Abstract
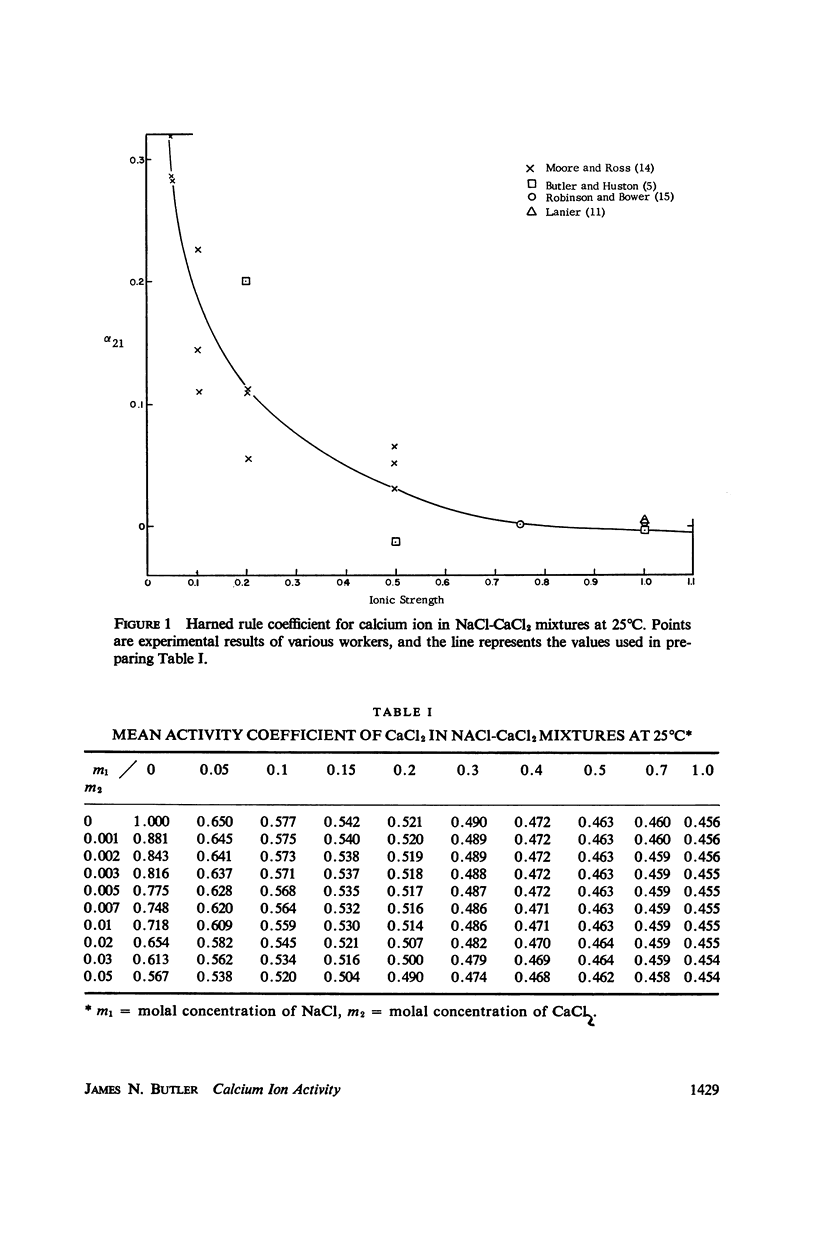
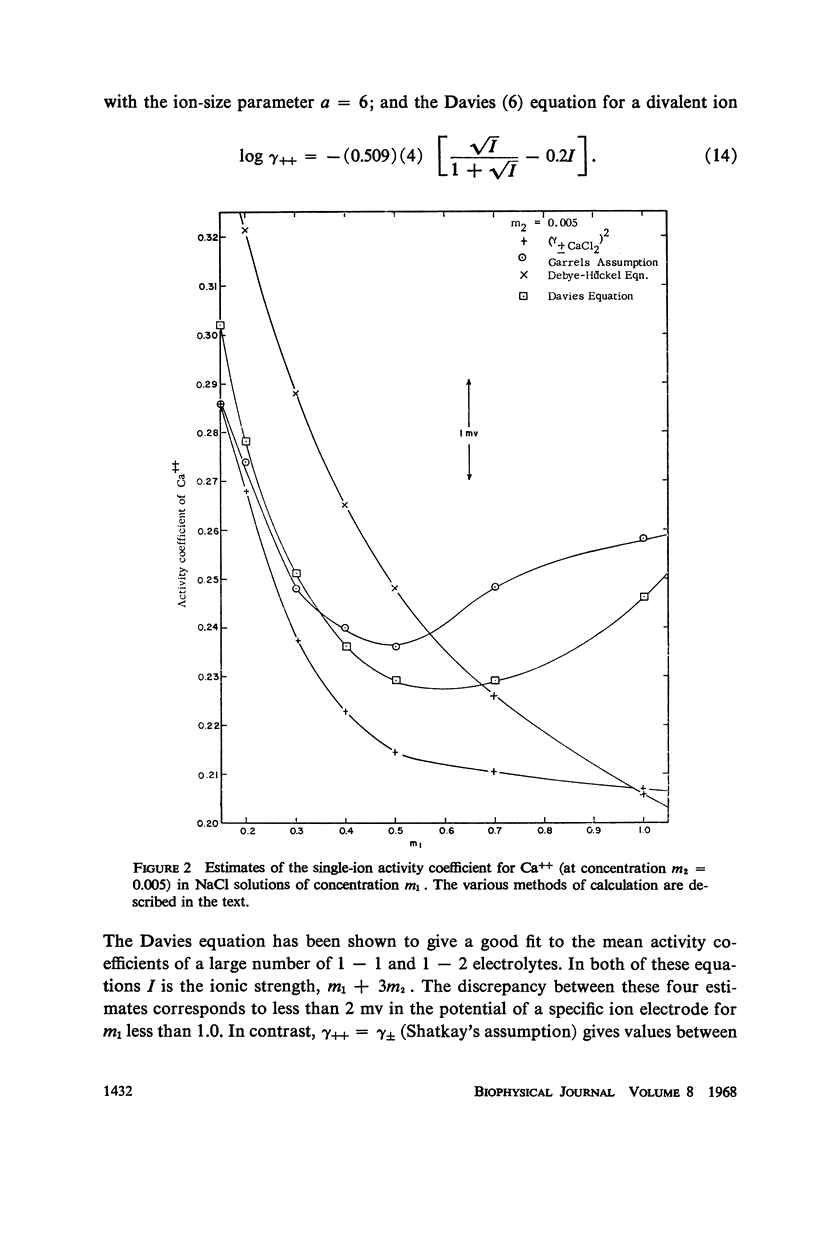
Experimental data on the mean activity coefficient of CaCl2 in NaCl-CaCl2 mixtures at ionic strengths below 1 m have been used to prepare a table of activity coefficients for Ca++ in solutions of physiological interest. The establishment of an empirical calcium ion activity scale is discussed, and a number of possible assumptions are examined. The assumption γ++ = (γ±)2 is suggested as being the simplest with a theoretical basis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETT I. M., FRASER G. P. A rapid micromethod for determining serum calcium. Clin Chim Acta. 1959 May;4(3):346–356. doi: 10.1016/0009-8981(59)90101-9. [DOI] [PubMed] [Google Scholar]
- ETTORI J., SCOGGAN S. M. Metal ions in biological systems; a determination of pM by spectrophotometry of metal indicators at two wavelengths. Arch Biochem Biophys. 1958 Nov;78(1):213–220. doi: 10.1016/0003-9861(58)90330-8. [DOI] [PubMed] [Google Scholar]
- MACINTYRE I. The flame-spectrophotometric determination of calcium in biological fluids and an isotopic analysis of the errors in the Kramer-Tisdall procedure. Biochem J. 1957 Sep;67(1):164–172. doi: 10.1042/bj0670164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCLORRY R. G. Serum calcium determination; a review and discussion of methods. Can J Med Technol. 1958 Sep;20(3):90–97. [PubMed] [Google Scholar]
- Ross J. W. Calcium-selective electrode with liquid ion exchanger. Science. 1967 Jun 9;156(3780):1378–1379. doi: 10.1126/science.156.3780.1378. [DOI] [PubMed] [Google Scholar]
- SCHIRARDIN H., METAIS P. [A simple method for the determination of plasma calcium fractions]. Ann Biol Clin (Paris) 1959 Jul-Sep;17:465–474. [PubMed] [Google Scholar]
- Shatkay A. Activity coefficients of calcium ions in mixed solutions. J Phys Chem. 1967 Nov;71(12):3858–3861. doi: 10.1021/j100871a021. [DOI] [PubMed] [Google Scholar]


