soluble TNFSF ligands can be constructed by fusing TNFSF extracellular domains with a multimerizing scaffold. One of the most interesting TNFSFs is the ligand for GITR, a receptor expressed on CD4+ CD25+ regulatory T cells (Tregs). Agonistic anti-GITR antibodies abrogate the immunosuppressive properties of Tregs (41, 59, 79, 86) and also act as a costimulatory factor for CD4+ CD25− effector T cells which express GITR following activation (42). To test if GITR ligand (GITRL) could augment the response to a DNA vaccine, pScGag was tested either alone or in combination with pSP-D-CD40L or pSP-D-GITRL. As shown in Fig. 5, pSP-D-GITRL significantly augmented both cytolytic activity (Fig. 5A) and IFN-γ ELISPOTs (Fig. 5B) in response to an MHC class I-restricted Gag peptide. Unlike pSP-D-CD40L, however, pSP-D-GITRL increased both proliferative (Fig. 5C) and antibody responses (Fig. 5D) to Gag protein.
Generation of long-lived memory CD8+ T cells in response to DNA vaccination.
As shown above, Gag peptide/MHC class I tetramer-positive spleen cells were detected 2 weeks after vaccination (Fig. 3C). To determine if these CD8+ T cells persisted, mice were vaccinated as before and then evaluated 3 months later. Flow cytometry gated on CD8+ cells demonstrated the long-term persistence of these antigen-specific T cells, especially when pSP-D-CD40L was used. Many of these cells were also CD62Lhi, which is the phenotype of long-lived central memory cells (data not shown).
Other studies.
In the experiments shown in Fig. 3 and 4, the DNA vaccines contained 80 μg of antigen plasmid DNA (either pScGag or pSyngp140JR-FL) and 20 μg of CD40L-containing plasmid DNA. As an initial dose-response study, combinations of 40 μg plus 10 μg and 20 μg plus 5 μg of pScGag and pSP-D-CD40L, respectively, were also studied. While the dose of 40 μg plus 10 μg preserved most of the CD8+ T-cell responses, there was a definite drop in immunogenicity when the lower dose of 20 μg plus 5 μg was tested. Thus, pSP-D-CD40L alone did not reduce the quantity of DNA needed to vaccinate mice in this initial experiment. Instead, other methods of DNA vaccination would be needed to provide a “DNA sparing” effect.
Given the different response profiles elicited by pSP-D-CD40L and pSP-D-GITRL, the possibility of combining them was studied. However, DNA vaccination with pScGag plus both of these 4-trimer TNFSF plasmids elicited significantly fewer CD8+ responses than pScGag plus pSP-D-CD40L used alone. This is likely due to the formation of heteromultimers of SP-D-CD40L and SP-D-GITRL, since heteromultimers of TNFSF ligands have been found to be inactive or even inhibitory upon engaging their cognate receptors (1, 85). However, it may be possible to combine these TNFSF adjuvants if they are delivered into separate vaccination sites or administered to the same site at different times.
DISCUSSION
The TNF superfamily (TNFSF) includes CD40L, GITRL, RANKL, OX40L, 4-1BBL, LIGHT, CD70, and at least 12 other molecules (9, 11, 53, 81). From an immunological point of view, CD40L (also called CD154) has special importance as an initiator and promoter of the immune response (47, 70). CD40L is considered to be the molecular embodiment of the “help” provided by activated CD4+ T cells in that it “licenses” dendritic cells to present processed antigen to CD8+ T cells (10, 73, 78, 82) and promotes CD8+ T-cell memory responses (38). CD40L also plays a role in antibody responses by promoting B-cell proliferation and immunoglobulin class switching (70). These immunostimulatory functions of CD40L have led to many strategies for using it as a vaccine adjuvant.
Despite the intense interest in CD40L as a key activator of immune responses (over 6,000 papers have been published that relate to either CD40L or its receptor), there are currently no clinically accepted means for applying CD40L or most other TNFSFs in vivo. A Phase I clinical trial of a 1-trimer soluble form of CD40L (sCD40LT) (63) was conducted in patients with cancer, but hepatotoxicity limited the feasibility of delivering this protein by the systemic route (95). In contrast, the fusion of the extracellular domain of a TNFSF with the body of a multimerizing scaffold molecule provides a new platform for producing multimeric soluble TNFSFs that may circumvent the previous difficulties in studying this important molecular family.
TNFSFs are usually produced as type II trimeric membrane proteins but may be proteolytically cleaved from the cell surface to form soluble trimers. TNF itself was discovered as a soluble protein, which led to the assumption that all TNFSFs might be active as soluble trimers. It was soon determined, however, that soluble TNF activates only one of the two TNF receptors, whereas the multimeric membrane form of TNF activates both receptors (27). The critical role of multimerization was proven by showing that the activities of membrane TNF could be replicated by a soluble, Flag-tagged form of TNF that was cross-linked by anti-Flag antibody (77). Parallel studies of FasL, another TNFSF, found that soluble FasL, unlike membrane FasL, did not induce apoptosis in fresh, resting CD4+ T cells. However, a spontaneously aggregating form of soluble FasL (WX1) acted like membrane FasL, again demonstrating the importance of TNFSF multimerization for activity (87).
A similar picture has arisen for CD40L, where cross-linking of the soluble form is needed to stimulate B cells to the same level as membrane CD40L. In the case of a CD8-CD40L fusion protein, cross-linking with anti-CD8 antibody was required to drive resting B cells to proliferate (46). Similarly, membrane CD40L expressed on baculovirus-transduced SF9 insect cells strongly stimulated B cells, but small vesicles (10 to 1,500 nm) from the same cells were much less stimulatory (43). These in vitro studies indicate that CD40L trimers are most active when presented in a multimeric form.
