Abstract
Little is known about the requirements for human T-cell leukemia virus type 1 (HTLV-1) entry, including the identity of the cellular receptor(s). Previous studies have shown that although the HTLV receptor(s) are widely expressed on cell lines of various cell types from different species, cell lines differ dramatically in their susceptibility to HTLV-Env-mediated fusion. Human cells (293, HeLa, and primary CD4+ T cells) showed higher levels of binding at saturation than rodent (NIH 3T3 and NRK) cells to an HTLV-1 SU immunoadhesin. A direct comparison of the binding of the HTLV-1 surface glycoprotein (SU) immunoadhesin and transduction by HTLV-1 pseudotyped virus revealed parallels between the level of binding and the titer for various cell lines. When cells were treated with phorbol myristate acetate (PMA), which down-modulates a number of cell surface molecules, the level of SU binding was markedly reduced. However, PMA treatment only slightly reduced the titer of murine leukemia virus(HTLV-1) on both highly susceptible and poorly susceptible cells. Treatment of target cells with trypsin greatly reduced binding, indicating that the majority of HTLV SU binding is to proteins. Polycations, which enhance the infectivity of several other retroviruses, inhibited HTLV-1 Env-mediated binding and entry on both human and rodent cells. These results suggest that factors other than the number of primary binding receptors are responsible for the differences in the titers of HTLV-1 pseudotypes between highly susceptible cells and poorly susceptible cells.
Human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus which is the etiological agent of a severe lymphocyte neoplasia called adult T-cell leukemia/lymphoma (ATL) (53, 77) and a progressive neurological disease known as HTLV-1-associated myelopathy/tropical spastic paraparesis (19, 48). The virus is endemic in southern Japan, the Caribbean basin, Central and South America, and portions of West Africa. HTLV-1 and the closely related human T-cell leukemia virus type 2 (HTLV-2) are uncommon in the general populations of the United States and Europe. However, one recent study revealed that HTLV is prevalent in the United States among paid blood donors, African-American health care clinic patients, Amerindians, intravenous drug users, and patients with other-than-low-grade non-Hodgkin's lymphoma (52).
ATL is a malignancy of CD4+ T cells. It has been generally believed that the majority of the cells infected by HTLV-1 in vivo are CD4+ T cells (30, 54). However, HTLV-1 can infect all subsets of human lymphocytes in vitro, and recent studies indicate that both CD4+ and CD8+ T cells serve as viral reservoirs in HTLV-1-associated myelopathy/tropical spastic paraparesis patients (42). Although capable of infecting a number of different cell types, HTLV-1 is poorly infectious in both primary cells and established cell lines in vitro.
As for all retroviruses, entry of HTLV-1 into target cells is mediated by the envelope glycoproteins (Env), a surface glycoprotein (SU), and a transmembrane glycoprotein (TM). The HTLV-1 Env proteins are initially synthesized as precursor proteins, which are subsequently glycosylated and cleaved in the Golgi apparatus by a furin-like cellular protease to yield the SU (gp46) and the TM (gp21) glycoproteins. Following cleavage, the SU and the TM remain associated with each other through noncovalent interactions (51). As for other retroviruses, it is believed that the HTLV-1 SU glycoprotein specifically binds to a cellular receptor, inducing a conformational change in the SU-TM complex. This change activates a fusion domain within TM, allowing fusion of the viral and cellular membranes (5, 9, 10, 37, 51, 55, 56). Recent work using HTLV/murine leukemia virus (MLV) envelope chimeras strongly suggests that the region of SU that interacts with the receptor is located within the N-terminal two-thirds of the protein (29). For HTLV-1, both SU and TM appear to play an additional role in a postfusion event critical for infectivity (11, 28).
The cellular receptor(s) for HTLV-1 have not yet been identified. Based on results from receptor interference assays, HTLV-1 is believed to share a common receptor with HTLV-2 and other primate T-cell leukemia/lymphoma viruses (64, 55). The gene encoding the receptor was mapped to chromosome 17 and further localized to 17q23.2-25.3 (18, 35, 55), although later studies have questioned this assignment (27, 47, 67).
A number of different candidates for the HTLV receptor have been proposed (reviewed in reference 63). Monoclonal antibody 23-34, directed against an antigen that maps to chromosome 17, has been shown to block HTLV-1 entry (17, 18). For the majority of the studies, receptor candidates were identified by screening for antibodies that block HTLV-1 syncytium formation. However, recent studies have shown that monoclonal antibodies directed against proteins highly expressed on the cell surface (e.g., major histocompatibility complex class II) can inhibit HTLV-1-induced syncytium formation (24, 44). These observations raised the possibility that the ability of various antibodies previously shown to prevent syncytium formation reflects steric hindrance rather than a direct block of Env-receptor binding (24). In addition, none of the studies have isolated a relevant cDNA that codes for a protein that demonstrates receptor function.
Early studies reported that HTLV-1 pseudotypes were unable to transduce many cell lines, including many of rodent origin, suggesting that these cells lack a functional receptor (65, 74, 75). However, more recent studies using highly sensitive cell fusion assays and high-titer retroviral vector systems indicate that the range of cells that express the HTLV-1 receptor is greater than originally believed (35, 47, 67, 73). These studies reveal that a number of cell lines previously believed to lack the receptor allow HTLV-1 Env-mediated fusion and viral entry, although at lower levels than the most susceptible cell lines. From these studies, it is not clear whether the same cell surface molecule(s) are involved in HTLV-1 Env-mediated fusion and transduction in highly and poorly susceptible cell lines. For example, it has been suggested that the very low titers of HTLV-1 pseudotypes on certain cell lines might reflect nonspecific interactions between the high-titer virus and target cells (63). Using an HTLV-1 SU immunoadhesin, we observed that human cells (293, HeLa, and primary CD4+ T cells) showed higher levels of binding at saturation than rodent (NIH 3T3 and NRK) cells.
To more directly evaluate the cell surface expression of molecule(s) critical for receptor-mediated fusion and entry, we compared the cell surface expression levels of HTLV SU binding proteins to the susceptibilities of these cell lines to HTLV-1 pseudotyped virions. These comparisons revealed a parallel between the level of binding and the titer on various cell lines. To better understand HTLV-1 viral entry, additional studies were performed following exposure of cells to substances previously shown to alter Env-mediated binding and entry of other retroviruses. Treatment of cells with the phorbol ester phorbol myristate acetate (PMA) markedly reduced the level of SU binding. However, PMA treatment only slightly reduced the titer of HTLV-1 pseudotyped virus on highly susceptible cells, suggesting that factors other than the number of primary binding receptors are responsible for the differences between highly and poorly susceptible cell lines.
MATERIALS AND METHODS
Cells and cell culture.
293, HeLa, NIH 3T3, NRK-49F, MDBK, MOLT-4, and Jurkat cells were obtained from American Type Culture Collection (Rockville, Md.). 293-T/17 is a highly transfectable subclone of a 293 line transformed with the simian virus 40 large T antigen (49). B5, a subclone of a fetal rhesus lung cell line, is highly permissive for HTLV-1 infection (12). MT-2 is an HTLV-1 producer cell line derived from cord blood cells (41). 729pH 6neo, an HTLV-2 producer cell line, was a gift from Patrick Green (Ohio State University Medical Center).
MDBK cells were maintained in Eagle's minimum essential medium (MEM) with 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, and 10% horse serum. 729pH 6neo cells were maintained in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum (FCS). The suspension cell lines MOLT-4, Jurkat, and MT-2 were maintained in RPMI 1640 supplemented with 10% FCS. All the other lines were maintained in Dulbecco's modified Eagle's medium MEM (DMEM) supplemented with 10% FCS.
CD4+ T lymphocytes were isolated from leukopaks of peripheral blood collected from adult human immunodeficiency virus type 1 (HIV-1)- and HTLV-1-negative healthy donors by the National Institutes of Health transfusion branch (Bethesda, Md.) according to the NIH-approved Institutional Review Board protocols. peripheral blood mononuclear cells were isolated by Ficoll-Hypaque gradient centrifugation. The light density fraction was collected and washed twice with phosphate-buffered saline (PBS). For enrichment of CD4+ cells, the CD8+ T cells and myeloid cells were removed as follows: 107 cells were resuspended in 760 μl of cold PBS-2% bovine serum albumin (BSA)-EDTA, and 20 μl each of anti-human CD8- and anti-human CD11c-labeled magnetic microbeads (Miltenyi Biotec Inc., Auburn, Ca.). After incubation for 15 min on ice, the cells were washed once in cold PBS-2% BSA-0.01% sodium azide buffer and then passed over a magnetically bound VS+ column (Miltenyi Biotec Inc.). The flow-through, containing CD4+ T cells enriched to >95%, was collected. After being washed in PBS, the cells were stimulated by culturing at 106/ml in RPMI 1640 medium containing 10% FCS and phytohemagglutin (PHA) (2 μg/ml; Abbott Diagnostics, Abbott Park, Ill.). Three days later, recombinant interleukin 2 (IL-2) (50 U/ml; Zeptometrix, Buffalo, N.Y.) was added to these cultures. The cells were then cultured for an additional 4 to 7 days prior to their use in the assays for binding. All cells were cultured in a humidified atmosphere at 37°C in an incubator containing 5% CO2.
Construction of plasmid encoding the HTLV-1 SU region fused to immunoglobulin G (HTSU-IgG).
