Abstract
PEPSCAN analysis has been used to characterize the immunogenic regions of the capsid protein (CP) in virions of plum pox potyvirus (PPV). In addition to the well-known highly immunogenic N- and C-terminal domains of CP, regions within the core domain of the protein have also shown high immunogenicity. Moreover, the N terminus of CP is not homogeneously immunogenic, alternatively showing regions frequently recognized by antibodies and others that are not recognized at all. These results have helped us to design efficient antigen presentation vectors based on PPV. As predicted by PEPSCAN analysis, a small displacement of the insertion site in a previously constructed vector, PPV-γ, turned the derived chimeras into efficient immunogens. Vectors expressing foreign peptides at different positions within a highly immunogenic region (amino acids 43 to 52) in the N-terminal domain of CP were the most effective at inducing specific antibody responses against the foreign sequence.
Commercial and academic interest in plants as heterologous expression systems has increased significantly, especially for the production of biomedically relevant proteins (22). There are two major strategies for the production of therapeutic molecules by plants: genetic transformation of the plant genome to create transgenic plants and manipulation of the genome of plant viruses (2). Although both strategies have been used successfully to express proteins of interest, in some cases viral vectors may have certain advantages over transgenic plants, including a short cycle time, ease of scale-up, and a generally wide host range that allows expression of a gene in different plant species by using the same vector construct (22).
Characterization of the antigenic determinants of pathogens has led to the concept of peptide vaccines. It has been reported that the immunogenicity of these peptides is increased by the use of epitope-presentation systems (10, 14). Engineering virus coat proteins to function as carrier molecules for immunogenic peptides has been one of the approaches exploited (18). These carrier proteins have the potential to assemble and form recombinant virus particles displaying the desired epitopes on their surfaces. Both filamentous and icosahedral plant viruses have been successfully developed as epitope presentation systems (13, 14, 20, 21, 30, 38, 40, 42, 43) and in some cases as an alternative to previous tissue culture-derived vaccines (9, 25).
Plum pox virus (PPV) belongs to the Potyvirus genus of plant viruses. The potyvirus genome consists of a single-stranded messenger polarity RNA molecule of about 10 kb, with a VPg protein at its 5′ end and a poly(A) tail at its 3′ end (Fig. 1). This genome is translated into a large polyprotein that is further processed by three virus-encoded proteases (32, 33, 41). The genome is encapsidated by ∼2,000 U of a single type of capsid protein (CP), encoded at the 3′ end of the genome (37).
FIG. 1.
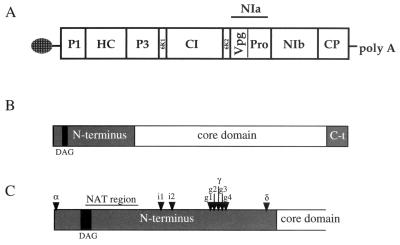
(A) Genome organization of PPV, with the viral open reading frame depicted as a box divided into the different viral products. (B) Schematic representation of PPV CP, showing its different domains. The gray boxes represent the N-terminal and C-terminal domains, and the white box represents the well-conserved core region. A black box indicates the localization of the DAG triad involved in aphid transmission. (C) Detailed representation of the N-terminal domain of PPV CP. The insertion points of the PPV-α, -γ, -δ, -i1, -i2, -g1, -g2, -g3, and -g4 antigen presentation vectors are indicated by arrowheads. The region deleted in NAT mutants is also indicated.
The potyvirus CP is involved in cell-to-cell and long-distance movement inside the plant. In particular, the N- and C-terminal parts of the CP are known to be involved in systemic movement (11, 12). Moreover, the N-terminal domain of the potyvirus CP holds an amino acid triad that is essential for aphid transmission (4, 5). Naturally appearing PPV mutants called NAT (non-aphid-transmissible) have been reported (27, 29). These mutants have a 15-amino-acid (aa) deletion that includes the last amino acid of the triad essential for aphid transmission. The N-terminal domain of the potyvirus CP, which is extremely variable among different virus species, has been previously described as being surface exposed on the potyvirus virions and highly immunogenic (1, 36).
Although promising results have been obtained with a PPV-derived antigen presentation vector, PPV-NATMluI (13), expressing different forms of a 15-aa peptide from the N terminus of the canine parvovirus (CPV) VP2 protein, this vector showed some restriction for the expression of other foreign peptides. Therefore, optimization studies were carried out to determine other appropriate insertion sites in the surface of PPV CP. A first generation of alternative PPV-derived vectors, with different insertion points within the CP N-terminal domain, was developed. Although these vectors were somehow tolerant to insertion of foreign sequences and chimeras derived from them were efficient as antigens, they failed to be good immunogens for evoking a specific response against foreign peptides. A second approach was based on the knowledge acquired from PEPSCAN analysis of PPV CP, particularly of its N-terminal domain. These data helped us to design novel vectors that rendered chimeras efficient at developing specific immune responses against foreign peptides.
MATERIALS AND METHODS
Synthesis of peptides linked to a cellulose membrane.
Multiple and simultaneous syntheses of peptides were performed. Peptides were synthesized linked to a pure-cellulose chromatographic paper. The synthesis was performed according to Fmoc (9-fluorenilmethoxy carbonyl) protocols (3). Peptides were linked to the membrane by their carboxy-terminal ends as previously described (15, 16). Application of the activated amino acids was performed automatically in a 24 × 16 matrix by using the robot Auto-Spot Robot ASP222 (Abimed). A membrane was prepared with a duplicated set of 10-aa-long overlapping peptides that covered the full-length sequence of PPV CP and the sequence of divergence between wild-type PPV CP and chimeric PPV-CPV CP (13), with displacements of 2 aa in the amino-carboxy sense.
Binding of specific antibodies to membrane-linked peptides.