More recently, the multimerization requirements for the activity of soluble CD40L were studied in greater detail using multimeric soluble TNFSF fusion proteins. C1q superfamily molecules (44, 45) and collectin superfamily molecules (15, 36, 37, 54, 93) are proteins that spontaneously multimerize into molecular structures with extended, trimeric, collagen-like arms. Acrp30 (adiponectin) is a C1q superfamily protein that forms a V-shaped molecule with two trimeric arms (67, 92), whereas pulmonary surfactant protein D (SP-D) is a collectin protein that forms a plus-sign-shaped molecule with four trimeric arms (15). By replacing the globular head groups of Acrp30 or the carbohydrate recognition domains of SP-D with the extracellular domain of CD40L, Holler et al. (34) and Haswell et al. (33) produced molecules carrying two and four trimers of CD40L, respectively. Whereas single-trimer soluble CD40L did not effectively stimulate B cells, these multimeric (i.e., many-trimer) soluble CD40L fusion proteins were strongly stimulatory in vitro (33, 34).
The current study aimed at determining the multimerization requirements of soluble CD40L in a relevant in vivo setting, namely immunization. Because the large Acrp30-CD40L and SP-D-CD40L protein molecules were difficult to purify (unpublished data), a DNA vaccine approach was adopted. An antigen plasmid encoding the Gag protein of HIV-1 was mixed with plasmids encoding either native membrane CD40L or one of three forms of soluble CD40L: 1-trimer (stabilized by an isoleucine zipper), 2-trimer (Acrp30-CD40L fusion protein), and 4-trimer (SP-D-CD40L fusion protein). Remarkably, the effectiveness of soluble CD40L as an adjuvant for CD8+ T-cell responses appeared to be directly related to the valence of the trimers, with the 2- and 4-trimer CD40L constructs being significantly stronger than that of the single trimer construct.
It is worth noting, however, that the scaffold moiety might have adjuvant-promoting effects independent of its effect on the valence of the CD40L trimers. For example, the collagen-like portion of SP-D has been reported to bind to gp340, a receptor on the surface of macrophages and possibly DCs (35). Since this portion of SP-D was included in SP-D-CD40L, binding of this fusion protein to gp340 could serve to concentrate SP-D-CD40L on the surface of antigen-presenting cells and thereby enhance its effectiveness.
The finding that membrane CD40L, pMemCD40L, did not serve as an effective DNA vaccine adjuvant stands in contrast to several previous reports (13, 31, 39, 57, 60, 80, 96). In all of these cases, the antigen used in the DNA vaccine was cell associated, i.e., located either in the cytoplasm, like β-galactosidase (13, 60), or on the cell membrane, like HIV-1 gp160 (39) or respiratory syncytial virus envelope (31). In contrast, the present report and two previous reports failed to find any adjuvant effects with pMemCD40L-like plasmids when secreted antigens were studied (e.g., TSSA from Treponema cruzi [61] or gp120 [88]). Antigen localization has been previously identified as an important variable for DNA vaccination (12, 62, 69, 75, 89) and has implications for the selection of adjuvanting molecules. When a DNA vaccine encodes nonsecreted, cell-associated antigen (either cytoplasmic or transmembrane), it may be crucial for dendritic cells to migrate to the vaccination site in order to take up antigen and transport it to the draining lymph node for presentation to T cells. Consistent with this model, chemotactic substances that attract DCs into the intramuscular vaccine site have been shown to significantly enhance the response to DNA vaccines for nonsecreted antigens (51, 88). While secreted antigens can be transported by DCs in the same way, lymphatic vessels can also carry these soluble proteins (exemplified by lysozyme and ovalbumin) to the draining lymph node, whereupon intranodal DCs take up and present these antigens to T cells within 3 to 6 h (40, 55). Applying these considerations to the data in this report, if secreted Gag generated by pScGag at the intramuscular vaccination site rapidly moved to the draining lymph node while membrane CD40L from pMemCD40L remained immobilized on myocytes at the vaccination site, this would explain why membrane CD40L (pMemCD40L) failed to adjuvant secreted Gag (pScGag). In contrast, soluble forms of CD40L such as that generated by pSP-D-CD40L can accompany this secreted antigen to the draining lymph node and thereby adjuvant the immune response more effectively.
It is also worth noting that DNA encoding a form of soluble CD40L trimer (sCD40LT) (63) was previously reported to be an effective adjuvant for a DNA vaccine encoding a nonsecreted antigen (β-galactosidase) (28). If sCD40LT was expressed as a single-trimer protein, this report would be inconsistent with the in vitro data described above showing that CD40L, like several other TNFSF ligands, is not very active unless multimerized beyond the 1-trimer form (33, 34). Previous reports that the sCD40LT protein is active on DCs in vitro could be explained if this protein were produced using a low-pH step, such as the pH 2.8 elution step used to remove Flag-tagged sCD40LT protein from an anti-Flag antibody affinity purification column (Srinivasan and Spriggs, U.S. patent 5,716,805). Under these low-pH conditions, the protein becomes disordered into a sticky “molten globule” structure that readily aggregates, thereby effectively multimerizing the purified protein into a many-trimer multimer (56). The effectiveness of the psCD40LT plasmid as a DNA vaccine adjuvant could also be explained if it contained a Flag tag, as described for another DNA vaccine made with this construct (97). If so, the formation of anti-Flag antibodies would be expected to multimerize the CD40L trimeric fusion protein into a high-molecular-weight, poorly diffusible immune complex (77) that could effectively adjuvant a nonsecreted antigen. To exclude this possibility, the Flag sequence was intentionally omitted from the pTrCD40L construct studied in this report, and this 1-trimer soluble CD40L construct was significantly less active than the 2- and 4-trimer forms, consistent with the in vitro studies (33, 34).