A DNA fragment encoding the HTLV-1 SU region was generated by PCR amplification, using the plasmid CMV-ENV-LTR, (11) which contains the 3′ end of the HTLV-1 genome (beginning at nucleotide 5012 of the sequence with GenBank accession number J02029) as template DNA (59). PCR amplification was performed using the oligonucleotide primers 5′-CGCTAGCTAGCACCATGGGTAAGTTTCTCGCCACTTTG-3′ and 5′-CTAGTTCTAGATCGGCGGGAGCGGGATCCTAG-3′. The resultant DNA fragment was cloned into the pCR2.1-Topo vector (Invitrogen, Carlsbad, Calif.). This vector was digested with NheI and XbaI to generate a fragment that contains the entire portion of the genome encoding the HTLV-1 SU protein (from three nucleotides before the start of SU to the position of the SU/TM cleavage; 5199 to 6138), and this fragment was then ligated to the plasmid pSK100 (gift of John Young, University of Wisconsin, Madison, Wis.), which had been digested with NheI and XbaI. The resultant plasmid, HTSU-IgG/pSK100, encodes a fusion protein containing the entire SU region of HTLV-1 (amino acids 1 to 313) fused in frame to the Fc region of a rabbit immunoglobulin heavy chain (amino acid residues 96 to 323). The authenticity of this open reading frame was confirmed by DNA sequence analysis using an ABI model 373 automated sequencer.
HTSU-IgG binding studies.
To generate the HTSU-IgG fusion protein, 293 cells were plated on a 100-mm-diameter plate and transfected 24 h later using FuGene 6 (Roche Molecular Biochemicals; Indianapolis, Ind.) and 8 μg of either the plasmid HTSU-IgG/pSK100 or a control plasmid encoding an immunoadhesin containing the SU protein from the avian retrovirus ALSV-A (SUA-rIgG) (78). The transfected cells were refed with OptiMEM (Invitrogen) containing 1% FCS 20 h prior to harvesting. Following harvesting, the cell culture supernatant was frozen at −80°C and the concentration of immunoadhesins was determined by enzyme-linked immunosorbent assay (ELISA), as described below. To examine the specific binding of HTSU-IgG, 106 target cells, resuspended in 0.3 ml of PBS-2% FCS-0.02% sodium azide, were incubated with supernatant (containing the appropriate amount of HTSU-IgG or, as a negative control, SUA-IgG) and medium to a final volume of 200 μl at room temperature for 30 min. The cells were then washed once with ice-cold PBS-1% FCS, resuspended in 0.5 ml of PBS-1% FCS and incubated for 30 min on ice with a 1:25 dilution of fluorescein isothiocyanate (FITC)-conjugated antibody specific for rabbit immunoglobulins (Sigma, St. Louis, Mo.). The cells were then washed once and resuspended in 400 μl of ice-cold PBS containing 4% paraformaldehyde. For experiments involving large numbers of samples and kinetic studies, cells were fixed in 4% paraformaldehyde for 30 min on ice prior to exposure to the immunoadhesin, and the binding to the immunoadhesins was carried out on ice rather than at room temperature. Kinetic experiments determined that HTSU-IgG binding stabilized at 30 min at 4°C, that binding of the control immunoadhesin SUA-IgG increased after 1 h, and that the FITC-conjugated secondary antibody binding stabilized at 20 min at 4°C. Therefore, studies measuring immunoadhesin binding at different concentrations were carried out at 4°C for 30 min for both immunoadhesin and secondary antibody.
For some experiments, 10,000 live cell events were measured on a Coulter EPICS XL-MCL flow cytometer and analyzed using Coulter System II software version 3.0. For other experiments, 10,000 live cell events were measured on a FACScan (BD PharMingen, San Diego, Calif.) and analyzed using Flowjo software (Treestar, Aurora Calif.). All experiments were performed a minimum of three times. The human monoclonal antibodies directed against the neutralizing epitopes of HTLV-1 SU used in the blocking studies (PRH-4, PRH-7A, and PRH-11A), and the isotype control R0-4, generous gifts of Ken Hadlock and Steve Foung, have been previously described (20).
Quantification of immunoadhesins by ELISA.
Paired antibodies to measure rabbit IgG-Fc and rabbit IgG (rIgG) were obtained from Research Diagnostics (Flanders, N.J.). Microtiter wells (Nunc) were coated overnight at 20°C with 100 μl of purified goat anti-rabbit IgG-Fc antibodies per well diluted 1:500 in 0.05 M sodium carbonate, pH 9.6. After the wells were washed three times with Tris-buffered saline (TBS)-Tween (50 mM TBS-0.05% Tween 20), samples (either undiluted or serially diluted fivefold) were added to wells (200 μl/well) and incubated at 20°C. Standards (rabbit IgG) were diluted in PBS-Tween to 10 ηg/ml and then serially diluted twofold down to 0.02 ηg/ml. After 2 h, the wells were washed with TBS-Tween and blocked by incubation in TBS-1% BSA for 10 min at 20°C. After three further washes, the wells were incubated for 2 h at 20°C with 100 μl of HRP-conjugated goat anti-rabbit IgG-Fc/well diluted 1:18,750 in PBS-Tween. After three washes, each well received 100 μl of a freshly prepared 3,3′,5,5′-tetramethylbenzidine solution (TMB microwell peroxidase substrate system; K&P Labs, Gaithersburg, Md.). The reaction was stopped after 15 min with 100 μl of 1.0 M H3PO4 per well, and the plates were examined at 450 nm. Absorbance values were corrected by using the mean value of the negative controls (reaction carried out in wells without antigen).
Generation of pseudotyped retroviral vectors.
MLV-based retroviral vectors were generated using three plasmids: (i) pCgp, an MLV gag-pol expression plasmid that generates the retroviral core; (ii) pCMMP.EGFP, which generates an MLV-based indicator provirus encoding the enhanced green fluorescent protein (EGFP), and (iii) one of several plasmids encoding envelope proteins, for pseudotyping the retroviral core. The plasmids pSCA (expressing the SU protein of amphotropic-MLV), pCG (expressing the G protein of VSV), and pCgp were gifts from P. Cannon (22). The pCMMP.EGFP plasmid was a gift from K. Bradley and J. A. T. Young (University of Wisconsin) (62). The HTLV-1 Env expressing the plasmid CMV-ENV-LTR and CMV-ENV-ΔPvuII, a control plasmid containing a stop codon inserted into the Env open reading frame, were gifts of M. C. Dokhelar (Institut Cochin de Genetique Moleculaire, Paris, France) (11).
To generate virus, 293-T cells were transfected with a total of 8 μg of DNA using FuGene 6, at a 0.2:1:1 ratio of Env:pCgp:pCMMP.EGFP (for CMV-ENV-LTR and CMV-ENV-ΔPvuII) or at a 1:1:1 ratio (for pSCA) as previously described (66). Twenty-four hours after transfection, the cells were refed. Forty-eight hours after transfection, viral supernatants were harvested, subjected to low-speed centrifugation, filtered through a 0.45-μm-pore-size filter, and used immediately.
Viral transduction.
Target cells were seeded in 6-well plates at 5 × 104 cells/well. Twenty-four hours later, medium was replaced with 1 ml of viral supernatant, an additional 0.5 ml of media was added to the wells, and cells were transduced using a modification of the spin infection method recently described for increasing the infectivity of HIV-1 (45). Cells were centrifuged in a Sorvall RT6000B using an H1000B rotor at 1,200 × g for 2 h at 25°C. The spin infection method increased the titer over standard transduction conditions 5- to 10-fold (data not shown). For experiments involving treatment (with trypsin, PMA, or polycations), cells were washed three times immediately after spinning and incubated at 37°C unless otherwise noted. For all other experiments, the cells were incubated at 37°C for 2 h and then washed twice in medium and incubated at 37°C. For determination of relative viral titer, target cells were incubated with 10-fold dilutions of viral supernatant. Negative control cultures (cells transduced with MLV vectors with no Env) were included in each experiment. Four days later, 50,000 live cells were analyzed by flow cytometry for the expression of EGFP to determine the percentage of transduced cells. Relative titers were determined from the well with the lowest percentage that was at least 5% positive using the following formula: (percent positive minus percent positive in negative control) × 105 (average number of cells in well on day of transduction). Titers were corrected to 1 ml. For all experiments, titers were determined in at least three independent experiments, and the standard error of the mean (SEM) was calculated.
Statistical analyses.
Pairwise comparisons were performed using the Tukey test to determine whether differences were significant at the 0.05 level. For the data in experiments comparing treated and untreated cells, all values were normalized to a mean control value and evaluated by analysis of variance (by F-test). Significance was evaluated using a 0.05 level.
RESULTS
The HTLV-1 SU immunoadhesin specifically binds to target cells of different species.
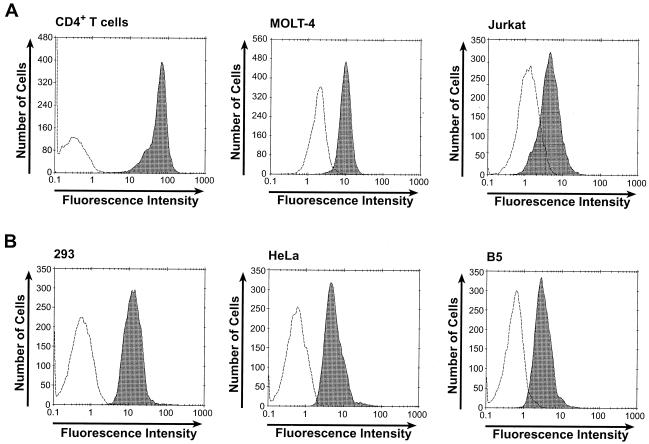
As a first step in examining whether the HTLV SU portion of the immunoadhesin binds to cellular molecules critical for entry, the ability of HTSU-IgG to bind to CD4+ T cells was examined. The generation of HTSU-IgG and its binding to target cells was carried out as described in Materials and Methods. The amount of bound immunoadhesin was determined by flow cytometry using FITC-labeled secondary antibody directed against the rabbit immunoglobulin chain. A dramatic increase in FITC-specific fluorescence was observed when CD4+ T cells were incubated with HTSU-IgG compared to results for cells incubated with a similar immunoadhesin containing the SU from an unrelated retrovirus (ALV subgroup A) (SUA-rIgG) (78) (Fig. 1A). Two CD4+ T-cell lines previously shown to be infectible by HTLV-1, MOLT-4 and Jurkat, were also positive for binding, as were adherent cell lines of primate origin previously shown to be highly susceptible to infection by HTLV-1 and/or transduction by HTLV pseudotyped vectors (Fig. 1).