Specific binding of PPV polyclonal antibodies (13) to membrane-bound PPV CP peptides was tested following the protocol described by R. Frank (15). All reactions were performed in 20 mM Tris (pH 7.5)-0.5 M NaCl. Membranes previously blocked with SuperBlock (Pierce) were incubated for at least 4 h at room temperature with different dilutions of the primary antibodies, depending on their affinities. Secondary antibodies conjugated to alkaline phosphatase were used in 1:10,000 dilutions. A commercial substrate for detection of the alkaline phosphatase enzymatic activity (Bio-Rad) was used. The high stability of the peptide linkages allowed us to reuse the membrane after stripping off all adsorbed material by subsequent treatments with dimethylformamide, 8 M urea, 1% sodium dodecyl sulfate, and 0.1% β-mercaptoethanol.
Construction of PPV-derived vectors.
Plasmid vectors pGPPV-α, pGPPV-γ, and pGPPV-δ were constructed according to the following strategy. Mutations that introduced the MluI restriction enzyme recognition sequence at particular positions were performed by PCR-based mutagenesis (19). The mutagenic oligodeoxynucleotides (oligos) used were mα (5′TCTTTCGTCACGCGTAGCTTG GTGC 3′), mγ (5′AGTTTGCAGACGCGTTTGAGGTCC 3′), and mδ (5′GTTGACTAGACGCGTCGCGTTTGAG 3′) (the MluI recognition sequence is shown in bold). Two flanking oligos from nucleotides (nt) 8067 to 8082 and the reverse one from nt 9114 to 9127 of PPV (23) were also used for the PCR amplifications. The ClaI-SacI fragments from the PCR-amplified products holding the respective mutations were introduced in the plasmid pGPPV (containing a PPV full-length cDNA under the control of a phage T7 promoter) (34) by triple ligation using SalI as the third enzyme.
A similar strategy was followed to construct the plasmid vectors pICPPV-g1, pICPPV-g2, pICPPV-g3, pICPPV-g4, pICPPV-i1, and pICPPV-i2. The mutagenic oligos used for the PCR-based mutagenesis were g1 (5′GCAGTTGAGGACGCGTTCCTGACACC 3′), g2 (5′ GTTTGCAGTTGACGCGTAGGTCCTGAC 3′), g3 (5′ CAAAAGTTTGACGCGTCAGTTGAGG 3′), g4 (5′ GTTCCAAAAGTACGCGTTTGCAGTTG 3′), i1 (5′ GAAAATGGGGTTACGCGTGAGCATTGACG 3′), and i2 (5′ GTTGCTGGCGTACGCGTGAAAATGGG 3′). The flanking oligos were the same as those described above. The mutated PCR fragments, digested with ClaI and SacI, were introduced in the plasmid pICPPV (containing a PPV full-length cDNA clone under the control of cauliflower mosaic virus 35S promoter) (28) by triple ligation using SalI as the third enzyme.
The accuracy of the sequences derived from PCR amplification was verified by DNA sequencing.
Insertion of foreign sequences in PPV-derived vectors.
The sequence encoding the 6L15 peptide of the canine parvovirus (CPV) (8) was created by hybridizing complementary oligos CPV-1, 5′ CGCGTGCAGTTCAACCAGACGGTGGTCAACCTGCTGTCAGAAATGAACGCG 3′ (forward), and CPV-2, 5′ CGCGCGCGTTCATTTCTGACAGCAGGTTGACCACCGTCTGGTTGAACTGCA 3′, (reverse). The sequence coding for a peptide of the envelope protein of feline immunodeficiency virus (FIV) (6) was created by hybridizing complementary oligos FIV-1, 5′ CGCGTGGTTGCAATCAAAACCAGTTCTTCTGCAAAG 3′ (forward), and FIV-2, from 5′ CGCGCTTTGCAGAAGAACTGGTTTTGATTGCAACCA 3′ (reverse). These pairs of oligos created MluI-compatible overhangs. The hybridization was carried out by incubating 1.5 μg of each oligo in a buffer containing 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 1 mM EDTA at 90°C for 5 min and then cooling the mixture down slowly to room temperature.
The hybridized oligos were cloned directly in the MluI sites of the different pICPPV-derived vectors. For cloning in pGPPV-α, pGPPV-γ, and pGPPV-δ, the hybridized oligos were inserted in pUC18-derived intermediate plasmids that contained PPV BamHI-SacI fragments holding the α, γ, and δ mutations. Then, the BamHI-SacI fragments of the resulting plasmids were introduced in pGPPV.
In vitro transcription and plant inoculation.
Capped transcripts from full-length cDNA clones derived from pGPPV were synthesized with the T7 Cap Scribe transcription kit (Roche Molecular Biochemicals), following the manufacturer's instructions. Twenty microliters of reaction mixture diluted 1:1 in 5 mM sodium phosphate (pH 7.5) were used to inoculate, by manual rubbing, eight Nicotiana clevelandii plants dusted with carborundum (three leaves per plant).
DNA from pICPPV-derived clones was directly used for mechanical inoculation (28). The plasmid DNA was diluted in 5 mM sodium phosphate (pH 7.5) to a concentration of between 100 and 500 ng/μl. Three leaves per N. clevelandii plant dusted with carborundum were inoculated with 10 μl of the diluted samples.
Viruses were propagated in N. clevelandii plants and were purified according to Laín et al. (24).
Western blot analysis.
Samples from infected plants homogenized in 5 mM sodium phosphate (pH 7.5) (2 ml/g) or from purified virions diluted in the same buffer were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and subjected to immunoreactions as described previously (17). A rabbit anti-PPV polyclonal antibody or a commercial mixture of anti-PPV monoclonal antibodies (MAbs) (Ingenasa, Madrid, Spain) was used for the detection of PPV capsid protein. An anti-CPV polyclonal antibody and the anti-CPV VP2 monoclonal antibody 3C9 (26) were used for detection of the VP2 peptide. Peroxidase-conjugated goat anti-mouse or anti-rabbit immunoglobulins G (IgG) purchased from Jackson ImmunoResearch Laboratories were used as secondary antibodies. The peroxidase reaction was developed either with the ECL kit (Amersham Pharmacia Biotech) or with 4-chloro-1-naphthol (Sigma).
Immunocapture-RT-PCR (IC-PCR).