Given the difficulties in producing a usable form of soluble CD40L, most studies have employed agonistic anti-CD40 antibodies to activate CD40-bearing cells. Agonistic anti-CD40 antibodies were used to prove the role of CD40-activated DCs in generating CD8+ T-cell responses (73, 78) and to induce anti-tumor responses (23, 83, 94). Agonistic anti-CD40 antibodies also act as a general vaccine adjuvant (8) and synergize with Toll-like receptor agonists to elicit profound vaccine-induced CD8+ T-cell responses (4). However, these antibodies induce splenomegaly and shock-like symptoms unless used in small amounts in a single administration (8, 94), which raises concerns about using agonistic anti-CD40 antibodies in humans.
In contrast, the multimeric soluble CD40L plasmids described in this report (pAcrp30-CD40L and pSP-D-CD40L) have no apparent toxicity. No signs of distress were seen at any point in the 6-week vaccination protocol, and no inflammation was induced in the muscle tissue 48 h after injecting these plasmids. As evidence that systemic immune activation was absent, the injection of the pScGag antigen plasmid and the pSP-D-CD40L adjuvant plasmid separately into opposite legs did not induce the augmented CD8+ T-cell responses that were seen when the two plasmids were premixed and injected together. Given that DNA vaccination appears to be safe in human volunteers (19), it is likely that plasmid DNA for multimeric soluble TNFSF fusion proteins could be added to DNA vaccines without incurring the risks associated with agonistic antibodies to TNF receptors.
While DNA vaccination may be safe, its efficacy has been called into question, particularly in humans. Although some DNA vaccines elicit strong immune responses in mice (29), most investigators have found it necessary to add cytokines or chemokines to enhance the response to immunization (2, 7, 26, 49). Alternative or complementary approaches include electroporation (65), gene gun delivery of DNA-coated gold particles (90), and delivery on polymer microparticles (64) or by polymer microencapsulation (52). The need for such modifications is exemplified by the very weak CD8+ T-cell responses seen after DNA vaccination in clinical studies of HIV vaccines (21). Another example in humans is the complete lack of antibodies induced by a DNA vaccine for malaria (19).
Importantly, however, the pSP-D-CD40L-containing vaccine described in this report generated strong CD8+ T-cell responses. While a head-to-head comparison of a pSP-D-CD40L-adjuvanted vaccine with other vaccines has not yet been performed, it is instructive to compare these results with those of other studies in BALB/c mice that employed exactly the same CD8+ T-cell target cell (AMQMLKETI peptide-pulsed P815 cells) for either CTL assays or as a stimulator in IFN-γ ELISPOT assays. By these measures, the results in this report equaled or exceeded those reported for chimpanzee adenovirus 68-based or vaccinia virus-based Gag vaccines (22), a flavivirus Kunjin Gag vaccine (32), or Gag delivered intraperitoneally using live Listeria monocytogenes as a vector (68). Stronger CD8+ T-cell responses using these assays were reported for a vesicular stomatitis virus vector (30) or a second-generation rabies vector (58). However, unlike DNA vaccines, these viral and bacterial vectors generate anti-vector immune responses that could limit booster immunizations with the same agent.
Despite the enhancing effect of pSP-D-CD40L on CD8+ T-cell responses, this adjuvant plasmid did not augment the proliferative and cytokine responses of CD4+ T cells or the production of IgG antibody. Agonistic anti-CD40 antibody was previously reported to increase CD4+ T-cell responses to a modest degree (25), but the experiments herein failed to detect any significant effects of multimeric soluble CD40L on CD4+ T-cell-proliferative responses and cytokine production. In support of the concept that CD40 stimulation is not linked to subsequent CD4+ T-cell responses, a previous study showed that lymphocytic choriomeningitis virus-exposed transgenic mice lacking either CD40 or CD40L maintained normal CD4+ T-cell-proliferative and cytokine responses to lymphocytic choriomeningitis virus antigens, indicating that the CD40L/CD40 system is not strictly necessary for CD4+ cell responses (66). It remains possible, however, that the response to antigens that contain stronger CD4+ T-helper epitopes would be enhanced by pSP-D-CD40L (3).
The lack of a consistent enhancing effect of pSP-D-CD40L on antibody responses was initially very surprising, given the known role of CD40 stimulation in B-cell activation (70). Even more unexpected were the data indicating that pSP-D-CD40L could suppress antibody induced by the pScGag antigen plasmid alone (Fig. 5D). However, these data are in accord with several reports showing that strong CD40 stimulation prevented the movement of B cells into germinal centers, blocked the development of memory B cells, and impaired B-cell differentiation into antibody-secreting plasma cells (6, 14, 20, 71). Thus, the negative effect of pSP-D-CD40L on antibody production described in this report supports the concept of strong CD40 stimulation provided by multimeric soluble CD40L.