FIG. 1.
HTSU-IgG binds to various cell types. (A) Binding of HTSU-IgG to primary CD4+ T cells and two T-cell lines. Primary CD4+ T cells were isolated and activated by PHA and IL-2 as described in Materials and Methods and assayed 4 days after the addition of IL-2. Primary CD4+ T cells, MOLT-4 cells, and Jurkat cells were incubated with 100 ηg of HTSU-IgG (shaded)/ml or, as a negative control, 500 ηg of SUA-rIgG (unshaded)/ml. The cells were then incubated with a FITC-conjugated antibody specific for rabbit IgG, and the amount of binding was determined by flow cytometry. (B) Binding of HTSU-IgG to non-T-cell primate cell lines. Binding assays were performed on 293, HeLa, and B5 cells as described for panel A.
Binding of HTSU-IgG to target cells is inhibited by neutralizing antibodies directed against HTLV-1 SU and by HTLV Env-expressing virions.
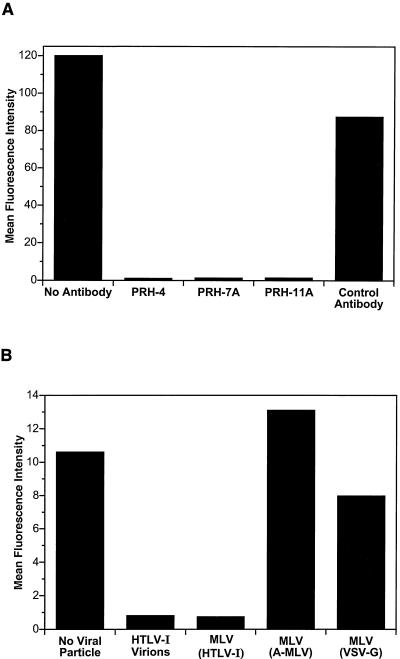
To further examine the specificity of the binding of the HTLV-1 SU immunoadhesin to target cells, HTSU-IgG was preincubated with monoclonal antibodies directed against neutralizing epitopes of SU (20, 21). When HTSU-IgG was incubated with PRH-4, PRH-7A, or PRH-11A, monoclonal antibodies that block HTLV-1-induced syncytium formation, the mean fluorescence intensity of binding to 293 cells was reduced to <0.5% of the positive control value (Fig. 2A). In contrast, preincubation of HTSU-IgG with R0-4, a human monoclonal antibody of the same isotype as the PRH antibodies directed against an unrelated cell surface antigen, had only a minor effect on the mean fluorescence intensity. Similar results were obtained when CD4+ T cells were used as targets (data not shown). These results indicate that the SU portion of the immunoadhesin is conformationally intact. These observations also strongly suggest that binding of HTSU-IgG to target cells involves specific interactions between SU and cell surface molecules critical for HTLV-1-Env-mediated fusion.
FIG. 2.
HTSU-IgG specifically binds to HTLV-1 SU binding protein(s). (A) Anti-HTLV-1 SU antibodies specifically inhibit HTSU-IgG binding. HTSU-IgG (500 ηg/ml) was incubated on ice with 10 μg/ml of one of the following human monoclonal antibodies to a final volume of 400 μl: PRH-4, PRH-7A, PRH-11A, or R0-4 (20, 21). The first three are directed against neutralizing epitopes of the HTLV-1 SU. The sample marked “Control Antibody” was incubated with R04, an isotype control directed against a 64-kDa protein of cytomegalovirus (17). The sample marked “No Antibody” was incubated on ice with no competing antibody. After 30 min, the immunoadhesin incubated with antibody was added to 5 × 105 293 cells in a final volume of 1 ml, and the binding studies were performed as described in Fig. 1. (B) Binding of HTSU-IgG is inhibited by viral particles containing HTLV-1 Env glycoproteins. MLV-based viral particles, pseudotyped with various envelopes, were generated as described in Materials and Methods, with the following modification: an MLV-based provirus that encodes β-galactosidase (pCnBg) was used in place of the plasmid pCMMP.EGFP. HeLa cells were incubated for 1 h on ice with cell-free viral particles, either pseudotyped MLV particles or HTLV-1 virions (from MT-2 cells). The amount of MLV particles was standardized by reverse transcriptase activity using a commercially available kit (C-type-RT activity assay; Cavidi Tech, Uppsala, Sweden); HTLV-1 virions were at a concentration of 120 ηg/ml, as determined using a p19 ELISA kit (Zeptometrix, Buffalo, N.Y.). After 1 h, HTSU-IgG was added to each sample, and the binding assays were performed using 100 ηg of HTSU-IgG/ml.
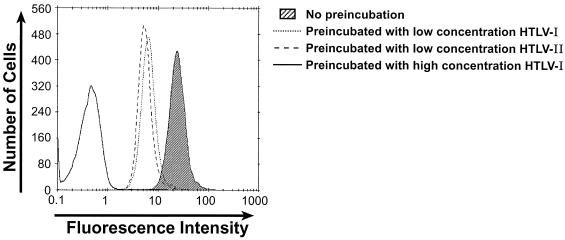
To explore further the hypothesis that binding of the HTLV-1 SU immunoadhesin is receptor mediated, studies were performed to demonstrate that HTLV-1 virions, as well as viral particles pseudotyped with HTLV-1 envelope proteins, would compete with HTSU-IgG for binding to target cells. HeLa cells were first incubated with viral particles pseudotyped with HTLV Env or with non-HTLV Env glycoproteins. After washing, the cells were incubated with HTSU-IgG, and flow cytometric analysis of binding was performed. Preincubation of the target cells either with HTLV-1 viral particles or with HTLV-pseudotyped viral particles reduced the mean fluorescence to <0.2% of that observed when the cells were incubated with HTSU-IgG alone (Fig. 2B). In contrast, preincubation with pseudotyped particles containing Env from amphotropic MLV or vesicular stomatitis virus G protein did not markedly reduce binding (Fig. 2B). In other studies, binding of the immunoadhesin was also significantly reduced following expression of the HTLV-1 Env glycoproteins in the target cells, either by infection with HTLV-1 virus or by transfection with an expression vector (data not shown). On the basis of receptor interference assays, HTLV-1 and HTLV-2 have been shown to share a common receptor (54). Therefore, the ability of HTLV-1 and HTLV-2 virions to block binding of the HTSU-IgG was compared. When target cells were preincubated with equal concentrations of either HTLV-1 or HTLV-2 virions, binding of the immunoadhesin was similarly inhibited (Fig. 3). These results provide further support for the concept that the HTLV-1 SU immunoadhesin binds to a cell surface molecule critical for binding on target cells in a manner similar to that of the HTLV SU in the context of viral particles.
FIG. 3.
HTLV-1 and HTLV-2 similarly inhibit the binding of HTSU-IgG. Cell-free HTLV-1 and HTLV-2 were harvested from the supernatant of producer cell lines (MT-2 and 729pH 6neo, respectively), and the relative amount of viral particles was determined using a p19 ELISA kit. HeLa cells were preincubated for 30 min on ice with a high concentration (100 ηg of p19/ml) of HTLV-1 (solid line), a low concentration (0.2 ηg of p19/ml) of HTLV-1 (dotted line), or HTLV-2 (dashed line) or media without viral particles (shaded). HTSU-IgG (400 ηg/ml) was then added to each sample, and binding assays were performed.
Specific binding of HTLV SU immunoadhesin parallels susceptibility to transduction by HTLV-1 pseudotypes in different cell lines.
Although a number of studies have demonstrated that various cell lines differ widely in their susceptibility to transduction by HTLV-1 pseudotyped virions (6, 43, 46, 65, 67, 73, 74, 75), the reasons for these differences are not clear. One approach to examine these differences would be to treat the cells with different reagents previously shown to alter Env-mediated entry of other retroviruses and compare the effect on HTSU-IgG binding and the titer of HTLV pseudotypes on various cell types.
As a first step in those studies, we wanted to optimize conditions to examine the relative titer of HTLV-1 pseudotyped virus. The titer of the HTLV pseudotypes was optimized using modifications recently developed for other retroviral systems (see Materials and Methods). Briefly, the highest titers were generated with a transient three-plasmid MLV-based expression system using a retroviral vector encoding the EGFP gene (pCMMP.EGFP) (62) and transduction using the spin infection method (45) (data not shown).
