Abstract
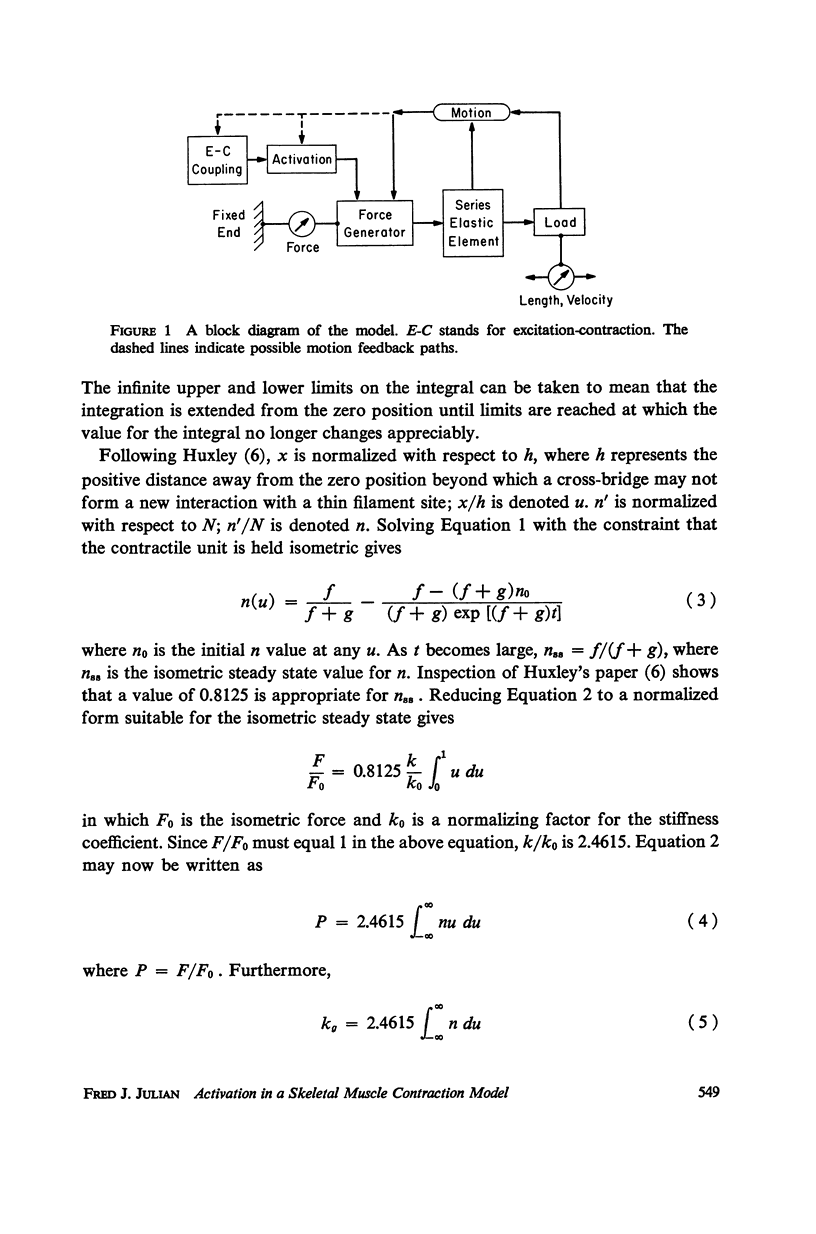
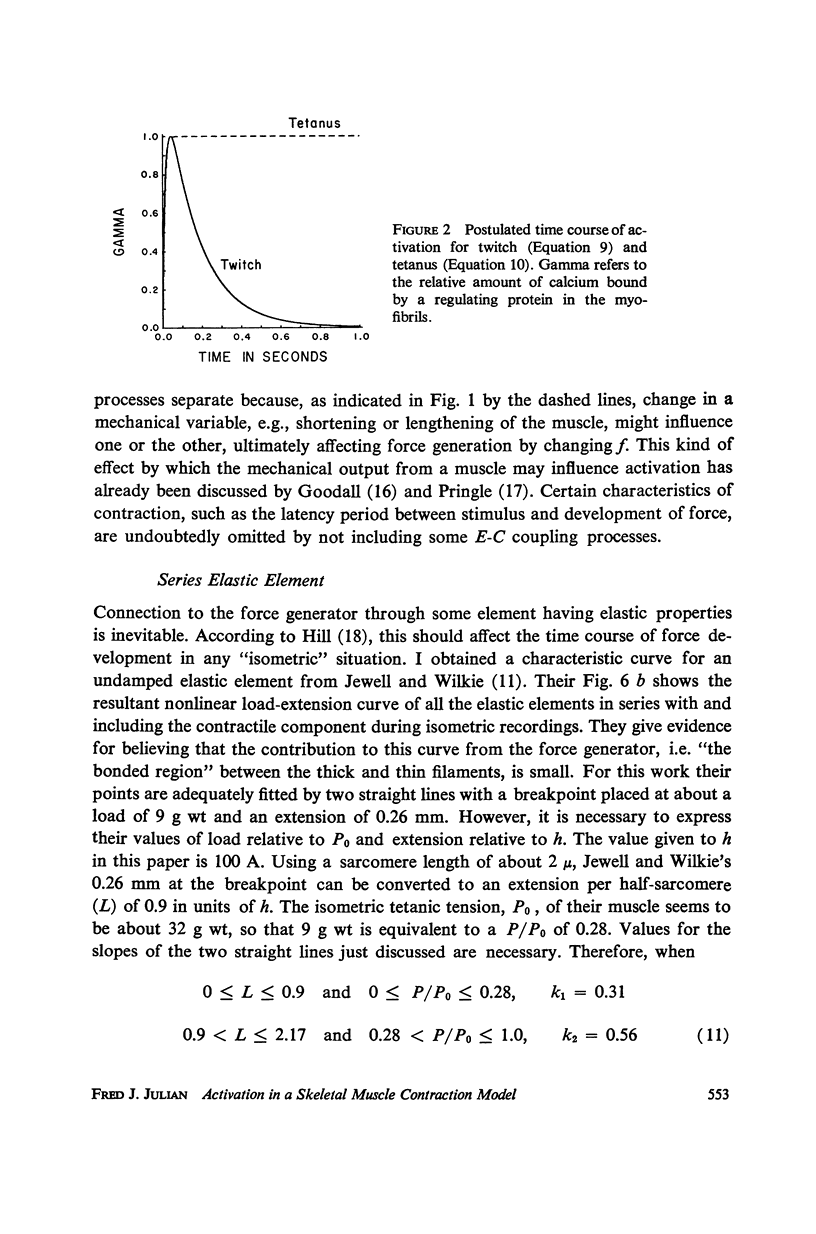
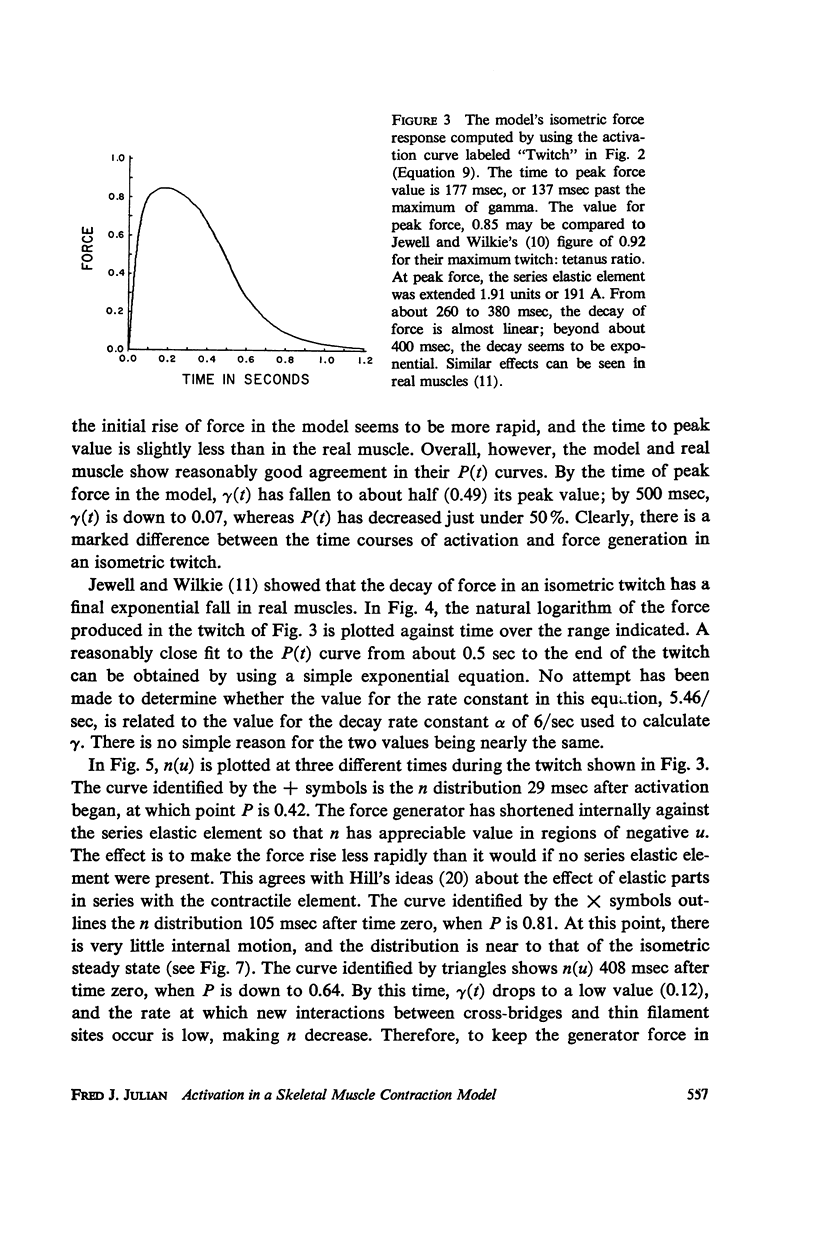
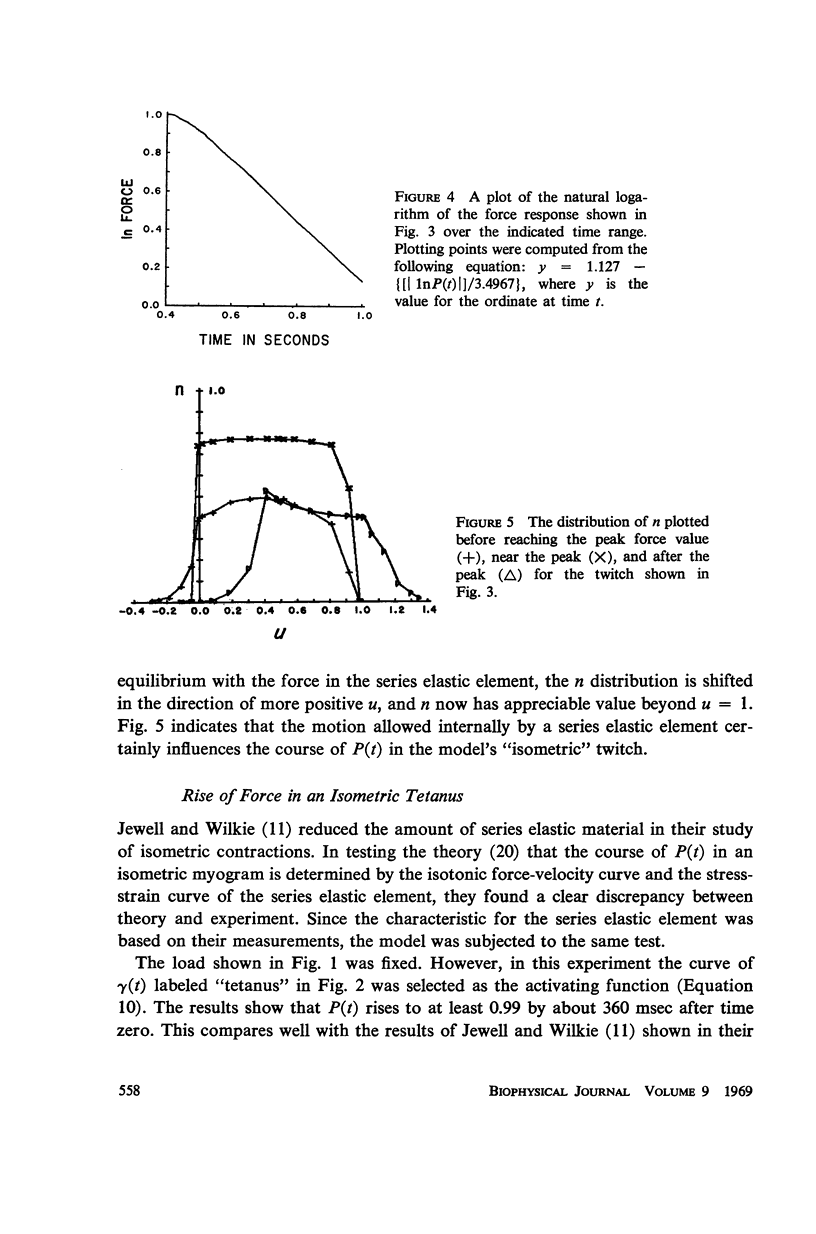
A sliding filament model for muscle contraction is extended by including an activation mechanism based on the hypothesis that the binding of calcium by a regulating protein in the myofibrils must occur before the rate constant governing the making of interactions between cross-bridges and thin filament sites can take on nonzero values. The magnitude of the rate constant is proportional to the amount of bound calcium. The model's isometric