This profile of responses (strong CD8+ T-cell responses but negligible CD4+ T-cell and antibody responses) contrasts with the responses elicited by most experimental vaccines under development. While few infections could benefit from such a skewed vaccine response, this unique response profile has considerable potential for a vaccine against HIV. Unlike most microbes, HIV replicates in activated CD4+ T cells and especially in HIV-specific CD4+ T cells (18, 24). Indeed, when macaques were vaccinated with an simian immunodeficiency virus (SIV) envelope vaccine that elicited anti-SIV CD4+ T cells but virtually no anti-SIV CD8+ T cells, the vaccinated macaques failed to control a subsequent SIV challenge and rapidly progressed to AIDS (84). This study revealed the need for a “CD8-focused” vaccine that would generate strong antiviral CD8+ T-cell responses but minimal CD4+ T-cell responses (24). The multimeric soluble CD40L vaccine of the present study is perhaps the most CD8-focused vaccine yet described and thus should be considered for further testing.
Since fusion with a multimeric scaffold is a generalizable method for producing multimeric soluble TNFSF ligands (34), this strategy provides a convenient way to test the in vivo effects of other TNFSFs. GITRL is an important TNFSF to test, given the critical role of Tregs in limiting immune responses and the characteristic presence of GITR on these cells (76). Depletion of CD4+ T cells prior to vaccination, including DNA vaccination, has led to enhanced CD8+ T-cell responses due to the removal of Tregs (50). Administration of agonistic anti-GITR antibodies reversed Treg suppression of CD4+ and CD8+ T-cell responses (16, 59, 79). Membrane GITRL expressed on 293 cells completely reversed immunosuppression by Tregs and strongly costimulated antigen-responsive T cells (42, 91). Recently, Ji et al. described the effects of Flag-tagged soluble GITRL on CD4+CD25+ Tregs and found that cross-linking with anti-Flag antibody significantly enhanced the activity of this TNFSF protein in vitro (C. Terhorst, personal communication) (41). This suggests that soluble GITRL, like FasL, TRAIL, and CD40L (33, 34), is most active as a multimeric protein.
Consistent with this hypothesis, pSP-D-GITRL was an effective DNA vaccine adjuvant. Immunization with pScGag plus pSP-D-GITRL led to strong CD8+ T-cell responses, although they were less than that with pSP-D-CD40L. Interestingly, the pSP-D-GITRL adjuvant plasmid significantly augmented proliferative responses and antibody production. It is likely that the augmentation of CD4+ T-cell responses by pSP-D-GITRL provided helper CD4+ T cells for B cells. Thus, GITRL, here used as pSP-D-GITRL, is an interesting new vaccine adjuvant, although further studies are needed to determine if it can be used safely without exacerbating autoimmunity.
In conclusion, these studies establish the adjuvant activity of two multimeric soluble TNFSF ligands in vivo when used as part of a DNA vaccine. Several other immunostimulatory TNFSFs remain to be tested in this manner, including RANKL, OX40L, 4-1BBL, CD70, and LIGHT. Using the multimeric scaffold fusion protein approach described here, these and other TNFSFs can now be tested in vivo in an expeditious manner.
Acknowledgments
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: V3 consensus envelope peptides; HIV-1IIIB p55 protein; HIV-1SF162 gp120 protein; pSyngp140JR-FL, donated by Eun-Chung Park and Brian Seed; and H-2Kd/AMQMLKETI tetramer, from the NIAID Tetramer Facility, Atlanta, GA. We thank Douglas D. Richman for stimulating discussions and encouragement. We gratefully acknowledge Kendra McCafferty and Mari V. Bray for training in veterinary procedures and Eyal Raz and Tomoko Hayashi for advice on immunological assays. We thank the Flow Cytometry Core and Molecular Biology Core of the UCSD Center for AIDS Research for assistance (supported by NIH grant P30 AI036214).
This study was supported by NIH grant R21AI52842, American Foundation for AIDS Research (amfAR) grant 02719-28-RGV, and the State of California’s Universitywide AIDS Research Program grant ID04-VMRF-031 (all to R.S.K.), and by the Research Center on AIDS and HIV Infection of the VA San Diego Healthcare System. C.A.S. is the recipient of a Merit Review award from the Department of Veterans Affairs. G.W.S. was supported by an NIH AIDS Training Grant to UCSD (T32AI007384).
REFERENCES
- 1.Abraham, E. 2003. Computational design of variant TNF molecules: a novel methodology for inhibition of proinflammatory cascades. Sci. STKE 2003:PE51. [DOI] [PubMed] [Google Scholar]
- 2.Ahlers, J. D., I. M. Belyakov, and J. A. Berzofsky. 2003. Cytokine, chemokine, and costimulatory molecule modulation to enhance efficacy of HIV vaccines. Curr. Mol. Med. 3:285-301. [DOI] [PubMed] [Google Scholar]