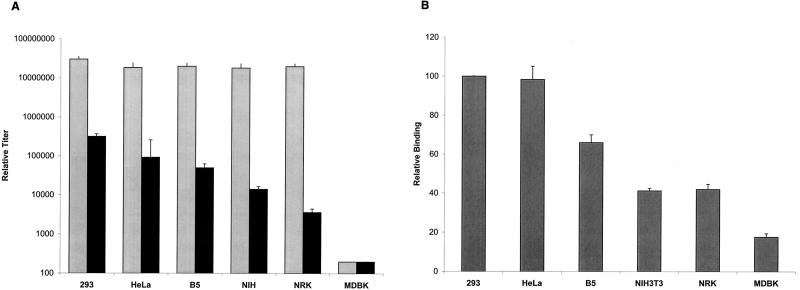
Using these conditions, the relative susceptibilities of different cells to transduction by HTLV-1 pseudotyped virus, MLV(HTLV-1), was determined (Fig. 4A). Target cells were incubated with serial dilutions of either MLV(HTLV-1) or, as a control, with MLV(A-MLV), the same retroviral core pseudotyped with amphotropic MLV envelope proteins. Each titer represents the mean of three to seven independent experiments. As expected from previous studies (46, 67, 73), the cell lines fell into three categories: 293, HeLa, and B5 were highly susceptible to MLV(HTLV-1), NIH 3T3 and NRK were poorly susceptible lines, and MDBK was not transduced above background levels. Pairwise analysis revealed that the titers of MLV(HTLV-1) for each of the highly susceptible lines (293, HeLa, and B5) were significantly different (P < 0.05) from those for each of the less susceptible lines (NIH 3T3, NRK, and MDBK). Similar analyses of the MLV(A-MLV) titer for the same cell lines revealed that only one of the cell lines (MDBK) was significantly different from any of the other cell lines. This observation is consistent with a previous report that MDBK cells were poorly transduced by HIV(A-MLV), even after they were transfected with the rat receptor for amphotropic virus (67).
FIG. 4.
Susceptibility of various cell lines to transduction by HTLV-1 pseudotyped virus parallels their ability to bind HTSU-IgG. (A) Titer of MLV(HTLV-1) and MLV(A-MLV) for various cell lines. Target cells were transduced with 10-fold dilutions of cell-free pseudotyped virus containing an EGFP indicator provirus. The relative titer was determined from the percentage of cells positive for the expression of EGFP as measured by flow cytometric analysis. These titers represent the means for three to seven independent experiments; each error bar represents the SEM. Pairwise comparison was performed using the Tukey test. Grey, titer of MLV(A-MLV); black, titer of MLV(HTLV). (B) Relative binding of HTSU-IgG to various cell lines. Binding of HTSU-IgG (200 ηg/ml) was determined by flow cytometric analysis, as described above. The amount of specific binding was determined by subtracting the MFI of SUA-IgG binding (500 ηg/ml) from the MFI of HTSU-IgG binding. For each experiment, the values were normalized to the amount of binding of HTSU-IgG to 293 cells within that experiment. The numbers represent the means for three independent experiments; error bars represent SEM). Pairwise comparison was performed using the Tukey test; significance was determined at the 0.05 level.
Similar studies to determine the susceptibility of T-cell lines to transduction by MLV(HTLV-1) revealed that the titers for Jurkat and MOLT-4 cells were 1.1 × 105 and 6.1 × 104, respectively. However, these could not be directly compared to the titers for the adherent cells, since the MLV(A-MLV) titer for these cells was different from the those of the adherent lines and from each other's (Jurkat, 8 × 105; MOLT-4, 7 × 106).
Next, the level of HTSU-IgG binding on the adherent cell lines was also determined (Fig. 4B), and pairwise analyses were performed to determine significance. As was seen for titer of MLV(HTLV-1), the level of relative binding to each of the highly susceptible lines was significantly different from that for each of the less susceptible lines. The ability of the HTSU-IgG to bind on different cell lines generally paralleled relative titers of MLV(HTLV-1) for those lines (compare Fig. 4A and B).
HTSU-IgG binding at equilibrium reveals differences in receptor expression among cell lines.
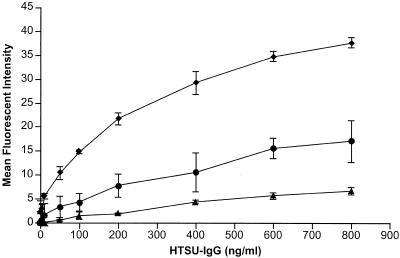
To further investigate the differences in binding among cell lines, we investigated HTSU-IgG binding at equilibrium. Measurement of binding of HTSU-IgG after incubation at different concentrations with identical numbers of cells revealed a saturable dose-response curve. Comparison of the binding curves for three representative cell lines (293, NIH 3T3, and MDBK) is shown in Fig. 5. All three cell lines showed saturable binding kinetics. The binding curves of HeLa and NRK were similar to those of 293 and NIH 3T3, respectively (data not shown), suggesting that cell lines on which the titer of MLV(HTLV-1) is higher have a higher number of receptors per cell than poorly susceptible cell lines. Moreover, the observation that half-maximal binding occurred at the same concentration (200 ηg/ml) for all cell lines tested suggests that the affinity of the binding receptor(s) for HTSU-IgG is similar for all the cell lines. Finally, the levels of binding at saturation paralleled the relative susceptibility to HTLV-1(MLV) transduction.
FIG. 5.
Cell lines with different susceptibilities to MLV(HTLV-1) transduction show different levels of binding at saturation. Cell lines with different levels of susceptibility to MLV(HTLV-1) transduction (293, NIH 3T3, and MDBK) were fixed in 4% paraformaldehyde for 30 min on ice, washed, and incubated with various concentrations of HTSU-IgG or SUA-IgG on ice, and binding assays performed with the mean fluorescent intensities calculated as described in Materials and Methods. Immunoadhesin and secondary antibody incubations were carried out under conditions shown not to be kinetically limited (see Materials and Methods). These titers represent the means for two independent experiments; error bars show the SEM. ♦, 293 cells; •, NIH 3T3 cells; ▴, MDBK cells.
PMA down-regulates the expression of HTLV SU binding protein(s).
Several recent studies have demonstrated that inefficient infection by retroviruses or transduction by retroviral vectors can occur when the appropriate receptor is present at a concentration below a threshold amount (32, 33, 34, 36). This appears to reflect the role of cooperativity of receptors in Env-mediated membrane fusion (reviewed in reference 14). It seemed possible that HTLV SU binding receptor(s) on cell lines poorly susceptible to MLV(HTLV-1) could be present at concentrations below the threshold required for efficient binding and fusion. As one approach to address this, we sought to identify substances that would differentially effect the expression of the SU binding proteins and the titer of the pseudotypes.
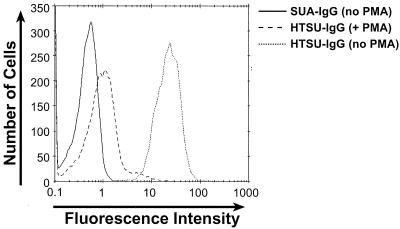
Previous studies have shown that exposure of cells to phorbol esters, including PMA, reduces the number of certain cell surface receptors, including the HIV receptor CD4 (26, 50, 68) and the HIV coreceptor CXCR4 (16, 60, 68). The effect of PMA on the expression of HTLV SU binding proteins was first examined by incubating primary human CD4+ cells in the presence or absence of PMA and determining the levels of binding of the immunoadhesin. Primary CD4+ cells were isolated and activated by PHA and IL-2 as described in Materials and Methods. Six days after the addition of IL-2, PMA (100 ηg/ml) was added. Eighteen hours later, binding of HTSU-IgG to cells was analyzed. Treatment of CD4+ cells with PMA dramatically reduced the binding of the soluble HTSU-IgG (Fig. 6). The mean fluorescence intensity (MFI) was reduced approximately 25-fold from that observed with no PMA treatment; the reduction in cell surface expression is similar in magnitude to that observed for the expression of CXCR4 following treatment with PMA (60).
FIG. 6.
PMA treatment reduces binding of HTSU-IgG to primary CD4+ T cells. CD4+ T cells were incubated overnight at 37°C in the presence or absence of 100 ηg of PMA/ml. Binding assays were performed on the following day with 200 ηg of HTSU-IgG/ml. Binding was also analyzed with untreated cells with 500 ηg of SUA-IgG/ml to determine the background level of binding. Solid line, binding of SUA-IgG to untreated cells; dashed line, binding of HTSU-IgG to PMA-treated cells; dotted line, binding of HTSU-IgG to untreated cells.
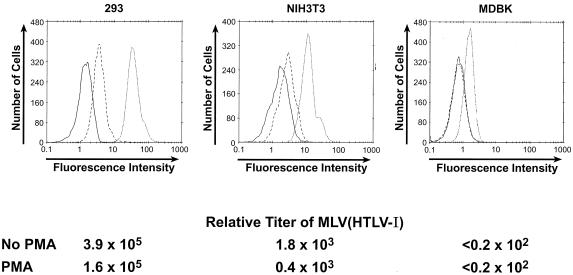
We next wanted to determine whether PMA differentially affected the level of the HTLV SU binding proteins and/or the titer of pseudotyped virus in cells highly and poorly susceptible to HTLV-1 viral transduction. PMA treatment of 293 cells reduced the MFI of binding to 9.5% of that of the untreated 293 cells (Fig. 7). For NIH 3T3 the effect was similar, reducing the MFI to 11% of the untreated cells. PMA treatment of MDBK cells reduced the mean fluorescence to background levels (Fig. 7). Thus, PMA regulates the expression of HTLV-1 SU binding proteins to a similar extent on cell lines with high and low susceptibilities to MLV(HTLV-1) transduction.
FIG. 7.