Samples from infected plants homogenized in 5 mM sodium phosphate (pH 7.5) or purified virions diluted in the same buffer were incubated for 2 h at 37°C in tubes previously coated with anti-PPV IgG, and then, after two washing steps with phosphate-buffered saline (PBS)-Tween buffer (1× PBS, 0.5 g of Tween 20 per liter), reverse transcription (RT)-PCR was performed either as previously described (7) or by using the Titan one-tube RT-PCR system (Roche Molecular Biochemicals) according to the manufacturer's instructions.
Immunization of mice.
Groups of four 3-week-old BALB/c mice received three injections of 10 μg of protein (purified virions) per mouse intraperitoneally at days 0, 30, and 45. The samples were emulsified in complete Freund's adjuvant for the first inoculation and in incomplete Freund's adjuvant for the boosters. Immunized mice were bled before immunization and on day 60, and the sera were pooled for serological characterization.
ELISAs.
To detect the presence of specific antibodies against FIV in the sera of cats, enzyme-linked immunosorbent assay (ELISA) plates (Nunc) were coated with 100 ng of PPV-γ-FIV purified virions diluted in sodium carbonate buffer (50 mM; pH 9.6) overnight at 4°C. Then, they were subsequently incubated for 30 min at 25°C with different dilutions of cat sera in PBS-Tween-350 mM NaCl-1% powdered skim milk and with anti-cat IgGs conjugated with peroxidase (Sigma) diluted 1:20,000 in the same buffer. Washes were performed with PBS-Tween. Peroxidase activity was detected by adding 3,3′,5,5′,tetramethylbenzidine as a substrate and stopped with sulfuric acid. The optical densities of samples were determined at 405 nm.
To analyze the antibody response in animals immunized with PPV/CPV chimeras, indirect ELISAs were performed according to the protocol described in reference 13.
In vitro neutralization assays.
To determine the ability of the specific mouse sera to neutralize CPV in vitro, a CRFK cell monolayer-protection assay was performed as described previously (26).
RESULTS
Characterization of PPV-derived antigen presentation vectors PPV-α, PPV-γ, and PPV-δ and of chimeras derived from them.
Initially, three vectors were designed to express foreign sequences as insertions in three different points within the N-terminal domain of PPV CP. The clones pGPPV-α, pGPPV-γ, and pGPPV-δ have insertions of 6 nt that constitute the recognition sequence of MluI restriction enzyme. This means that mutants PPV-α, PPV-γ, and PPV-δ have insertions of two amino acids (threonine and arginine) in the corresponding positions within CP (Fig. 1). PPV-α holds the insertion point just after the first CP amino acid, which is known to be important for the cleavage event that splits off CP from the viral polyprotein. PPV-γ bears the insertion site between aa 68 and 69. We assumed that this place could be surface exposed because it resides near a site recognized by cellular proteases (24). Finally, the insertion place of PPV-δ lies between aa 87 and 88, very close to the beginning of the PPV CP conserved core domain (36). A point mutation, which gives rise to a serine-to-proline change at position 85 of the CP, was accidentally introduced during the construction of pGPPV-δ.
PPV-α, PPV-γ, and PPV-δ were able to establish a systemic infection in N. clevelandii plants. The time course of infection and symptomatology of PPV-γ and PPV-δ were similar to those of wild-type-PPV. Symptoms of PPV-α were mild and appeared with a delay of 6 to 7 days compared to wild-type virus. In addition, systemic accumulation of PPV-α was quite low (data not shown). For these reasons PPV-α was discarded as a candidate for peptide expression.
As a first attempt to test the capability of PPV-γ and PPV-δ as antigen presentation vectors, the 6L15 peptide from the N-terminal domain of VP2 protein of CPV was inserted in them. This peptide had previously shown its ability to induce neutralizing antibodies against CPV (8) and had been expressed in former PPV-derived chimeras (13). Capped transcripts synthesized from chimeric clones pGPPV-γ-CPV and pGPPV-δ-CPV were inoculated onto N. clevelandii plants. Both chimeras were able to infect the plants. The time course of infection and symptomatology were similar to those of wild-type-infected plants. Chimeric particles were good antigens, as they could be detected by a polyclonal antibody against CPV in Western blot assays and ELISA (data not shown). The stability of the mutated sequence in the progeny virus was corroborated by nucleotide sequencing of appropriate IC-PCR-amplified DNA fragments.
To further explore the usefulness of chimeras derived from these vectors as a source of antigen reagents, we decided to express a 10-aa peptide from a transmembrane protein of FIV in the PPV-γ vector. Different sequences including this peptide have failed to be expressed in PPV-NATMluI vector (M. R. Fernández-Fernández and J. A. García, unpublished results). There is great interest in the expression of this peptide in heterologous systems for diagnosis, as it contains an epitope that is universally recognized by the sera of FIV-infected cats (6). PPV-γ-FIV was infectious, and as is the case for PPV-γ-CPV, its infection resembled the wild-type one.
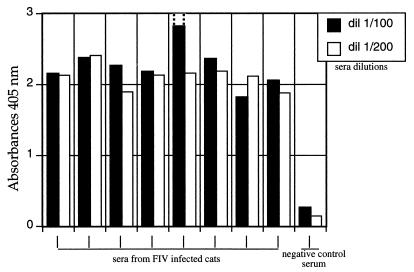
Wells of ELISA plates were coated with virions purified from PPV-γ-FIV-infected plants, and sera from FIV-infected and -noninfected animals were used to test the viability of the diagnosis system. Figure 2 shows that the PPV-γ-FIV chimera allows clear-cut discrimination between seronegative and seropositive animals even at a 1/200 serum dilution.
FIG. 2.
Detection of FIV-specific antibodies in cat sera. An indirect ELISA was performed by coating the plates with PPV-γ-FIV purified virions (100 ng per well). Two different dilutions of sera were tested, 1/100 (black bars) and 1/200 (white bars). Dotted lines represent saturation of that sample measurement.
In order to assess the immunogenicity of peptides expressed in the γ and δ sites, mice were immunized with PPV-γ-CPV and PPV-δ-CPV purified virions. No specific anti-CPV antibodies were detected in the immunized animals, despite their developing a strong antibody response against PPV (Table 1 and data not shown).