- 3.Ahlers, J. D., I. M. Belyakov, E. K. Thomas, and J. A. Berzofsky. 2001. High-affinity T helper epitope induces complementary helper and APC polarization, increased CTL, and protection against viral infection. J. Clin. Investig. 108:1677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahonen, C. L., C. L. Doxsee, S. M. McGurran, T. R. Riter, W. F. Wade, R. J. Barth, J. P. Vasilakos, R. J. Noelle, and R. M. Kedl. 2004. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J. Exp. Med. 199:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andre, S., B. Seed, J. Eberle, W. Schraut, A. Bultmann, and J. Haas. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 72:1497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arpin, C., J. Banchereau, and Y. J. Liu. 1997. Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J. Exp. Med. 186:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch, D. H., N. L. Letvin, and R. A. Seder. 2004. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol. Rev. 202:266-274. [DOI] [PubMed] [Google Scholar]
- 8.Barr, T. A., A. L. McCormick, J. Carlring, and A. W. Heath. 2003. A potent adjuvant effect of CD40 antibody attached to antigen. Immunology 109:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazzoni, F., and B. Beutler. 1996. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 334:1717-1725. [DOI] [PubMed] [Google Scholar]
- 10.Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 11.Bodmer, J. L., P. Schneider, and J. Tschopp. 2002. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27:19-26. [DOI] [PubMed] [Google Scholar]
- 12.Boyle, J. S., C. Koniaras, and A. M. Lew. 1997. Influence of cellular location of expressed antigen on the efficacy of DNA vaccination: cytotoxic T lymphocyte and antibody responses are suboptimal when antigen is cytoplasmic after intramuscular DNA immunization. Int. Immunol. 9:1897-1906. [DOI] [PubMed] [Google Scholar]
- 13.Burger, J. A., R. B. Mendoza, and T. J. Kipps. 2001. Plasmids encoding granulocyte-macrophage colony-stimulating factor and CD154 enhance the immune response to genetic vaccines. Vaccine 19:2181-2189. [DOI] [PubMed] [Google Scholar]
- 14.Callard, R. E., J. Herbert, S. H. Smith, R. J. Armitage, and K. E. Costelloe. 1995. CD40 cross-linking inhibits specific antibody production by human B cells. Int. Immunol. 7:1809-1815. [DOI] [PubMed] [Google Scholar]
- 15.Crouch, E., A. Persson, D. Chang, and J. Heuser. 1994. Molecular structure of pulmonary surfactant protein D (SP-D). J. Biol. Chem. 269:17311-17319. [PubMed] [Google Scholar]
- 16.Dittmer, U., H. He, R. J. Messer, S. Schimmer, A. R. Olbrich, C. Ohlen, P. D. Greenberg, I. M. Stromnes, M. Iwashiro, S. Sakaguchi, L. H. Evans, K. E. Peterson, G. Yang, and K. J. Hasenkrug. 2004. Functional impairment of CD8(+) T cells by regulatory T cells during persistent retroviral infection. Immunity 20:293-303. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly, J. J., B. Wahren, and M. A. Liu. 2005. DNA vaccines: progress and challenges. J. Immunol. 175:633-639. [DOI] [PubMed] [Google Scholar]
- 18.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 19.Epstein, J. E., E. J. Gorak, Y. Charoenvit, R. Wang, N. Freydberg, O. Osinowo, T. L. Richie, E. L. Stoltz, F. Trespalacios, J. Nerges, J. Ng, V. Fallarme-Majam, E. Abot, L. Goh, S. Parker, S. Kumar, R. C. Hedstrom, J. Norman, R. Stout, and S. L. Hoffman. 2002. Safety, tolerability, and lack of antibody responses after administration of a PfCSP DNA malaria vaccine via needle or needle-free jet injection, and comparison of intramuscular and combination intramuscular/intradermal routes. Hum. Gene Ther. 13:1551-1560. [DOI] [PubMed] [Google Scholar]
- 20.Erickson, L. D., B. G. Durell, L. A. Vogel, B. P. O'Connor, M. Cascalho, T. Yasui, H. Kikutani, and R. J. Noelle. 2002. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. J. Clin. Investig. 109:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estcourt, M. J., A. J. McMichael, and T. Hanke. 2004. DNA vaccines against human immunodeficiency virus type 1. Immunol. Rev. 199:144-155. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416-1422. [DOI] [PubMed] [Google Scholar]
- 23.French, R. R., H. T. Chan, A. L. Tutt, and M. J. Glennie. 1999. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 5:548-553. [DOI] [PubMed] [Google Scholar]
- 24.Garber, D. A., G. Silvestri, and M. B. Feinberg. 2004. Prospects for an AIDS vaccine: three big questions, no easy answers. Lancet Infect. Dis. 4:397-413. [DOI] [PubMed] [Google Scholar]
- 25.Gerloni, M., S. Xiong, S. Mukerjee, S. P. Schoenberger, M. Croft, and M. Zanetti. 2000. Functional cooperation between T helper cell determinants. Proc. Natl. Acad. Sci. USA 97:13269-13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giri, M., K. E. Ugen, and D. B. Weiner. 2004. DNA vaccines against human immunodeficiency virus type 1 in the past decade. Clin. Microbiol. Rev. 17:370-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grell, M., E. Douni, H. Wajant, M. Lohden, M. Clauss, B. Maxeiner, S. Georgopoulos, W. Lesslauer, G. Kollias, K. Pfizenmaier, et al. 1995. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell 83:793-802. [DOI] [PubMed] [Google Scholar]
- 28.Gurunathan, S., K. R. Irvine, C. Y. Wu, J. I. Cohen, E. Thomas, C. Prussin, N. P. Restifo, and R. A. Seder. 1998. CD40 ligand/trimer DNA enhances both humoral and cellular immune responses and induces protective immunity to infectious and tumor challenge. J. Immunol. 161:4563-4571. [PMC free article] [PubMed] [Google Scholar]