PMA treatment reduces HTLV-1 SU binding and titer of MLV(HTLV-1) for various cell types. Target cells were incubated for 2 h at 37°C in the presence or absence of 100 ηg of PMA/ml, and studies of binding and transduction were performed in parallel. Top, binding assay. The cells were washed once and used as targets in the binding assay with 200 ηg of HTSU-IgG/ml. Solid line, binding of SUA-IgG to untreated cells; dashed line, binding of HTSU-IgG to PMA-treated cells; dotted line, binding of HTSU-IgG to untreated cells. Bottom, transduction assay. After 2 h of PMA treatment, the medium was removed, viral supernatants containing PMA (100 ηg/ml) were added, spin infection was performed, and the relative titers determined as described in the legend to Fig. 4. The mean and SEM for this and two other independent experiments were as follows: 293 cells (no PMA), 3.6 × 105 ± 1.0 × 105; 293 cells (plus PMA), 1.7 × 105 ± 0.7 × 105; NIH 3T3 cells (no PMA), 3.6 × 103 ± 1.1 × 103; NIH 3T3 cells (plus PMA), 1.2 × 103 ± 0.5 × 103. Analysis of variance using values normalized to the mean demonstrated significance between the PMA-treated and untreated cells (P < 0.05).
In parallel to the binding assays, the effect of PMA on the titers of HTLV pseudotypes for these target cells was determined. The titers and binding shown in Fig. 7 were simultaneously determined. These studies revealed that PMA reduced the titer of HTLV-1 pseudotypes, although the effect was less dramatic than that observed for binding. Analyses of several independent experiments confirmed that the difference in titer with PMA treatment was statistically significant. The effect on MDBK cells could not be determined because, as shown in Fig. 4A, the titer of MLV(HTLV-1) on these cells was below level of detection of this assay.
HTSU-IgG binds to a trypsin-sensitive component.
Recently two laboratories reported that pretreatment of target cells with trypsin resulted in a relatively small (less than fourfold) decrease in the titer of HTLV-1 pseudotyped virions (46, 73). These studies, along with previous reports that a lipid-like component is important for HTLV-1 Env-mediated fusion (57, 58), raised the possibility that a nonprotein component may play a major role in HTLV SU binding.
To address this, the effects of trypsin treatment on the relative titer of MLV(HTLV-1) and on binding of HTSU-IgG were determined. HeLa cells (2 × 105 cells/ml) were incubated for 20 min at room temperature either in serum-free DMEM containing 0.1% trypsin or in serum-free DMEM alone. Trypsination was stopped by addition of FCS (15% final), and the cells were washed twice prior to exposure to the pseudotyped virus. Analysis of variance by the F-test demonstrated significant difference between the titer of MLV(HTLV-1) on treated and untreated cells (P = 0.043). The effects of trypsin on the relative titer of HTLV pseudotypes obtained in this study were similar to those recently reported. Treatment of HeLa cells with trypsin reduced the titer of MLV(HTLV-1) (1.4 × 104 ± 0.3 × 104) relative to the titer on untreated cells (3.6 × 104 ± 0.9 × 104). This reduction was similar to that observed for MLV(A-MLV), which uses the PiT-2 transmembrane protein as its receptor, in experiments performed in parallel (titer with trypsin treatment, 0.95 × 107 ± 0.4 × 107; titer with no treatment, 1.9 × 107 ± 0.4 × 107).
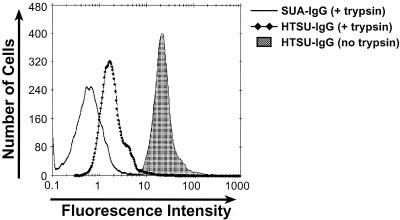
To directly examine the trypsin sensitivity of the cell surface component(s) that binds HTLV SU, experiments were performed to determine the effect of pretreatment with trypsin on the ability of HeLa cells to bind HTSU-IgG. Binding of the HTLV SU fusion protein was dramatically reduced after trypsin treatment of HeLa cells; the MFI was reduced approximately 20-fold from that observed with no trypsin treatment (Fig. 8). Additional studies revealed that even brief (5 min) exposure to trypsin reduced HTSU-IgG binding on HeLa and other cell lines (NIH 3T3 and MDBK) (data not shown). These results strongly suggest that the majority of specific binding of the immunoadhesin reflects interaction with a cell surface protein.
FIG. 8.
Trypsin pretreatment of HeLa cells dramatically reduces the binding of HTSU-IgG. HeLa cells were treated with trypsin as described in the text and used as targets in the HTSU-IgG binding assay, with 400 ηg of HTSU-IgG/ml. Solid line, binding of SUA-IgG to trypsin-treated cells; line with diamonds, binding of HTSU-IgG to trypsin treated cells; shaded area, binding of HTSU-IgG to untreated cells.
HTLV-1 Env-mediated entry and HTSU-IgG binding is inhibited by polycations.
We next investigated the effect of polycations, which have been shown to enhance the infectivity of other retroviruses (8, 38, 72), on the relative titer of MLV(HTLV-1) and HTLV-1 SU binding on different cell lines. Such studies might provide insight into whether the differences among cell lines involve a charge-mediated interaction. Target cells (293, NIH 3T3, and NRK) were incubated with different concentrations of polybrene for 30 min prior to and during transduction. For all three cell types, polybrene significantly decreased the relative titer of MLV(HTLV-1) (Fig. 9A). Incubation with various concentrations of DEAE-dextran (1 to 10 μg/ml) was shown to have a similar effect (data not shown). Preincubation of both the virus and the cell with polybrene prior to transduction slightly enhanced the decrease in titer over preincubation with the cells alone (data not shown). In some experiments, a slight enhancement of relative titer (⩽2-fold) was observed at low concentrations of polybrene (⩽1 μg/ml) and DEAE-dextran (<0.5 μg/ml) (data not shown).
FIG. 9.
Inhibition of HTLV-1 Env-mediated entry and HTSU-IgG binding by polybrene. (A) Effect of polybrene on titer of MLV(HTLV-1). Target cells (293, NIH 3T3, and NRK) were incubated with 0, 4, 8, or 16 μg of polybrene/ml for 30 min at 37°C. Cells were then exposed to MLV(HTLV-1) in DMEM to which the appropriate concentration of polybrene had been added and transduced by spin infection. Cells were harvested, and titers were determined as described in Materials and Methods. Titers obtained in the absence of polybrene were nor-malized to 100, and the relative titer was determined using the following formula: (titer with polybrene/titer without polybrene) × 100. The results are the means for two independent experiments; the error bars represent SEM. (B) Effect of transduction conditions on polybrene and DEAE-dextran inhibition of MLV(HTLV-1) titer. 293 cells were incubated either with MLV(HTLV-1) alone, with 4 μg of polybrene/ml, or with 10 μg of DEAE-dextran/ml. Cells were then transduced for 2 h by spin infection and then moved to a 37°C environment for 1 h (spin) or left for 3 h at 37°C (no spin). Cells were then washed, and the titer was determined and normalized as described above. White, no treatment; grey, polybrene; black, DEAE-dextran. (C) Effect of polybrene on binding of HTSU-IgG. Target cells (293 or NRK) were incubated for 30 min with 0, 4, 8, or 16 μg of polybrene/ml, and binding to HTSU-IgG (200 ηg/ml) or SUA-IgG (200 ηg/ml) was determined.
To rule out the possibility that this inhibition of transduction was an artifact of the spin infection method, 293 cells were preincubated with either polybrene (4 μg/ml) or DEAE-dextran (10 μg/ml) and transduced at either 1 × g or 1,200 × g. Polybrene and DEAE-dextran reduced the relative titer of MLV(HTLV-1) to similar extents under the two conditions (Fig. 9B).
To determine whether this inhibition of titer also reflected a difference in HTLV Env-specific binding, the effect of polybrene on binding of the HTSU-IgG was examined. Target cells (293 or NIH 3T3) were incubated for 30 min with 0, 4, 8, or 16 μg/ml of polybrene, and the level of HTSU-IgG binding was determined. Polybrene inhibited the binding of the HTLV SU immunoadhesin to both cell lines (Fig. 9C); this effect paralleled the effect on relative titer (compare Fig. 9A and C). No significant effect of polybrene on the background binding of the SUA-IgG was observed (data not shown). It therefore seems likely that the polycation inhibition of HTLV-1 Env-mediated transduction reflects an inhibition of specific interactions between the HTLV-1 SU and molecules on the cell surface.
DISCUSSION
Despite efforts from a number of laboratories, little is known about the requirements for human T-cell leukemia virus (HTLV-1) entry, including the identity of the cellular receptor(s). Early studies of the distribution of cell surface components important for HTLV-1 binding compared the abilities of different hematopoietic cells to bind labeled HTLV-1 virions (31, 61, 76). A number of later studies inferred receptor levels on different cell types from their ability to form syncytia with cells expressing HTLV-1 Env or from the relative titer of viral vectors pseudotyped with HTLV-1 Env on these cells (35, 47, 65, 67, 73, 74, 75).
These studies revealed two basic facts about HTLV Env-mediated binding and entry. First, HTLV receptor(s) are widely expressed on cell lines of various cell types from different species. Second, various cell lines differ dramatically in their susceptibility to HTLV Env-mediated fusion. It is not yet clear whether the differences among cell lines reflect qualitative or quantitative differences in the HTLV receptor(s) and/or the presence of additional molecules that enhance or inhibit binding and/or entry. In the present study, we directly compared the cell surface expression level of HTLV SU binding proteins and the susceptibility to HTLV-1 pseudotyped virions for various cell lines. These studies were performed under standard conditions and following exposure to substances previously shown to alter Env-mediated binding and entry of other retroviruses.