TABLE 1.
Antibody response to chimeric PPV particles in mice
| Antigen | ELISA titera
|
Neutralization titerb | ||
|---|---|---|---|---|
| 6L15 peptide | CPV | PPV | ||
| PPV-g1-CPV | 2.9 | 2.6 | 5.9 | 1.6 |
| PPV-g2-CPV | 3.2 | 2.6 | 5.9 | 1.9 |
| PPV-g3-CPV | 2.9 | 2 | 5.9 | 1.3 |
| PPV-g4-CPV | 3.5 | 2.9 | 5.9 | 1.6 |
| PPV-γ-CPV | —c | — | 5.9 | — |
| PPV | — | — | 5.9 | — |
Expressed as −log of the serum dilution that gave absorption values three times the blank value (preimmune serum).
Expressed as log of the highest serum dilution that causes a 50% reduction of the cell monolayer.
—, below detection threshold.
Preferential immunogenic regions of PPV CP.
The null capacity of the foreign peptides expressed by chimeras described above to develop an antibody response suggests that, in spite of the overall surface exposure of the N-terminal domain of potyvirus CP, there could be drastic differences in immunogenicity along this region. Thus, we decided to perform a PEPSCAN (term derived from peptide scanning) analysis of PPV CP to identify highly immunogenic regions that could help us in the design of new PPV antigen presentation vectors. The PEPSCAN analysis detects linear epitopes by assessing the reactivity of the antibody against short overlapping peptides derived from the corresponding protein.
Overlapping peptides covering the full-length PPV CP sequence were synthesized as spots in a cellulose membrane. Sera from rabbits and mice immunized with purified wild-type PPV or chimeric PPV (PPV-CPV and PPV-2CPV) virions were tested for binding to the immobilized peptides. A schematic representation of the amino acids recognized by the different antibodies is shown in Fig. 3. The results confirm that the region of preferential recognition by the sera is the N-terminal domain, although there were also other sequences highly immunogenic in the C-terminal domain, as well as in the central domain, which had usually been considered a nonexposed region (36). As we presumed, the N-terminal domain appeared not to be uniformly immunogenic. Within this region, stretches of preferential recognition alternated with others that were not recognized at all.
FIG. 3.
Reactivity of different sera tested against overlapping PPV CP peptides immobilized in a cellulose membrane. Bars show amino acids that reacted with the serum in at least two consecutive peptides. Black, rabbit 73 immunized with four doses of 200 μg of PPV; dark gray, rabbit 271 immunized with two doses of 50 μg of PPV; gray, rabbit 353 immunized with two doses of 500 μg of PPV; light gray, rabbit 347 immunized with two doses of 500 μg of PPV-CPV chimera (13); white, rabbit 349 immunized with two doses of 500 μg of the chimera PPV-2CPV (13); ▧, group of mice immunized with two doses of 50 μg of PPV; ▨, group of mice immunized with two doses of 5 μg of PPV; ▥, group of mice immunized with two doses of 5 μg of PPV-CPV chimera. All rabbit sera were used at a 1:500 dilution, and the mice sera were used at a 1:1,000 dilution. Sera from animals immunized with PPV-CPV chimeras recognize peptides with CPV sequences that are present in the chimera in positions equivalent to those flanked by asterisks. The serum from a rabbit immunized with a PPV-CPV chimera recognized PPV sequences (flanked by black dots) that are not present in the chimera sequence. The serum from a rabbit immunized with wild-type PPV recognized peptides with CPV sequences (represented by flanking white dots). The 15-aa sequence that is deleted in NAT mutants is boxed. Amino acids forming PRS are signaled by asterisks. The white arrow signals the limit between the N- and C-terminal domains (in italics) and the core domain of the CP sequence. Black arrows show the insertion sites of different PPV antigen presentation vectors.
According to these results, 13 preferential recognition sites (PRS), defined as sequences of four or more amino acids that were recognized by four or more of the tested sera, were identified. These PRS do not have to correspond exactly with 13 epitopes, since each site could contain more than one epitope. Four PRS reside in the N-terminal domain, one at the N terminus/core border, seven in the core domain, and one within the C-terminal domain (Table 2).
TABLE 2.
Identification of preferential immunogenic regions in the PPV CP sequencea
| Domain and PRS sequence (positions)b | Serum from rabbit no.c
|
Sera from group of mice immunized with 2 doses of:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 73 | 271 | 353 | 347 | 349 | 50 μg of PPV | 5 μg of PPV | 5 μg of PPV-CPV | |
| N terminus | ||||||||
| SPILQPPP (25-32) | + | + | − | + | + | + | − | − |
| SMLNPIFTPA (43-52) | + | + | + | + | + | + | + | + |
| VSQVSGPQ (61-68) | + | + | + | + | + | − | − | − |
| PQLQTFGT (67-74) | + | − | − | + | + | + | − | − |
| Core | ||||||||
| VNTNRDRDVDAG (89-100)d | + | +/− | + | + | + | + | + | + |
| SVGTFTVP (101-108) | + | − | + | + | + | − | − | − |
| SPAQVDLS (137-144) | + | − | + | + | + | + | + | + |
| KRDYDVTD (161-168) | + | + | + | + | − | − | − | − |
| TSPNINGM (187-194) | + | − | + | + | + | + | − | − |
| IQRNLTDY (249-256) | + | +/− | + | + | + | + | − | + |
| YAFDFY (261-264) | + | +/− | + | + | + | + | + | + |
| NRLFGL (293-298) | + | − | + | + | − | − | + | − |
| C terminus | ||||||||
| TAGDVN (313-318) | + | − | + | + | + | − | + | − |
+, reactivity of the serum against that region; −, no reactivity of the serum against that region; +/− reactivity of the sera with an overlapping, but slightly displaced sequence.
Sequences of four or more amino acids recognized by at least four sera.
See legend to Fig. 3 for details.
This PRS includes amino acids from the N-terminal region.
Development of alternative vectors with insertion sites near the γ site, and characterization of chimeras derived from them.