- 29.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 30.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. High-level primary CD8(+) T-cell response to human immunodeficiency virus type 1 gag and env generated by vaccination with recombinant vesicular stomatitis viruses. J. Virol. 76:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harcourt, J. L., M. P. Brown, L. J. Anderson, and R. A. Tripp. 2003. CD40 ligand (CD154) improves the durability of respiratory syncytial virus DNA vaccination in BALB/c mice. Vaccine 21:2964-2979. [DOI] [PubMed] [Google Scholar]
- 32.Harvey, T. J., I. Anraku, R. Linedale, D. Harrich, J. Mackenzie, A. Suhrbier, and A. A. Khromykh. 2003. Kunjin virus replicon vectors for human immunodeficiency virus vaccine development. J. Virol. 77:7796-7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haswell, L. E., M. J. Glennie, and A. Al-Shamkhani. 2001. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur. J. Immunol. 31:3094-3100. [DOI] [PubMed] [Google Scholar]
- 34.Holler, N., A. Tardivel, M. Kovacsovics-Bankowski, S. Hertig, O. Gaide, F. Martinon, A. Tinel, D. Deperthes, S. Calderara, T. Schulthess, J. Engel, P. Schneider, and J. Tschopp. 2003. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol. Cell. Biol. 23:1428-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmskov, U., J. Mollenhauer, J. Madsen, L. Vitved, J. Gronlund, I. Tornoe, A. Kliem, K. B. Reid, A. Poustka, and K. Skjodt. 1999. Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D. Proc. Natl. Acad. Sci. USA 96:10794-10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmskov, U., S. Thiel, and J. C. Jensenius. 2003. Collectins and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547-578. [DOI] [PubMed] [Google Scholar]
- 37.Hoppe, H. J., and K. B. Reid. 1994. Collectins-soluble proteins containing collagenous regions and lectin domains-and their roles in innate immunity. Protein Sci. 3:1143-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 101:5610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ihata, A., S. Watabe, S. Sasaki, A. Shirai, J. Fukushima, K. Hamajima, J. Inoue, and K. Okuda. 1999. Immunomodulatory effect of a plasmid expressing CD40 ligand on DNA vaccination against human immunodeficiency virus type-1. Immunology 98:436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itano, A. A., S. J. McSorley, R. L. Reinhardt, B. D. Ehst, E. Ingulli, A. Y. Rudensky, and M. K. Jenkins. 2003. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19:47-57. [DOI] [PubMed] [Google Scholar]
- 41.Ji, H. B., G. Liao, W. A. Faubion, A. C. Abadia-Molina, C. Cozzo, F. S. Laroux, A. Caton, and C. Terhorst. 2004. Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J. Immunol. 172:5823-5827. [DOI] [PubMed] [Google Scholar]
- 42.Kanamaru, F., P. Youngnak, M. Hashiguchi, T. Nishioka, T. Takahashi, S. Sakaguchi, I. Ishikawa, and M. Azuma. 2004. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J. Immunol. 172:7306-7314. [DOI] [PubMed] [Google Scholar]
- 43.Kehry, M. R., B. E. Castle, and P. D. Hodgkin. 1992. B-cell activation mediated by interactions with membranes from helper T cells. Adv. Exp. Med. Biol. 323:139-148. [DOI] [PubMed] [Google Scholar]
- 44.Kishore, U., C. Gaboriaud, P. Waters, A. K. Shrive, T. J. Greenhough, K. B. Reid, R. B. Sim, and G. J. Arlaud. 2004. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 25:551-561. [DOI] [PubMed] [Google Scholar]
- 45.Kishore, U., and K. B. Reid. 1999. Modular organization of proteins containing C1q-like globular domain. Immunopharmacology 42:15-21. [DOI] [PubMed] [Google Scholar]
- 46.Klaus, G. G., M. Holman, C. Johnson-Léger, J. R. Christenson, and M. R. Kehry. 1999. Interaction of B cells with activated T cells reduces the threshold for CD40-mediated B cell activation. Int. Immunol. 11:71-79. [DOI] [PubMed] [Google Scholar]
- 47.Kornbluth, R. S. 2002. An expanding role for CD40L and other tumor necrosis factor superfamily ligands in HIV infection. J. Hematother. Stem Cell Res. 11:787-801. [DOI] [PubMed] [Google Scholar]
- 48.Kornbluth, R. S., K. Kee, and D. D. Richman. 1998. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive beta-chemokines. Proc. Natl. Acad. Sci. USA 95:5205-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowalczyk, D. W., and H. C. J. Ertl. 1999. Immune responses to DNA vaccines. Cell. Mol. Life Sci. 55:751-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kursar, M., K. Bonhagen, J. Fensterle, A. Kohler, R. Hurwitz, T. Kamradt, S. H. Kaufmann, and H. W. Mittrucker. 2002. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J. Exp. Med. 196:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kutzler, M. A., and D. B. Weiner. 2004. Developing DNA vaccines that call to dendritic cells. J. Clin. Investig. 114:1241-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Little, S. R., D. M. Lynn, Q. Ge, D. G. Anderson, S. V. Puram, J. Chen, H. N. Eisen, and R. Langer. 2004. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc. Natl. Acad. Sci. USA 101:9534-9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 54.Lu, J., C. Teh, U. Kishore, and K. B. Reid. 2002. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim. Biophys. Acta 1572:387-400. [DOI] [PubMed] [Google Scholar]