To initiate these studies, conditions for examination of HTLV-1 Env binding and fusion were established. For part of this work, a soluble form of HTLV-1 SU (the immunoadhesin HTSU-rIgG) was generated by using an approach previously described for other retroviral SU proteins (1-4, 25, 78). The immunoadhesin bound at high levels to CD4+ T cells, the primary in vivo target cell of HTLV-1, as well as to a number of other cell lines (Fig. 1 and data not shown). Several studies validated that this binding reflects specific interactions between the SU component of the immunoadhesin and cell surface molecules involved in HTLV-1 virus binding and entry. Monoclonal antibodies directed against neutralizing epitopes of HTLV-1 SU, but not isotype controls, blocked binding of HTSU-rIgG to target cells (Fig. 2A), as did preincubation of target cells with HTLV virions and retroviral vectors pseudotyped with HTLV-1 (Fig. 2B and 3).
In other studies, titers of the HTLV-1 pseudotypes were optimized using modifications recently developed for other retroviral systems. Under the conditions used in this study, the relative titer of cell-free MLV(HTLV-1) for the most susceptible cell line (293) was in the range of 2 to 5 × 105. This represents a multiplicity of infection of >1 for the conditions used and is approximately 1 log higher than the cell-free titers of HTLV-1 pseudotyped virus for highly susceptible cells using other systems (46, 67). The titer of MLV(HTLV-1) on poorly susceptible cells (NIH 3T3, NRK, C127LT, and BHK-21) was more than 1 log higher than the level of detection of this assay (Fig. 4A and 7; data not shown); this was high enough to allow us to quantify the effect of substances that down-regulate HTLV-1 Env-mediated binding and entry.
Direct comparison of binding of HTLV-1 SU immunoadhesin and transduction by HTLV-1 pseudotyped virus with various cell lines revealed a relationship between the level of binding and the titer (Fig. 4, data not shown). Another group recently reported specific binding of HTLV-1 SU to a wide variety of mammalian and nonmammalian vertebrate cells, using a similar reagent produced in insect cells (27). Here, we extended this previous work by examining HTLV SU binding and titer of HTLV-1 pseudotyped virus in parallel. It was not clear from the studies in Fig. 4 or published studies (27) whether the different levels of binding reflected different numbers of binding receptors or species and/or cell type differences in the binding affinities of the receptors.
As an initial step in addressing this question, experiments to determine equilibrium binding on different cell lines were performed. At both nonsaturating and saturating concentrations, the cell lines which are the most susceptible to transduction by HTLV pseudotyped virions (293 and HeLa) or infection by HTLV-1 virions (primary CD4+ T cells) bound the highest levels of HTSU-IgG (Fig. 1, 4, and 5; M. Nath et al., submitted for publication). The plateau level of binding was several-fold higher than that observed for the poorly susceptible cell lines NIH 3T3 and NRK (Fig. 5 and data not shown). The observation that cell lines differ in their levels of HTSU-IgG binding could reflect differences in the number and/or affinity of the binding for different species and cell types. The observation that the concentration of immunoadhesin for half-maximal binding was the same for all the cell lines tested suggests that the affinities of the HTLV-1 SU binding on various cell types are similar. Since these studies were performed with an indirect fluorescence assay for binding, Scatchard analyses could not be used to determine the precise number of binding sites.
These observations and those of others (27) strongly suggest that cell lines poorly susceptible to transduction by HTLV-1 pseudotypes have significant levels of cell surface molecules that specifically bind SU. The observation that cell lines poorly susceptible to MLV(HTLV-1) bound lower levels of soluble SU than highly susceptible cells raised the possibility that the receptor(s) might be present at concentrations below the threshold required for efficient binding and fusion on poorly susceptible cells. One approach to address this was to reduce the cell surface expression of the SU binding proteins and to compare the effect on titers of MLV(HTLV-1) on different cell lines. We determined that treatment with the phorbol ester PMA, which has previously been shown to down-modulate a number of cell surface receptors, reduced binding by HTSU-IgG (Fig. 6 and 7). These experiments revealed that under conditions where 293 cells bound less soluble SU than NIH 3T3 cells (PMA-treated 293 cells [MFI = 2.3] versus untreated NIH 3T3 cells [MFI = 10.3]), the titer of MLV(HTLV-1) was still nearly 2 logs higher on the PMA-treated 293 cells (Fig. 7). These results suggest that factors other than the number of binding receptors on the cell surface are responsible for the differences in titers of MLV(HTLV-1) for these cell lines.
From previous studies, it was not clear whether or not the same cell surface molecules are involved in HTLV-1-mediated binding and fusion in highly and poorly susceptible cell lines. For example, it has been suggested that the low level of entry on poorly susceptible cells observed in previous studies could reflect the high level of expression of the HTLV Env glycoproteins on pseudotyped virions, resulting in artificially high and potentially nonspecific levels of virus binding and entry (63). In the present study, we observed that treating cells with PMA reduced binding to HTSU-IgG and titers of MLV(HTLV-1) to a similar extent on highly susceptible cells (CD4+ T cells and 293 cells) and poorly susceptible cells (NIH 3T3 and MDBK). Although PMA can down-regulate a large number of molecules, this observation provides further evidence that the molecules that bind SU on poorly susceptible cells are the same as, or closely related to, the SU binding molecules present on highly susceptible cells.
Two previous studies showed that trypsin treatment of target cells only moderately reduced the titer of HTLV-1 pseudotyped virions (46, 73). These studies, along with reports that nonprotein components are involved in HTLV-1-induced syncytium formation (57, 58), raised the possibility that both protein and nonprotein components may play major roles in HTLV-1 binding. We observed that trypsin treatment dramatically reduced HTSU-IgG binding to HeLa cells, suggesting that the majority of the binding of the immunoadhesin reflects binding to a cell surface proteins.
In contrast, but consistent with previous results using other HTLV pseudotypes, trypsin treatment of HeLa cells reduced the titer of MLV(HTLV-1) by about 50%. The discrepancy between the effects of trypsin on binding and on viral titer may reflect differences in the assay systems used. For example, nonprotein components may bind HTLV pseudotyped virions (in an Env-dependent or -independent manner) but not soluble SU. The difference could also reflect a more trivial difference in our assay systems (e.g., the increased time [2 h versus 30 minutes] to allow binding after trypsin is removed in the transduction assay). Alternatively, it is possible that nonprotein components may play a role in HTLV Env-mediated fusion. The low titer of HTLV pseudotyped virus for certain cell lines despite the presence of SU binding protein(s) may reflect one or several of a number of factors. As discussed above, our studies suggest that this does not appear to be primarily due to the lower number of HTLV SU binding proteins on those cells or a lower affinity of the binding receptor(s). It is possible that the poorly susceptible cell lines have cell surface molecules which sterically block binding of virions (see further below) and/or that the more susceptible cell lines have additional cell surface molecules which enhance binding and/or fusion. Recent studies have revealed that a number of molecules other than CD4 and coreceptors are capable of binding HIV (recently reviewed in reference 7); it is possible that cell surface molecules other than the primary binding receptor(s) enhance the binding of virions with HTLV-1 glycoproteins in highly susceptible cell lines. The recent observation that HTLV-1-induced cell fusion requires intact lipid rafts (44) raises the possibility that variations in the composition of these membrane microdomains could explain the differences between cell lines.
Alternatively, various cell lines could bind similar levels of virions but differ in their ability to support fusion and/or entry. For example, it is possible that the low titer of the HTLV-1 pseudotyped virus on certain cell lines could reflect the ability of HTLV-1 to utilize alternate entry pathways with different levels of efficiency. It is also possible that cell lines differ in their levels of cell surface molecules necessary for fusion.
For some retroviruses, the resistance of certain cell lines to viral entry can be overcome by pretreatment with tunicamycin, an inhibitor of N-linked glycosylation (13, 15, 39, 40, 69, 70, 71). Our preliminary studies suggests that glycosylation does not play a major role in the poorer susceptibility of rodent cell lines to transduction by MLV(HTLV-1) (data not shown).
Cationic polymers, such as polybrene and DEAE-dextran, have frequently been used to enhance both retroviral transduction and infection with oncogenic retroviruses. In contrast to the positive effect that polycations have on the infection of those viruses, we observed that the titer of MLV(HTLV-1) was dramatically reduced when polybrene and DEAE-dextran were present during transduction (Fig. 9A and B). In transductions performed in parallel, polybrene slightly increased the titer of MLV(A-MLV) (⩽2.5-fold); this modest effect is consistent with previous reports that the spin infection method largely replaces the enhancement effect of polybrene (45). Inhibition of transduction of HTLV-1 pseudotyped VSV by polycations has been previously reported (23). Further studies revealed that polybrene also inhibited the binding of HTSU-IgG; this effect mirrored the effect on titer (Fig. 9C). The polycations inhibited binding and entry to similar extents with different cell types (Fig. 9A and C; also data not shown). Although the specific nature of the interaction inhibited by polycations is not yet clear, it seems likely that this interaction does not explain differences in binding between highly susceptible cells and poorly susceptible cells. The inhibition by polycations raises the possibility that a positively charged molecule(s) on the cell surface plays an important role in HTLV SU binding and HTLV-Env-mediated entry. It is also possible that the HTLV SU interaction with cell surface receptor(s) involves negatively charged residues that might be masked by the positively charged polycations. Additional studies to investigate the role of charged interactions in HTLV-1 binding, including determining the effect of soluble heparin on MLV(HTLV-1) titers, are currently in progress.