Given the null immunogenicity of PPV-γ-CPV and the existence in the neighborhood of the γ site (aa 68 to 69) of sequences recognized by anti-PPV sera in the PEPSCAN analysis, it was tempting to speculate that slight displacements of the insertion point from the γ site could improve the capability of derived chimeras to induce an efficient B-cell response. Four different mutant clones, pICPPV-g1, -g2, -g3, and -g4, were constructed. These clones have insertions of 6 nt that represent MluI restriction sites between sequences coding for aa 66 to 67 (g1), 67 to 68 (g2), 69 to 70 (g3), and 70 to 71 (g4) of PPV CP (Fig. 1). After direct mechanical inoculation with DNA of these plasmids, systemic infections were established in N. clevelandii plants with time courses and symptomatologies similar to those of wild-type PPV infections.
The sequence encoding the CPV 6L15 peptide was cloned in the MluI sites of these vectors. Inoculation of N. clevelandii plants with DNA from the resulting plasmids, pICPPV-g1-CPV, g2-CPV, g3-CPV, and g4-CPV, caused infections indistinguishable from that caused by wild-type PPV.
As expected, Western blot analysis with antibodies against PPV showed that chimeric capsids had a slightly reduced electrophoretic mobility regarding wild-type CP due to the insertion they hold (Fig. 4A). Chimeric capsids were specifically recognized by antibodies against CPV peptide, while wild-type PPV capsids were not (Fig. 4A). The DNA fragments amplified by IC-PCR corresponding to the CP N-terminal domain of the chimeras showed the expected increased size compared to the wild-type one, suggesting that no deletions were taking place in the sequence inserted in the chimeric viruses (Fig. 4B). This conclusion was confirmed by nucleotide sequencing of the IC-PCR-amplified products.
FIG. 4.
Characterization of chimeric PPV of the g series. (A) Western blot analysis with the anti-PPV mixture of monoclonal antibodies Ingezym (Ingenasa) (lanes 1 to 6) and anti-CPV MAb 3C9 (lanes 7 to 12) of plants infected with PPV-g1-CPV (lanes 1 and 7), PPV-g2-CPV (lanes 2 and 8), PPV-γ-CPV (lanes 3 and 9), PPV-g3-CPV (lanes 4 and 10), PPV-g4-CPV (lanes 5 and 11), and wild-type PPV (lanes 6 and 12). Prestained molecular weight markers (Bio-Rad) were run in lanes M. (B) IC-PCR products (nt 8390 to 8900 of the PPV genome [23]) amplified from a healthy plant (lane 1) and from plants infected with PPV-g1-CPV (lane 3), PPV-g2-CPV (lane 4), PPV-γ-CPV (lane 5), PPV-g3-CPV (lane 6), PPV-g4-CPV (lane 7), and wild-type PPV (lane 8). HindIII restriction fragments of phage φ29 DNA were used as size markers (lane 2).
BALB/c mice were immunized with the four types of chimeric virions presenting the foreign peptide around the γ region (PPV-g1-CPV, g2-CPV, g3-CPV, and g4-CPV). As negative controls, mice were inoculated with wild-type PPV and with PPV-γ-CPV virions.
The anti-PPV, anti-CPV peptide, and anti-VP2 ELISA titers of the sera of the immunized mice are shown in Table 1. All chimeric viruses, as well as wild-type PPV, developed high antibody titers against PPV, indicating an efficient immunization of the animals. The mice immunized with the four new chimeras, but not those immunized with PPV-γ-CPV or wild-type PPV, were able to develop an efficient CPV-specific antibody response. Antibody titers were quite similar for all chimeras, with the best results being obtained for mice immunized with PPV-g4-CPV.
The neutralizing titers were in partial agreement with the ELISA results. The four PPV-gx-CPV chimeras induced detectable levels of CPV-neutralizing antibodies (Table 1). Interestingly, mice immunized with PPV-g2-CPV, which had a lower antibody response than those immunized with PPV-g4-CPV, showed a slightly more efficient neutralizing activity.
Characterization of vectors expressing foreign peptides within the highly immunogenic region spanning aa 43 to 52 of PPV CP.
We were interested in addressing whether the highly immunogenic region spanning aa 43 to 52 of PPV CP was tolerant to modifications and whether chimeras derived from insertions in that region could be competent as immunogens. Two clones, pICPPV-i1 and pICPPV-i2, which hold insertions of 6 nt that represent MluI restriction sites between sequences coding for aa 45 to 46 and 49 to 50 of PPV CP, respectively (Fig. 1), were constructed. PPV-i1 and PPV-i2 were able to establish systemic infections in N. clevelandii plants with time courses and symptomatologies similar to those of wild-type infections. Chimeras expressing the 6L15 CPV peptidic sequence in these two vectors, PPV-i1-CPV and PPV-i2-CPV, were constructed. These chimeras were viable and caused infections of N. clevelandii plants also similar to wild-type infections.
Western blot analysis showed that PPV-i1-CPV and PPV-i2-CPV CPs had a slightly reduced mobility compared to that of wild-type CP due to the insertion they hold (Fig. 5). A specific polyclonal antibody against CPV recognized chimeric capsids (Fig. 5). Nucleotide sequencing of IC-PCR-amplified viral cDNA confirmed the stable retention of the foreign inserts in the chimeric viruses (not shown).
FIG. 5.
Characterization of chimeric PPV-i1-CPV and PPV-i2-CPV. Western blot analysis with an anti-PPV polyclonal antibody (lanes 1 to 3) and an anti-CPV polyclonal antibody (lanes 4 to 6) of samples from purified wild-type PPV (lanes 1 and 4), PPV-i1-CPV (lanes 2 and 5) and PPV-i2-CPV (lanes 3 and 6) virions. The sizes (in kilodaltons) of prestained molecular weight markers (Bio-Rad) run in the same gel are indicated beside the panel.