- 55.Manickasingham, S., and C. Reis e Sousa. 2000. Microbial and T cell-derived stimuli regulate antigen presentation by dendritic cells in vivo. J. Immunol. 165:5027-5034. [DOI] [PubMed] [Google Scholar]
- 56.Matsuura, J. E., A. E. Morris, R. R. Ketchem, E. H. Braswell, R. Klinke, W. R. Gombotz, and R. L. Remmele, Jr. 2001. Biophysical characterization of a soluble CD40 ligand (CD154) coiled-coil trimer: evidence of a reversible acid-denatured molten globule. Arch. Biochem. Biophys. 392:208-218. [DOI] [PubMed] [Google Scholar]
- 57.Maue, A. C., W. R. Waters, M. V. Palmer, D. L. Whipple, F. C. Minion, W. C. Brown, and D. M. Estes. 2004. CD80 and CD86, but not CD154, augment DNA vaccine-induced protection in experimental bovine tuberculosis. Vaccine 23:769-779. [DOI] [PubMed] [Google Scholar]
- 58.McGettigan, J. P., R. J. Pomerantz, C. A. Siler, P. M. McKenna, H. D. Foley, B. Dietzschold, and M. J. Schnell. 2003. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. J. Virol. 77:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McHugh, R. S., M. J. Whitters, C. A. Piccirillo, D. A. Young, E. M. Shevach, M. Collins, and M. C. Byrne. 2002. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16:311-323. [DOI] [PubMed] [Google Scholar]
- 60.Mendoza, R. B., M. J. Cantwell, and T. J. Kipps. 1997. Immunostimulatory effects of a plasmid expressing CD40 ligand (CD154) on gene immunization. J. Immunol. 159:5777-5781. [PubMed] [Google Scholar]
- 61.Miyahira, Y., H. Akiba, M. Katae, K. Kubota, S. Kobayashi, T. Takeuchi, A. Garcia-Sastre, Y. Fukuchi, K. Okumura, H. Yagita, and T. Aoki. 2003. Cutting edge: a potent adjuvant effect of ligand to receptor activator of NF-kappa B gene for inducing antigen-specific CD8+ T cell response by DNA and viral vector vaccination. J. Immunol. 171:6344-6348. [DOI] [PubMed] [Google Scholar]
- 62.Morel, P. A., D. Falkner, J. Plowey, A. T. Larregina, and L. D. Falo. 2004. DNA immunisation: altering the cellular localisation of expressed protein and the immunisation route allows manipulation of the immune response. Vaccine 22:447-456. [DOI] [PubMed] [Google Scholar]
- 63.Morris, A. E., R. L. Remmele, Jr., R. Klinke, B. M. Macduff, W. C. Fanslow, and R. J. Armitage. 1999. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154). J. Biol. Chem. 274:418-423. [DOI] [PubMed] [Google Scholar]
- 64.O'Hagan, D., M. Singh, M. Ugozzoli, C. Wild, S. Barnett, M. Chen, M. Schaefer, B. Doe, G. R. Otten, and J. B. Ulmer. 2001. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J. Virol. 75:9037-9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otten, G., M. Schaefer, B. Doe, H. Liu, I. Srivastava, J. zur Megede, D. O'Hagan, J. Donnelly, G. Widera, D. Rabussay, M. G. Lewis, S. Barnett, and J. B. Ulmer. 2004. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 22:2489-2493. [DOI] [PubMed] [Google Scholar]
- 66.Oxenius, A., K. A. Campbell, C. R. Maliszewski, T. Kishimoto, H. Kikutani, H. Hengartner, R. M. Zinkernagel, and M. F. Bachmann. 1996. CD40-CD40 ligand interactions are critical in T-B cooperation but not for other anti-viral CD4+ T cell functions. J. Exp. Med. 183:2209-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pajvani, U. B., X. Du, T. P. Combs, A. H. Berg, M. W. Rajala, T. Schulthess, J. Engel, M. Brownlee, and P. E. Scherer. 2003. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J. Biol. Chem. 278:9073-9085. [DOI] [PubMed] [Google Scholar]
- 68.Peters, C., X. Peng, D. Douven, Z. K. Pan, and Y. Paterson. 2003. The induction of HIV Gag-specific CD8+ T cells in the spleen and gut-associated lymphoid tissue by parenteral or mucosal immunization with recombinant Listeria monocytogenes HIV Gag. J. Immunol. 170:5176-5187. [DOI] [PubMed] [Google Scholar]
- 69.Qiu, J. T., B. Liu, C. Tian, G. N. Pavlakis, and X. F. Yu. 2000. Enhancement of primary and secondary cellular immune responses against human immunodeficiency virus type 1 gag by using DNA expression vectors that target Gag antigen to the secretory pathway. J. Virol. 74:5997-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quezada, S. A., L. Z. Jarvinen, E. F. Lind, and R. J. Noelle. 2004. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22:307-328. [DOI] [PubMed] [Google Scholar]
- 71.Randall, T. D., A. W. Heath, L. Santos-Argumedo, M. C. Howard, I. L. Weissman, and F. E. Lund. 1998. Arrest of B lymphocyte terminal differentiation by CD40 signaling: mechanism for lack of antibody-secreting cells in germinal centers. Immunity 8:733-742. [DOI] [PubMed] [Google Scholar]
- 72.Raz, E., A. Watanabe, S. M. Baird, R. A. Eisenberg, T. B. Parr, M. Lotz, T. J. Kipps, and D. A. Carson. 1993. Systemic immunological effects of cytokine genes injected into skeletal muscle. Proc. Natl. Acad. Sci. USA 90:4523-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 74.Robinson, H. L., and T. M. Pertmer. 2000. DNA vaccines for viral infections: basic studies and applications. Adv. Virus Res. 55:1-74. [DOI] [PubMed] [Google Scholar]
- 75.Rush, C., T. Mitchell, and P. Garside. 2002. Efficient priming of CD4+ and CD8+ T cells by DNA vaccination depends on appropriate targeting of sufficient levels of immunologically relevant antigen to appropriate processing pathways. J. Immunol. 169:4951-4960. [DOI] [PubMed] [Google Scholar]
