Abstract
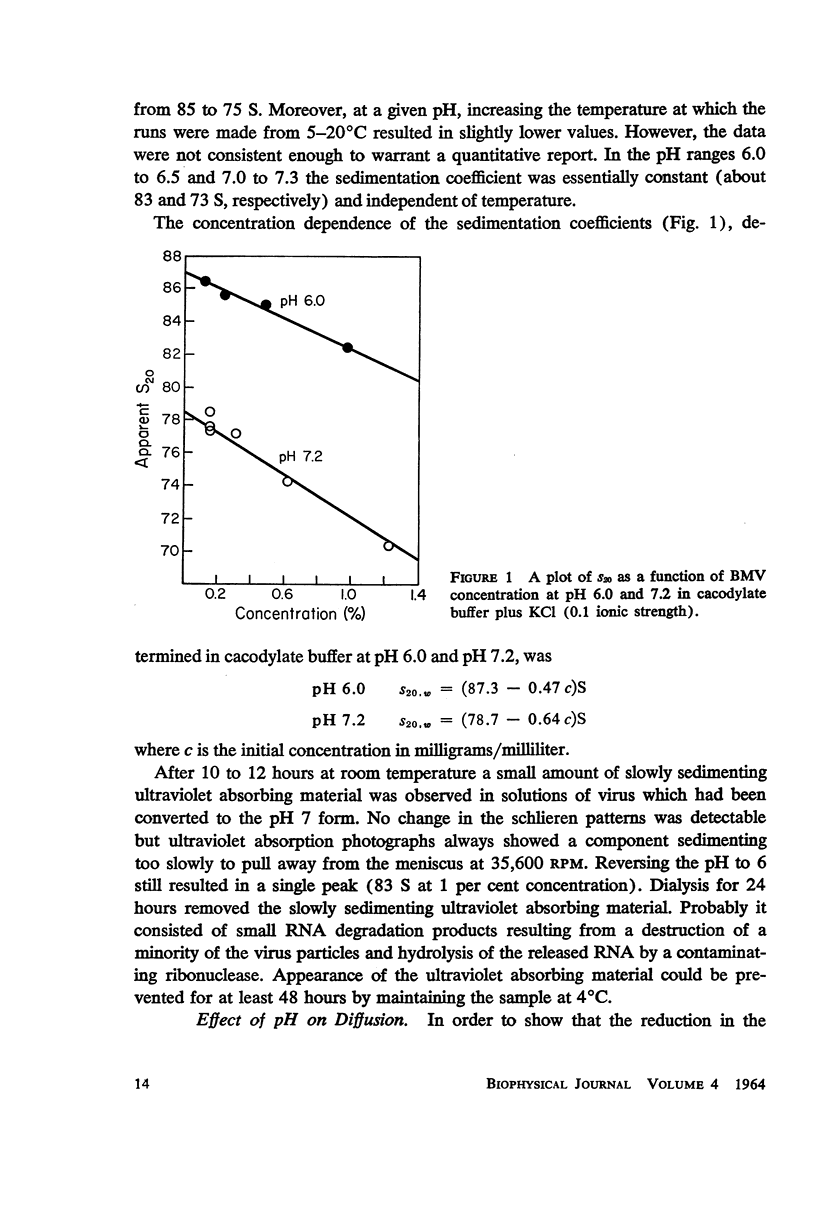
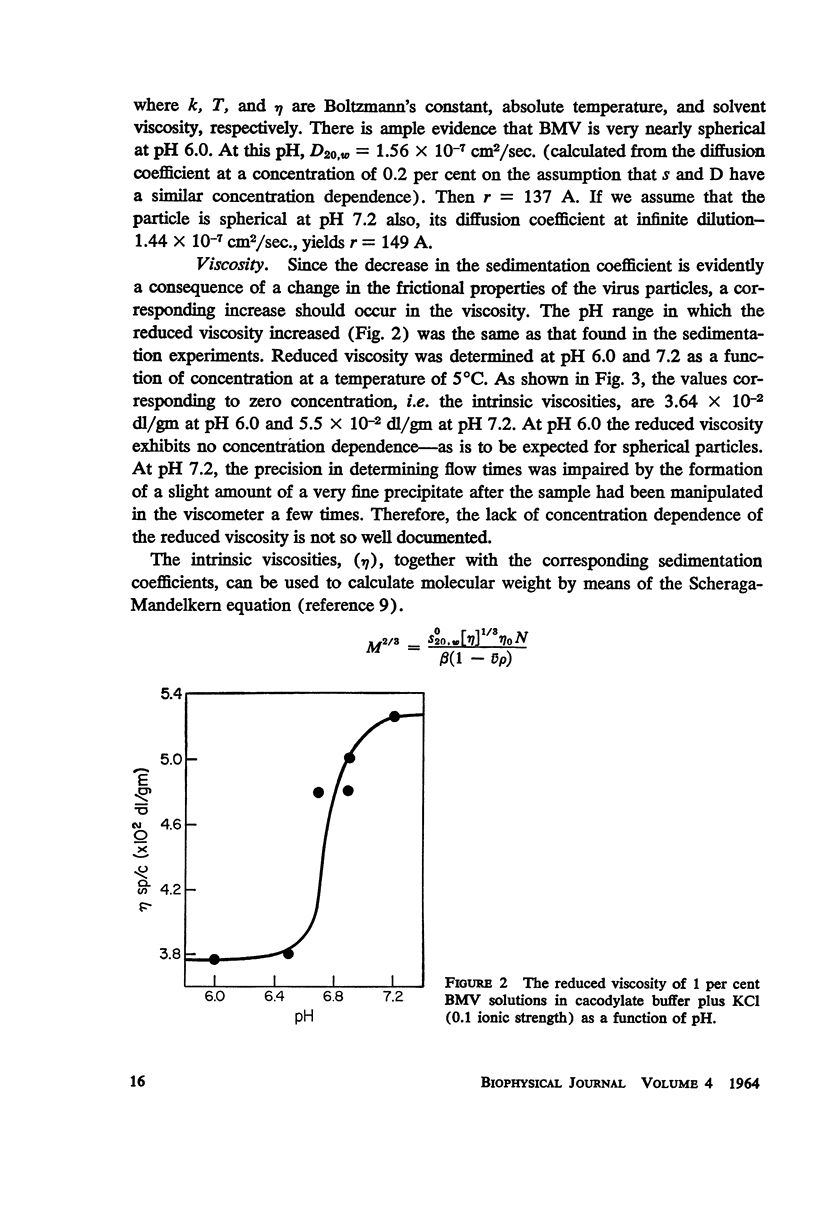
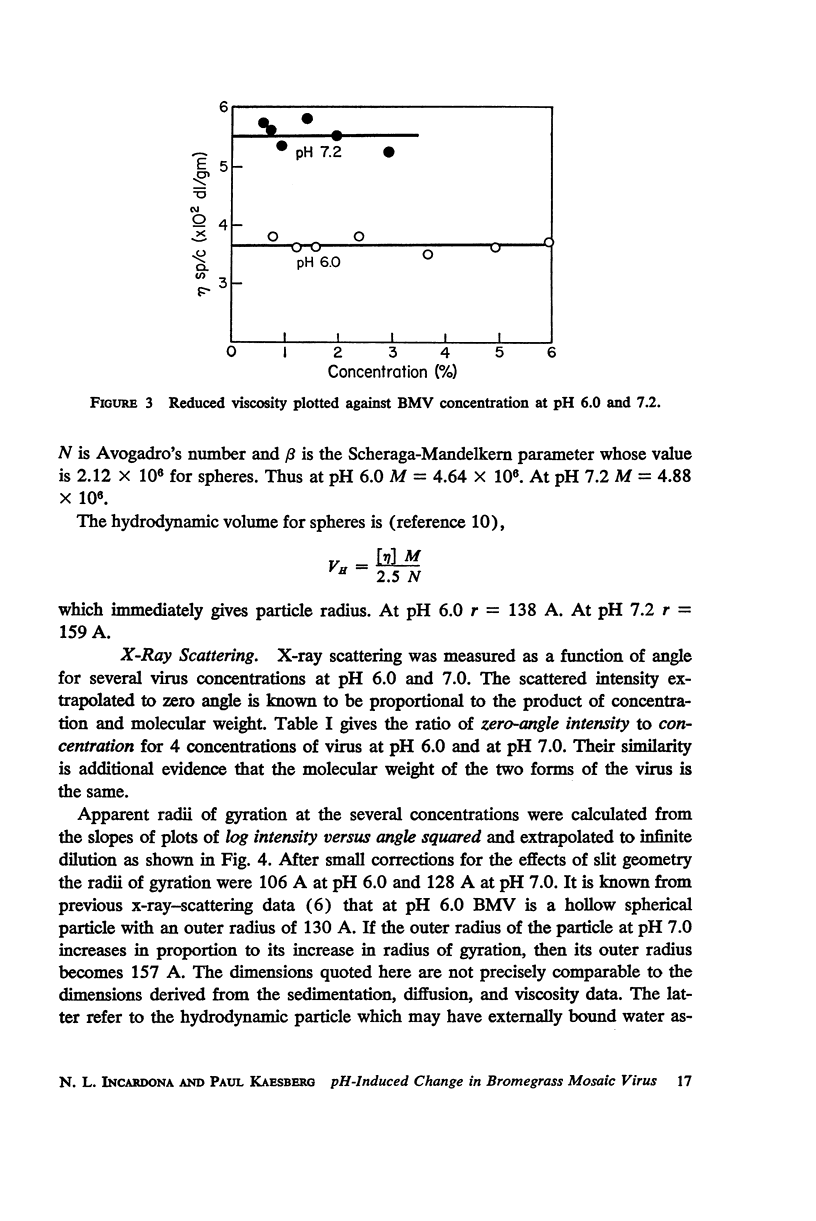
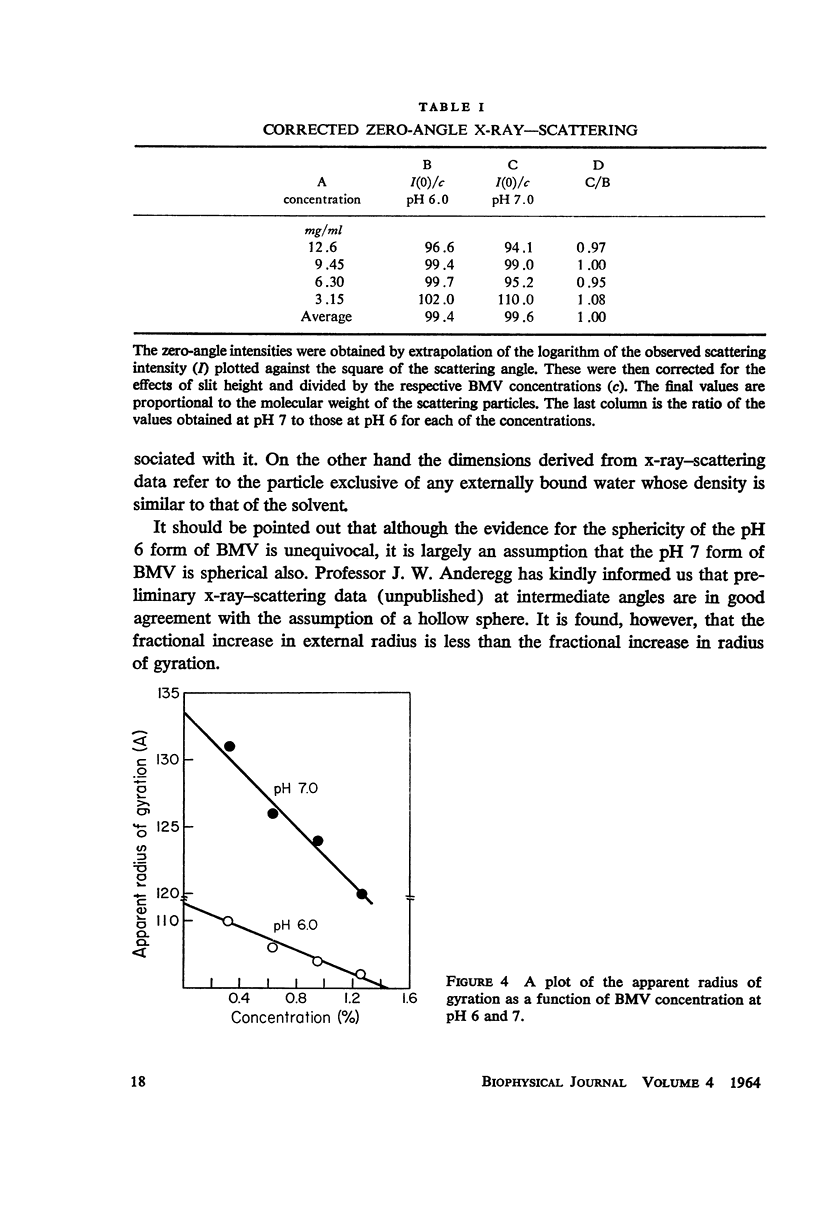
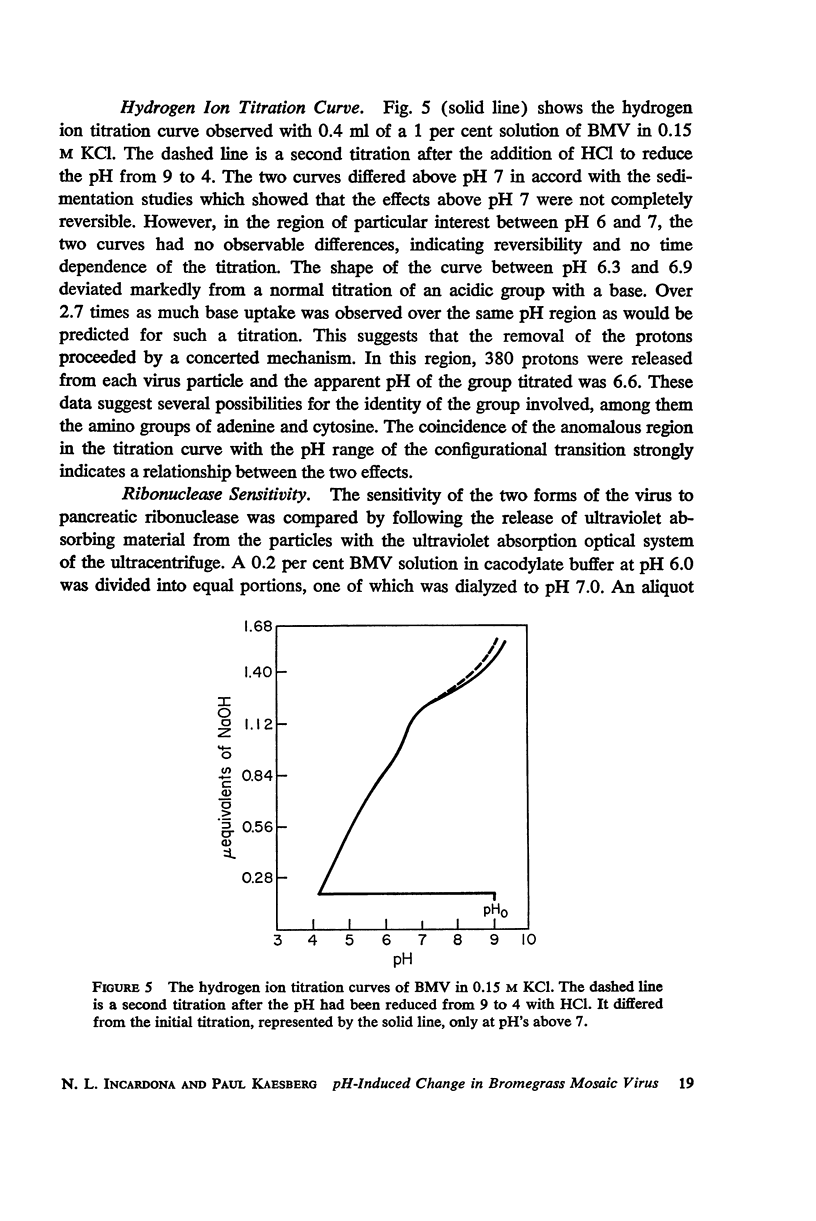
Bromegrass mosaic virus undergoes a reversible decrease in its sedimentation coefficient when the pH is raised above pH 6.7. At pH 6 the sedimentation coefficient is 87 S, at pH 7 it is 79 S. Intrinsic viscosities determined at pH 6 and 7 are 3.64 and 5.5 × 10-2 dl/gm. Diffusion coefficients are 1.56 × 10-7 