To evaluate the immunogenic properties of the chimeric PPV-i1-CPV and PPV-i2-CPV particles, BALB/c mice were immunized with purified virions. As a negative control, a group of animals was immunized with wild-type PPV. The antibody titers obtained in the immunization experiment are shown in Table 3. All immunized animals developed a high antibody response against PPV, indicating an efficient mice immunization. The specific antibody response against CPV in animals immunized with either chimera was remarkable. The best results were obtained with PPV-i2-CPV. This induced the highest anti-CPV response, despite the fact that the anti-PPV response was slightly weaker than that of the other two groups of animals (PPV-i1-CPV and wild-type PPV). Antibodies induced by both chimeras, PPV-i1-CPV and PPV-i2-CPV, had CPV-neutralizing activity. Also, at the neutralization level, the response against PPV-i2-CPV appeared to be somewhat stronger than that against PPV-i1-CPV.
TABLE 3.
Antibody response to chimeric PPV particles in mice
| Antigen | ELISA titera
|
Neutralization titerb | ||
|---|---|---|---|---|
| 6L15 peptide | CPV | PPV | ||
| PPV-i1CPV | 4 | 2.8 | 5.8 | 2.5 |
| PPV-i2CPV | 4.6 | 3.4 | 5.5 | 3.1 |
| PPV | —c | — | 5.8 | — |
Expressed as log of the serum dilution that gave absorption values three times the blank value (preimmune serum).
Expressed as log of the highest serum dilution that causes a 50% reduction of the cell monolayer.
—, below detection threshold.
DISCUSSION
The usefulness of viral capsids as carriers for small antigenic peptides has been illustrated for several plant viruses (for a review, see reference 22), and vaccines based on chimeric virions have been shown to be highly effective (9, 25). However, the design of these capsid-based epitope presentation systems is still rather empirical. PPV-NATMluI, a PPV-derived antigen presentation system previously developed in our laboratory (13), expresses foreign peptides as a substitution of the 15-aa sequence lost in NAT naturally appearing PPV mutants (27, 29). Different forms of the 6L15 CPV peptide cloned in this vector were shown to be highly immunogenic (13). However, some restrictions have been found for the expression of some other foreign peptides at the NAT site (Fernández-Fernández and García, unpublished).
We started to search for new appropriate cloning sites within the N-terminal region of PPV CP not only in order to overcome the cloning restrictions of the NAT site, but also in an attempt to make available several insertion sites to allow us to develop multivalent antigen presentation systems. The first three new PPV-based vectors that we designed, PPV-α, PPV-γ, and PPV-δ, were not fully satisfactory. PPV-α itself had infectivity restrictions that prevented it from being a good expression vector. γ and δ sites appeared to be more tolerant to insertions than the NAT site, and chimeras derived from PPV-γ and PPV-δ proved to be good antigens that could be useful for diagnostic purposes as shown for PPV-γ-FIV (Fig. 2). However, chimeras PPV-γ-CPV and PPV-δ-CPV failed to develop efficient specific antibody responses against foreign peptides. This may suggest that γ and δ sites are in hidden locations and unable to trigger an immunogenic response. As demonstrated in the development of expression vectors based on other plant viruses (31), the availability of three-dimensional data on the virion structure facilitates the identification of potentially immunogenic surface-exposed CP regions. The lack of this kind of data for any potyviral CP precluded the use of this approach for the development of PPV vectors. Thus, a PEPSCAN analysis was performed to identify, in a direct way, preferential immunogenic regions within PPV CP (Fig. 3 and Table 2). The results confirm that the C-terminal domain, and especially the N-terminal domain of PPV CP, are immunodominant regions of the protein. However, we were able to localize highly immunogenic regions within the core domain of CP, which were thought not to be exposed on the virion surface (36).
The N-terminal domain of CP is encoded by the most variable region, both in size and sequence, of the potyviral genome and has been described as surface exposed and highly immunogenic (36). Our PEPSCAN analysis shows that, in spite of its immunodominance, the N-terminal domain is not uniformly immunogenic. It includes regions that are preferentially recognized by sera. For instance, sequences around the NAT site are well recognized by anti-PPV antibodies, in concordance with the high immunogenicity of foreign inserts expressed with the PPV-NATMluI vector (13). However, these immunogenic regions alternate with others that are not recognized at all by the sera and consequently are probably not accessible from outside the virion. The N-terminal domain of PPV CP is exceptionally long, and we do not know if our results could be extrapolated to other potyvirus CPs with shorter N-terminal domains.
The PEPSCAN data suggest a possible explanation for the failure of PPV-γ-CPV and PPV-δ-CPV to induce the production of antibodies against the heterologous peptide. The γ and δ insertion sites are located in positions not specially recognized by the sera, but in the proximity of highly immunogenic regions. The γ site appears to be in a depression of immunogenicity, just in the hinge between two highly immunogenic regions. It is therefore not surprising that displacements of only 1 or 2 aa in the insertion sites could cause the shift from the null immunogenicity of foreign sequences cloned in the γ site to the efficient induction of antibodies of sequences cloned in any of the g sites (Table 1). A precedent of minor displacements in the insertion site causing drastic changes in the immunogenicity of the inserted sequences has been previously reported for epitope expression on the surface of chimeric parvovirus (35) and cowpea mosaic virus (39) particles. These results illustrate the utility of structure and immunogenicity predictions and fine-tuning experimental approaches in the development of antigen presentation systems based on virion capsids.
The PEPSCAN analysis showed that, in general, mice rendered poorer immunogenic responses than did rabbits and that some immunogenic regions were recognized exclusively by rabbit sera. However, other regions were recognized by all or almost all sera. The region located around aa 43 to 52 appears to be especially highly immunogenic (Fig. 3). Two positions within this region were selected as insertion sites for new PPV-based expression vectors, and foreign sequences cloned in these sites were shown to be able to mount a relevant and very efficient specific immune response, which, in the case of PPV-i2-CPV has rather improved the best results obtained with chimeras derived from the PPV-NATMluI vector (Table 3) (13). These results further validate the relevance of PEPSCAN analysis in the accurate definition of peptidic immunogenic regions, although it can identify only linear and not conformational epitopes.
It would be interesting to assess the level of tolerance of these successful vectors for the expression of a variety of peptides, as well as to check the feasibility of concurrent expression of different peptides by using several insertion points at the N-terminal domain of PPV CP.