- 76.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22:531-562. [DOI] [PubMed] [Google Scholar]
- 77.Schneider, P., N. Holler, J. L. Bodmer, M. Hahne, K. Frei, A. Fontana, and J. Tschopp. 1998. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187:1205-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schoenberger, S. P., R. E. Toes, E. I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 79.Shimizu, J., S. Yamazaki, T. Takahashi, Y. Ishida, and S. Sakaguchi. 2002. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135-142. [DOI] [PubMed] [Google Scholar]
- 80.Sin, J. I., J. J. Kim, D. Zhang, and D. B. Weiner. 2001. Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum. Gene Ther. 12:1091-1102. [DOI] [PubMed] [Google Scholar]
- 81.Smith, C. A., T. Farrah, and R. G. Goodwin. 1994. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76:959-962. [DOI] [PubMed] [Google Scholar]
- 82.Smith, C. M., N. S. Wilson, J. Waithman, J. A. Villadangos, F. R. Carbone, W. R. Heath, and G. T. Belz. 2004. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat. Immunol. 5:1143-1148. [DOI] [PubMed] [Google Scholar]
- 83.Sotomayor, E. M., I. Borrello, E. Tubb, F. M. Rattis, H. Bien, Z. Lu, S. Fein, S. Schoenberger, and H. I. Levitsky. 1999. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 5:780-787. [DOI] [PubMed] [Google Scholar]
- 84.Staprans, S. I., A. P. Barry, G. Silvestri, J. T. Safrit, N. Kozyr, B. Sumpter, H. Nguyen, H. McClure, D. Montefiori, J. I. Cohen, and M. B. Feinberg. 2004. Enhanced SIV replication and accelerated progression to AIDS in macaques primed to mount a CD4 T cell response to the SIV envelope protein. Proc. Natl. Acad. Sci. USA 101:13026-13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steed, P. M., M. G. Tansey, J. Zalevsky, E. A. Zhukovsky, J. R. Desjarlais, D. E. Szymkowski, C. Abbott, D. Carmichael, C. Chan, L. Cherry, P. Cheung, A. J. Chirino, H. H. Chung, S. K. Doberstein, A. Eivazi, A. V. Filikov, S. X. Gao, R. S. Hubert, M. Hwang, L. Hyun, S. Kashi, A. Kim, E. Kim, J. Kung, S. P. Martinez, U. S. Muchhal, D. H. Nguyen, C. O'Brien, D. O'Keefe, K. Singer, O. Vafa, J. Vielmetter, S. C. Yoder, and B. I. Dahiyat. 2003. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science 301:1895-1898. [DOI] [PubMed] [Google Scholar]
- 86.Stephens, G. L., R. S. McHugh, M. J. Whitters, D. A. Young, D. Luxenberg, B. M. Carreno, M. Collins, and E. M. Shevach. 2004. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 173:5008-5020. [DOI] [PubMed] [Google Scholar]
- 87.Suda, T., H. Hashimoto, M. Tanaka, T. Ochi, and S. Nagata. 1997. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J. Exp. Med. 186:2045-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sumida, S. M., P. F. McKay, D. M. Truitt, M. G. Kishko, J. C. Arthur, M. S. Seaman, S. S. Jackson, D. A. Gorgone, M. A. Lifton, N. L. Letvin, and D. H. Barouch. 2004. Recruitment and expansion of dendritic cells in vivo potentiate the immunogenicity of plasmid DNA vaccines. J. Clin. Investig. 114:1334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Svanholm, C., L. Bandholtz, A. Lobell, and H. Wigzell. 1999. Enhancement of antibody responses by DNA immunization using expression vectors mediating efficient antigen secretion. J. Immunol. Methods 228:121-130. [DOI] [PubMed] [Google Scholar]
- 90.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 91.Tone, M., Y. Tone, E. Adams, S. F. Yates, M. R. Frewin, S. P. Cobbold, and H. Waldmann. 2003. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc. Natl. Acad. Sci. USA 100:15059-15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsao, T. S., E. Tomas, H. E. Murrey, C. Hug, D. H. Lee, N. B. Ruderman, J. E. Heuser, and H. F. Lodish. 2003. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J. Biol. Chem. 278:50810-50817. [DOI] [PubMed] [Google Scholar]
- 93.van de Wetering, J. K., L. M. van Golde, and J. J. Batenburg. 2004. Collectins: players of the innate immune system. Eur. J. Biochem. 271:1229-1249. [DOI] [PubMed] [Google Scholar]
- 94.van Mierlo, G. J., A. T. den Boer, J. P. Medema, E. I. van der Voort, M. F. Fransen, R. Offringa, C. J. Melief, and R. E. Toes. 2002. CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc. Natl. Acad. Sci. USA 99:5561-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vonderheide, R. H., J. P. Dutcher, J. E. Anderson, S. G. Eckhardt, K. F. Stephans, B. Razvillas, S. Garl, M. D. Butine, V. P. Perry, R. J. Armitage, R. Ghalie, D. A. Caron, and J. G. Gribben. 2001. Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 19:3280-3287. [DOI] [PubMed] [Google Scholar]
- 96.Wienhold, D., E. Armengol, A. Marquardt, C. Marquardt, H. Voigt, M. Buttner, A. Saalmuller, and E. Pfaff. 2005. Immunomodulatory effect of plasmids coexpressing cytokines in classical swine fever virus subunit gp55/E2-DNA vaccination. Vet. Res. 36:571-587. [DOI] [PubMed] [Google Scholar]
- 97.Xiang, R., F. J. Primus, J. M. Ruehlmann, A. G. Niethammer, S. Silletti, H. N. Lode, C. S. Dolman, S. D. Gillies, and R. A. Reisfeld. 2001. A dual-function DNA vaccine encoding carcinoembryonic antigen and CD40 ligand trimer induces T cell-mediated protective immunity against colon cancer in carcinoembryonic antigen-transgenic mice. J. Immunol. 167:4560-4565. [DOI] [PubMed] [Google Scholar]