cm2/sec. and 1.44 × 10-7 cm2/sec., respectively. Radii of gyration, measured by x-ray scattering, are 106 and 128 A. However, appropriate combination of sedimentation, diffusion, and viscosity coefficients at pH 6 and 7 yield the same molecular weight. Also, the zero-angle value of x-ray-scattered intensity, which is a function of molecular weight, is the same at the two pH's. These results suggest that bromegrass mosaic virus particles undergo a pH-induced change in structure. This change causes, among other things, an increase in the susceptibility of the particles to degradation by pancreatic ribonuclease. The shape of the titration curve between pH 6.3 and 6.9 is anomalous.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockstahler L. E., Kaesberg P. The Molecular Weight and Other Biophysical Properties of Bromegrass Mosaic Virus. Biophys J. 1962 Jan;2(1):1–9. doi: 10.1016/s0006-3495(62)86836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINGS D. J., KOZLOFF L. M. Biophysical properties of bacteriophage T2. Biochim Biophys Acta. 1960 Nov 18;44:445–458. doi: 10.1016/0006-3002(60)91599-7. [DOI] [PubMed] [Google Scholar]
- FUJIMURA R., KAESBERG P. The adsorption of bacteriophage phi-X174 to its host. Biophys J. 1962 Nov;2:433–449. doi: 10.1016/s0006-3495(62)86866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARD W. J., Jr, FOSTER J. F. Changes in optical rotation in the acid transformations of plasma albumin. Evidence for the contribution of tertiary structure to rotatory behavior. J Biol Chem. 1961 Oct;236:2662–2669. [PubMed] [Google Scholar]