Acknowledgments
We thank Juan Pablo Albar and Ignacio Casal for helpful advice regarding the PEPSCAN analysis and the immunogenicity assays, respectively.
This work was supported by grants BIO2001-1434 from CICYT and QLK2-2000-00739 and QLK2-CT-2002-01050 from the European Union.
REFERENCES
- 1.Allison, R. F., W. G. Dougherty, T. D. Parks, J. Willis, R. E. Johnston, M. E. Kelly, and F. B. Armstrong. 1985. Biochemical analysis of the capsid protein gene and capsid protein of tobacco etch virus: N-terminal amino acids are located on the virion's surface. Virology 147:309-316. [DOI] [PubMed] [Google Scholar]
- 2.Arntzen, C. J. 1997. High-tech herbal medicine: plant-based vaccines. Nat. Biotechnol. 15:221-222. [DOI] [PubMed] [Google Scholar]
- 3.Atherton, E., and R. D. Sheppard. 1987. Peptides, vol. 9, p. 1-38. Academic Press, New York, N.Y. [Google Scholar]
- 4.Atreya, C. D., B. Raccah, and T. D. Pirone. 1990. A point mutation in the coat protein abolishes aphid transmissibility of a potyvirus. Virology 178:161-165. [DOI] [PubMed] [Google Scholar]
- 5.Atreya, P. L., C. D. Atreya, and T. P. Pirone. 1991. Amino acid substitutions in the coat protein results in loss of insect transmissibility of a plant virus. Proc. Natl. Acad. Sci. USA 88:7887-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avrameas, A., A. D. Strosberg, A. Moraillon, P. Sonigo, and G. Pancino. 1993. Serological diagnosis of feline immunodeficiency virus infection based on synthetic peptides from Env glycoproteins. Res. Virol. 144:209-218. [DOI] [PubMed] [Google Scholar]
- 7.Candresse, T., G. Macquaire, M. Lanneau, M. Bousalem, L. Quiot-Douine, J. B. Quiot, and J. Dunez. 1995. Analysis of plum pox virus variability and development of strain-specific PCR assays. Acta Hortic. 386:357-369. [Google Scholar]
- 8.Casal, J. I., J. P. M. Langeveld, E. Cortés, W. W. M. Schaaper, E. van Dijk, C. Vela, S. Kamstrup, and R. H. Meloen. 1995. Peptide vaccine against canine parvovirus: identification of two neutralization subsites in the N terminus of VP2 and optimization of the amino acid sequence. J. Virol. 69:7274-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalsgaard, K., A. Uttenthal, T. D. Jones, F. Xu, A. Merryweather, W. D. O. Hamilton, J. P. M. Langeveld, R. S. Boshuizen, S. Kamstrup, G. P. Lomonossoff, C. Porta, C. Vela, J. I. Casal, R. H. Meloen, and P. B. Rodgers. 1997. Plant-derived vaccine protects target animals against a viral disease. Nat. Biotechnol. 15:248-252. [DOI] [PubMed] [Google Scholar]
- 10.Delpeyroux, F., N. Chenciner, A. Lim, Y. Malpiece, B. Blondel, R. Crainic, S. van der Werf, and R. E. Streeck. 1986. A poliovirus neutralization epitope expressed on hybrid hepatitis B surface antigen particles. Science 233:472-475. [DOI] [PubMed] [Google Scholar]
- 11.Dolja, V. V., R. Haldeman, N. L. Robertson, W. G. Dougherty, and J. C. Carrington. 1994. Distinct functions of capsid protein in assembly and movement of tobacco etch potyvirus in plants. EMBO J. 13:1482-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolja, V. V., R. Haldeman-Cahill, A. E. Montgomery, K. A. Vandenbosch, and J. C. Carrington. 1995. Capsid protein determinants involved in cell-to-cell and long distance movement of tobacco etch potyvirus. Virology 206:1007-1016. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Fernández, M. R., J. L. Martínez-Torrecuadrada, J. I. Casal, and J. A. García. 1998. Development of an antigen presentation system based on plum pox potyvirus. FEBS Lett. 427:229-235. [DOI] [PubMed] [Google Scholar]
- 14.Fitchen, J., R. N. Beachy, and M. B. Hein. 1995. Plant virus expressing hybrid coat protein with added murine epitope elicits autoantibody response. Vaccine 13:1051-1057. [DOI] [PubMed] [Google Scholar]
- 15.Frank, R. 1992. Spot-synthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron 48:9217-9232. [Google Scholar]
- 16.Frank, R., and H. Overwin. 1996. SPOT synthesis. Epitope analysis with arrays of synthetic peptides prepared on cellulose membranes. Methods Mol. Biol. 66:149-169. [DOI] [PubMed] [Google Scholar]
- 17.García, J. A., M. T. Martín, M. T. Cervera, and J. L. Riechmann. 1992. Proteolytic processing of the plum pox potyvirus polyprotein by the NIa protease at a novel cleavage site. Virology 188:697-703. [DOI] [PubMed] [Google Scholar]
- 18.Haynes, J. R., J. Cunningham, A. von Seefried, M. Lennick, R. T. Garvin, and S.-H. Shen. 1986. Development of a genetically-engineered, candidate polio vaccine employing the self-assembling properties of the tobacco mosaic virus coat protein. Biotechnology 4:637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herlitze, S., and M. Koenen. 1990. A general and rapid mutagenesis method using polymerase chain reaction. Gene 91:143-147. [DOI] [PubMed] [Google Scholar]
- 20.Joelson, T., L. Akerblom, P. Oxelfelt, B. Strandberg, K. Tomenius, and T. J. Morris. 1997. Presentation of a foreign peptide on the surface of tomato bushy stunt virus. J. Gen. Virol. 78:1213-1217. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, J., T. Lin, and G. Lomonossoff. 1997. Presentation of heterologous peptides on plant viruses: genetics, structure, and function. Annu. Rev. Phytopathol. 35:67-86. [DOI] [PubMed] [Google Scholar]