It seems likely that the immunoadhesin, especially in combination with infection studies using HTLV virions and HTLV pseudotyped viruses, will be useful in providing additional insight into interactions between the HTLV-1 Env glycoproteins and their receptor(s). We have recently used these approaches to examine the regulation of expression of the HTLV-1 SU binding proteins during activation of primary T cells (Nath et al., submitted) and to characterize the role of specific residues of HTLV-1 SU in entry (A. C. Baines et al., unpublished data). The immunoadhesin could also prove useful in further characterizing molecules involved in HTLV binding and entry.
Acknowledgments
We thank John Young, Ken Bradley, Sara Klucking, and Jessica Bernestrom for reagents, helpful suggestions, and encouragement; Lorraine Albritton, Monica Roth, Pat Green, David Saunders, Vineet KewalRamani, and Sandra Ruscetti for useful discussions; Ken Hadlock and Steve Foung for providing the monoclonal antibodies directed against HTLV SU; Paula Cannon and Una O'Doherty for providing technical advice; Louise Finch and Refika Turnier for performing the flow cytometry; and Robert Jones and James Kaiser for providing statistical support.
Portions of this work were supported with funds from the NCI under contract NO1-CO-124000.
The content of this publication does not reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
REFERENCES
- 1.Adkins, H. B., J. Brojatsch, J. Naughton, M. M. Rolls, J. M. Pesola, and J. A. Young. 1997. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc. Natl. Acad. Sci. USA 94:11617-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. [DOI] [PubMed] [Google Scholar]
- 3.Byrn, R. A., J. Mordenti, C. Lucas, D. Smith, S. A. Marsters, J. S. Johnson, P. Cossum, S. M. Chamow, F. M. Wurm, T. Gregory, et al. 1990. Biological properties of a CD4 immunoadhesin. Nature 344:667-670. [DOI] [PubMed] [Google Scholar]
- 4.Capon, D. J., S. M. Chamow, J. Mordenti, S. A. Marsters, T. Gregory, H. Mitsuya, R. A. Byrn, C. Lucas, F. M. Wurm, J. E. Groopman, et al. 1989. Designing CD4 immunoadhesins for AIDS therapy. Nature 337:525-531. [DOI] [PubMed] [Google Scholar]
- 5.Center, R. J., B. Kobe, K. A. Wilson, T. Teh, G. J. Howlett, B. E. Kemp, and P. Poumbourios. 1998. Crystallization of a trimeric human T cell leukemia virus type 1 gp21 ectodomain fragment as a chimera with maltose-binding protein. Protein Sci. 7:1612-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapham, P., K. Nagy, R. Cheingsong-Popov, M. Exley, and R. A. Weiss. 1983. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science 222:1125-1127. [DOI] [PubMed] [Google Scholar]
- 7.Clapham, P. R., and A. McKnight. 2001. HIV-1 receptors and cell tropism. Br. Med. Bull. 58:43-59. [DOI] [PubMed] [Google Scholar]
- 8.Coelen, R. J., D. G. Jose, and J. T. May. 1983. The effect of hexadimethrine bromide (polybrene) on the infection of the primate retroviruses SSV 1/SSAV 1 and BaEV. Arch. Virol. 75:307-311. [DOI] [PubMed] [Google Scholar]
- 9.Daenke, S., and S. Booth. 2000. HTLV-1-induced cell fusion is limited at two distinct steps in the fusion pathway after receptor binding. J. Cell Sci. 113:37-44. [DOI] [PubMed] [Google Scholar]
- 10.Daenke, S., and S. Booth. 2000. Molecular mechanisms affecting HTLV type 1-dependent fusion at the cell membrane: implications for inhibiting viral transmission. AIDS Res. Hum. Retrovir. 16:1731-1736. [DOI] [PubMed] [Google Scholar]
- 11.Delamarre, L., A. R. Rosenberg, C. Pique, D. Pham, and M. C. Dokhelar. 1997. A novel human T-leukemia virus type 1 cell-to-cell transmission assay permits definition of SU glycoprotein amino acids important for infectivity. J. Virol. 71:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derse, D., J. Mikovits, D. Waters, S. Brining, and F. Ruscetti. 1996. Examining the molecular genetics of HTLV-I with an infectious molecular clone of the virus and permissive cell culture systems. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 12:1-5. [DOI] [PubMed] [Google Scholar]
- 13.Dirks, C., and A. D. Miller. 2001. Many nonmammalian cells exhibit postentry blocks to transduction by gammaretroviruses pseudotyped with various viral envelopes, including vesicular stomatitis virus G glycoprotein. J. Virol. 75:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 15.Eiden, M. V., K. Farrell, and C. A. Wilson. 1994. Glycosylation-dependent inactivation of the ecotropic murine leukemia virus receptor. J. Virol. 68:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forster, R., E. Kremmer, A. Schubel, D. Breitfeld, A. Kleinschmidt, C. Nerl, G. Bernhardt, and M. Lipp. 1998. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J. Immunol. 160:1522-1531. [PubMed] [Google Scholar]
- 17.Gavalchin, J., N. Fan, M. J. Lane, L. Papsidero, and B. J. Poiesz. 1993. Identification of a putative cellular receptor for HTLV-I by a monoclonal antibody, Mab 34-23. Virology 194:1-9. [DOI] [PubMed] [Google Scholar]
- 18.Gavalchin, J., N. Fan, P. G. Waterbury, E. Corbett, B. D. Faldasz, S. M. Peshick, B. J. Poiesz, L. Papsidero, and M. J. Lane. 1995. Regional localization of the putative cell surface receptor for HTLV-I to human chromosome 17q23.2-17q25.3. Virology 212:196-203. [DOI] [PubMed] [Google Scholar]
- 19.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed]
- 20.Hadlock, K. G., J. Rowe, S. Perkins, P. Bradshaw, G. Y. Song, C. Cheng, J. Yang, R. Gascon, J. Halmos, S. M. Rehman, M. S. McGrath, and S. K. Foung. 1997. Neutralizing human monoclonal antibodies to conformational epitopes of human T-cell lymphotropic virus type 1 and 2 gp46. J. Virol. 71:5828-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadlock, K. G., J. Rowe, and S. K. H. Foung. 1999. The humoral immune response to human T-cell lymphotropic virus type 1 envelope glycoprotein gp46 is directed primarily against conformational epitopes. J. Virol. 72:1205-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han, J. Y., Y. Zhao, W. F. Anderson, and P. M. Cannon. 1998. Role of variable regions A and B in receptor binding domain of amphotropic murine leukemia virus envelope protein. J. Virol. 72:9101-9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haraguchi, Y., Y. Takeuchi, and H. Hoshino. 1997. Inhibition of plating of human T cell leukemia virus type I and syncytium-inducing types of human immunodeficiency virus type 1 by polycations. AIDS Res. Hum. Retrovir. 13:1517-1523. [DOI] [PubMed] [Google Scholar]
- 24.Hildreth, J. E. 1998. Syncytium-inhibiting monoclonal antibodies produced against human T-cell lymphotropic virus type 1-infected cells recognize class II major histocompatibility complex molecules and block by protein crowding. J. Virol. 71:9544-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmen, S. L., and M. J. Federspiel. 2000. Selection of a subgroup A avian leukosis virus [ALV(A)] envelope resistant to soluble ALV(A) surface glycoprotein. Virology 273:364-373. [DOI] [PubMed] [Google Scholar]
- 26.Hoxie, J. A., J. L. Rackowski, B. S. Haggarty, and G. N. Gaulton. 1988. T4 endocytosis and phosphorylation induced by phorbol esters but not by mitogen or HIV infection. J. Immunol. 140:786-795. [PubMed] [Google Scholar]
- 27.Jassal, S. R., R. G. Pohler, and D. W. Brighty. 2001. Human T-cell leukemia virus type 1 receptor expression among syncytium-resistant cell lines revealed by a novel surface glycoprotein-immunoadhesin. J. Virol. 75:8317-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jinno, A., T. Haraguchi, H. Shiraki, and H. Hoshino. 1999. Inhibition of cell-free human T-cell leukemia virus type 1 infection at a postbinding step by the synthetic peptide derived from an ectodomain of the gp21 transmembrane glycoprotein. J. Virol. 73:9683-9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, F. J., I. Seiliez, C. Denesvre, D. Lavillette, F. L. Cosset, and M. Sitbon. 2000. Definition of an amino-terminal domain of the human T-cell leukemia virus type 1 envelope surface unit that extends the fusogenic range of an ecotropic murine leukemia virus. J. Biol. Chem. 275:23417-23420. [DOI] [PubMed] [Google Scholar]
- 30.Koyanagi, Y., Y. Itoyama, N. Nakamura, K. Takamatsu, J. Kira, T. Iwamasa, I. Goto, and N. Yamamoto. 1993. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology 196:25-33. [DOI] [PubMed] [Google Scholar]
- 31.Krichbaum-Stenger, K., B. J. Poeisz, P. Keller, G. Ehrlich, J. Gavalchin, B. H. Davis, and J. L. Moore. 1987. Specific adsorption of HTLV-I to various target human and animal cells. Blood 70:1303-1311. [PubMed] [Google Scholar]
- 32.Kuhmann, S. E., E. Platt, S. L. Kozak, and D. Kabat. 2000. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74:7005-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurre, P., H.-P. Kiem, J. Morris, S. Heyward, J.-L. Battini, and A. D. Miller. 1999. Efficient transduction by an amphotropic retrovirus vector is dependent on high-level expression of the cell surface virus receptor. J. Virol. 73:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.P. Kurre, J. Morris, A. D. Miller, and H.-P. Kiem. 2001. Envelope fusion protein binding studies in an inducible model of retrovirus receptor expression and in CD34+ cells emphasize limited transduction at low receptor levels. Gene Ther. 8:593-599. [DOI] [PubMed] [Google Scholar]