- 22.Koprowski, H., and V. Yusibov. 2001. The green revolution: plants as heterologous expression vectors. Vaccine 19:2735-2741. [DOI] [PubMed] [Google Scholar]
- 23.Laín, S., J. L. Riechmann, and J. A. García. 1989. The complete nucleotide sequence of plum pox potyvirus RNA. Virus Res. 13:157-172. [DOI] [PubMed] [Google Scholar]
- 24.Laín, S., J. L. Riechmann, E. Méndez, and J. A. García. 1988. Nucleotide sequence of the 3′ terminal region of plum pox potyvirus RNA. Virus Res. 10:325-342. [DOI] [PubMed] [Google Scholar]
- 25.Langeveld, J. P., F. R. Brennan, J. L. Martinez-Torrecuadrada, T. D. Jones, R. S. Boshuizen, C. Vela, J. I. Casal, S. Kamstrup, K. Dalsgaard, R. H. Meloen, M. M. Bendig, and W. D. Hamilton. 2001. Inactivated recombinant plant virus protects dogs from a lethal challenge with canine parvovirus. Vaccine 19:3661-3670. [DOI] [PubMed] [Google Scholar]
- 26.López de Turiso, J. A., E. Cortes, A. Ranz, J. Garcia, A. Sanz, C. Vela, and J. I. Casal. 1991. Fine mapping of canine parvovirus B cell epitopes. J. Gen. Virol. 72:2445-2456. [DOI] [PubMed] [Google Scholar]
- 27.López-Moya, J. J., T. Canto, J. R. Díaz-Ruíz, and D. López-Abella. 1995. Transmission by aphids of a naturally non-transmissible plum pox virus isolate with the aid of potato virus Y helper component. J. Gen. Virol. 76:2293-2297. [DOI] [PubMed] [Google Scholar]
- 28.López-Moya, J. J., and J. A. García. 2000. Construction of a stable and highly infectious intron-containing cDNA clone of plum pox potyvirus, and its use to infect plants by particle bombardment. Virus Res. 68:99-107. [DOI] [PubMed] [Google Scholar]
- 29.Maiss, E., U. Timpe, A. Briske-Rode, D.-E. Leseman, and R. Casper. 1992. Infectious in vivo transcripts of a plum pox potyvirus full-length cDNA clone containing the cauliflower mosaic virus 35S RNA promoter. J. Gen. Virol. 73:709-713. [DOI] [PubMed] [Google Scholar]
- 30.Marusic, C., P. Rizza, L. Lattanzi, C. Mancini, M. Spada, F. Belardelli, E. Benvenuto, and I. Capone. 2001. Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J. Virol. 75:8434-8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porta, C., V. E. Spall, T. Lin, J. E. Johnson, and G. P. Lomonossoff. 1996. The development of cowpea mosaic virus as a potential source of novel vaccines. Intervirology 39:79-84. [DOI] [PubMed] [Google Scholar]
- 32.Revers, F., O. Le Gall, T. Candresse, and A. Maule. 1999. New advances in understanding the molecular biology of plant/potyvirus interactions. Mol. Plant-Microbe Interact. 12:366-376. [Google Scholar]
- 33.Riechmann, J. L., S. Laín, and J. A. García. 1992. Highlights and prospects of potyvirus molecular biology. J. Gen. Virol. 73:1-16. [DOI] [PubMed] [Google Scholar]
- 34.Riechmann, J. L., S. Laín, and J. A. García. 1990. Infectious in vitro transcripts from a plum pox potyvirus cDNA clone. Virology 177:710-716. [DOI] [PubMed] [Google Scholar]
- 35.Rueda, P., A. Hurtado, M. del Barrio, J. L. Martinez-Torrecuadrada, S. Kamstrup, C. Leclerc, and J. I. Casal. 1999. Minor displacements in the insertion site provoke major differences in the induction of antibody responses by chimeric parvovirus-like particles. Virology 263:89-99. [DOI] [PubMed] [Google Scholar]
- 36.Shukla, D. D., P. M. Strike, S. L. Tracy, K. H. Gough, and C. W. Ward. 1988. The N and C termini of the coat proteins of potyviruses are surface-located and the N-terminus contains the major virus-specific epitopes. J. Gen. Virol. 69:1497-1508. [Google Scholar]
- 37.Shukla, D. D., C. W. Ward, and A. A. Brunt. 1994. The Potyviridae. CAB International, Wallingford, United Kingdom.
- 38.Sugiyama, Y., H. Hamamato, S. Takemoto, Y. Watanabe, and Y. Okada. 1995. Systemic production of foreign peptides on the particle surface of tobacco mosaic virus. FEBS Lett. 359:247-250. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, K. M., T. Lin, C. Porta, A. G. Mosser, H. A. Giesing, G. P. Lomonossoff, and J. E. Johnson. 2000. Influence of three-dimensional structure on the immunogenicity of a peptide expressed on the surface of a plant virus. J. Mol. Recognit. 13:71-82. [DOI] [PubMed] [Google Scholar]
- 40.Turpen, T. H., S. J. Reinl, Y. Charoenvit, S. L. Hoffman, V. Fallarme, and L. K. Grill. 1995. Malarial epitopes expressed on the surface of recombinant tobacco mosaic virus. Biotechnology 13:53-57. [DOI] [PubMed] [Google Scholar]
- 41.Urcuqui-Inchima, S., A. L. Haenni, and F. Bernardi. 2001. Potyvirus proteins: a wealth of functions. Virus Res. 74:157-175. [DOI] [PubMed] [Google Scholar]
- 42.Usha, R., J. B. Rohll, V. E. Spall, M. Shanks, A. J. Maule, J. E. Johnson, and G. P. Lomonossoff. 1993. Expression of an animal antigenic site on the surface of a plant virus particle. Virology 197:366-374. [DOI] [PubMed] [Google Scholar]
- 43.Yusibov, V., A. Modelska, K. Steplewski, M. Agadjanyan, D. Weiner, D. C. Hooper, and H. Koprowski. 1997. Antigens produced in plants by infection with chimeric plant viruses immunize against rabies virus and HIV-1. Proc. Natl. Acad. Sci. USA 94:5784-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]