- 35.Li, Q. X., D. Camerini, Y. Xie, M. Greenwald, D. R. Kuritzkes, and I. S. Chen. 1996. Syncytium formation by recombinant HTLV-II envelope glycoprotein. Virology 218:279-284. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald, C., S. Walker, M. Watts, S. Ings, D. C. Linch, and S. Devereux. 2000. Effect of changes in expression of the amphotropic retroviral receptor PiT-2 on transduction efficiency and viral titer: implications for gene therapy. Hum. Gene Ther. 11:587-595. [DOI] [PubMed] [Google Scholar]
- 37.Maerz, A. L., R. J. Center, B. E. Kemp, B. Kobe, and P. Poumbourios. 2000. Functional implications of the human T-lymphotropic virus type 1 transmembrane glycoprotein helical hairpin structure. J. Virol. 74:6614-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manning, J. S., A. J. Hackett, and N. B. Darby, Jr. 1971. Effect of polycations on sensitivity of BALB-3T3 cells to murine leukemia and sarcoma virus infectivity. Appl. Microbiol. 22:1162-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, D. G., and A. D. Miller. 1992. Tunicamycin treatment of CHO cells abrogates multiple blocks to retrovirus infection, one of which is due to a secreted inhibitor. J. Virol. 66:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, D. G., and A. D. Miller. 1993. Inhibitors of retrovirus infection are secreted by several hamster cell lines and are also present in hamster sera. J. Virol. 67:5346-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 42.Nagai, M., B. Brennan, J. Sakai, C. A. Carlos, and S. Jacobson. 2001. CD8+ T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood 98:1858-1861. [DOI] [PubMed] [Google Scholar]
- 43.Nagy, K., P. Clapham, R. Cheingsong-Popov, and R. A. Weiss. 1983. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int. J. Cancer 32:321-328. [DOI] [PubMed] [Google Scholar]
- 44.Niyogi, K., and J. E. Hildreth. 2001. Characterization of new syncytium-inhibiting monoclonal antibodies implicates lipid rafts in human T-cell leukemia virus type 1 syncytium formation. J. Virol. 75:7351-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okuma, K., Y. Matsuura, H. Tatsuo, Y. Inagaki, M. Nakamura, N. Yamamoto, and Y. Yanagi. 2001. Analysis of the molecules involved in human T-cell leukaemia virus type 1 entry by a vesicular stomatitis virus pseudotype bearing its envelope glycoproteins. J. Gen. Virol. 82:821-830. [DOI] [PubMed] [Google Scholar]
- 47.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 48.Osame, M., S. Izumo, A. Igata, M. Matsumoto, T. Matsumoto, S. Sonoda, M. Tara, and Y. Shibata. 1986. Blood transfusion and HTLV-I associated myelopathy. Lancet ii:104-105. [DOI] [PubMed]
- 49.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelchen-Matthews, A., I. J. Parsons, and M. Marsh. 1993. Phorbol ester-induced down-regulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J. Exp. Med. 178:1209-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pique, C., D. Pham, T. Tursz, and M. C. Dokhelar. 1993. The cytoplasmic domain of the human T-cell leukemia virus type I envelope can modulate envelope functions in a cell type-dependent manner. J. Virol. 67:557-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poiesz, B. J., L. D. Papsidero, G. Ehrlich, M. Sherman, S. Dube, M. Poiesz, K. Dillon, F. W. Ruscetti, D. Slamon, C. Fang, A. Williams, D. Duggan, J. Glaser, A. Gottlieb, J. Goldberg, L. Ratner, P. Phillips, T. Han, A. Friedman-Kien, F. Siegal, K. Rai, A. Sawitsky, L. W. Sheremata, H. Dosik, and R. Montagna. 2001. Prevalence of HTLV-I-associated T-cell lymphoma. Am. J. Hematol. 66:32-38. [DOI] [PubMed] [Google Scholar]
- 53.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenberg, A. R., L. Delamarre, C. Pique, D. Pham, and M. C. Dokhelar. 1997. The ectodomain of the human T-cell leukemia virus type 1 TM glycoprotein is involved in postfusion events. J. Virol. 71:7180-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg, A. R., L. Delamarre, A. Preira, and M. C. Dokhelar. 1998. Analysis of functional conservation in the surface and transmembrane glycoprotein subunits of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J. Virol. 72:7609-7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sagara, Y., Y. Inoue, E. Kojima, C. Ishida, H. Shiraki, and Y. Maeda. 2001. Phosphatidylglycerol participates in syncytium formation induced by HTLV type 1-bearing cells. AIDS Res. Hum. Retrovir. 17:125-135. [DOI] [PubMed] [Google Scholar]
- 58.Sagara, Y., C. Ishida, Y. Inoue, H. Shiraki, and Y. Maeda. 1997. Trypsin-sensitive and -resistant components in human T-cell membranes required for syncytium formation by human T-cell lymphotropic virus type 1-bearing cells. J. Virol. 71:601-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seiki, M., S. Hattori, and M. Yoshida. 1982. Human adult T-cell leukemia virus: molecular cloning of the provirus DNA and the unique terminal structure. Proc. Natl. Acad. Sci. USA 79:6899-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Signoret, N., J. Oldridge, A. Pelchen-Matthews, P. J. Klasse, T. Tran, L. F. Brass, M. M. Rosenkilde, T. W. Schwartz, W. Holmes, W. Dallas, M. A. Luther, T. N. Wells, J. A. Hoxie, and M. Marsh. 1997. Phorbol esters and SDF-1 induce rapid endocytosis and down modulation of the chemokine receptor CXCR4. J. Cell Biol. 139:651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinangil, F., S. Harada, D. T. Purtilo, and D. J. Volsky. 1985. Host cell range of adult T-cell leukemia virus. I. Viral infectivity and binding to various cells as detected by flow cytometry. Int. J. Cancer 36:191-198. [DOI] [PubMed] [Google Scholar]
- 62.Snitkovsky, S., T. M. Niederman, B. S. Carter, R. C. Mulligan, and J. A. Young. 2000. A TVA-single-chain antibody fusion protein mediates specific targeting of a subgroup A avian leukosis virus vector to cells expressing a tumor-specific form of epidermal growth factor receptor. J. Virol. 74:9540-9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sommerfelt, M. A. 1999. Retrovirus receptors. J. Gen. Virol. 80:3049-3064. [DOI] [PubMed] [Google Scholar]
- 64.Sommerfelt, M. A., and R. A. Weiss. 1990. Receptor interference groups of 20 retroviruses plating on human cells. Virology 176:58-69. [DOI] [PubMed] [Google Scholar]
- 65.Sommerfelt, M. A., B. P. Williams, P. R. Clapham, E. Solomon, P. N. Goodfellow, and R. A. Weiss. 1988. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science 242:1557-1559. [DOI] [PubMed] [Google Scholar]
- 66.Soneoka, Y., P. M. Cannon, E. E. Ramsdale, J. C. Griffiths, G. Romano, S. M. Kingsman, and A. J. Kingsman. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sutton, R. E., and D. R. Littman. 1996. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J. Virol. 70:7322-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tahara-Hanaoka, S., Y. Ushijima, H. Tarui, M. Wada, T. Hara, S. Imanishi, T. Yamaguchi, T. Hattori, H. Nakauchi, and A. Koito. 2000. Differential level in co-down-modulation of CD4 and CXCR4 primed by HIV-1 gp120 in response to phorbol ester, PMA, among HIV-1 isolates. Microbiol. Immunol. 44:489-498. [DOI] [PubMed] [Google Scholar]
- 69.Tailor, C. S., A. Nouri, and D. Kabat. 2000. Cellular and species resistance to murine amphotropic, gibbon ape, and feline subgroup C leukemia viruses is strongly influenced by receptor expression levels and by receptor masking mechanisms. J. Virol. 74:9797-9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Talbot, S. J., R. A. Weiss, and T. F. Schulz. 1995. Reduced glycosylation of human cell lines increases susceptibility to CD4-independent infection by human immunodeficiency virus type 2 (LAV-2/B). J. Virol. 69:3399-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tavoloni, N., and A. Rudenholz. 1997. Variable transduction efficiency of murine leukemia retroviral vector on mammalian cells: role of cellular glycosylation. Virology 229:49-56. [DOI] [PubMed] [Google Scholar]
- 72.Toyoshima, K., and P. K. Vogt. 1969. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology 38:414-426. [DOI] [PubMed] [Google Scholar]
- 73.Trejo, S. R., and L. Ratner. 2000. The HTLV receptor is a widely expressed protein. Virology 268:41-48. [DOI] [PubMed] [Google Scholar]
- 74.Vile, R. G., T. F. Schulz, O. F. Danos, M. K. Collins, and R. A. Weiss. 1991. A murine cell line producing HTLV-I pseudotype virions carrying a selectable marker gene. Virology 180:420-424. [DOI] [PubMed] [Google Scholar]
- 75.Wilson, C., M. S. Reitz, H. Okayama, and M. V. Eiden. 1989. Formation of infectious hybrid virions with gibbon ape leukemia virus and human T-cell leukemia virus retroviral envelope glycoproteins and the gag and pol proteins of Moloney murine leukemia virus. J. Virol. 63:2374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto, N., T. Chosa, Y. Koyanagi, T. Tochikura, J. Schneider, and Y. Hinuma. 1984. Binding of adult T-cell leukemia virus to various hematopoietic cells. Cancer Lett. 21:261-268. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zingler, K., and J. A. Young. 1996. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J. Virol. 70:7510-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]