twitch and rise of force in an isometric tetanus are similar to the curves produced by real muscles. It redevelops force after a quick release in an isometric tetanus faster than the initial rise. Quick release experiments on the model during an isometric twitch show that the “active state” curve produced is different from the postulated calcium binding curve. The force developed by the model can be increased by a small quick stretch delivered soon after activation to values near the maximum generated in an isometric tetanus. Following the quick stretch, the force remains near the tetanic maximum for a long time even though the calcium binding curve rises to a peak and subsequently decays by about 50%. The model satisfies the constraint of shortening with a constant velocity under a constant load. Modifications can be made in the model so that it produces the delayed force changes following step length changes characteristic of insect fibrillar muscle.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLOSE R. The pattern of activation in the sartorius muscle of the frog. J Gen Physiol. 1962 Sep;46:1–18. doi: 10.1085/jgp.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES R. E. A MOLECULAR THEORY OF MUSCLE CONTRACTION: CALCIUM-DEPENDENT CONTRACTIONS WITH HYDROGEN BOND FORMATION PLUS ATP-DEPENDENT EXTENSIONS OF PART OF THE MYOSIN-ACTIN CROSS-BRIDGES. Nature. 1963 Sep 14;199:1068–1074. doi: 10.1038/1991068a0. [DOI] [PubMed] [Google Scholar]
- EBASHI S. THIRD COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF 'NATURAL ACTOMYOSIN'. Nature. 1963 Dec 7;200:1010–1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Ebashi F., Kodama A. Troponin as the Ca++-receptive protein in the contractile system. J Biochem. 1967 Jul;62(1):137–138. doi: 10.1093/oxfordjournals.jbchem.a128628. [DOI] [PubMed] [Google Scholar]
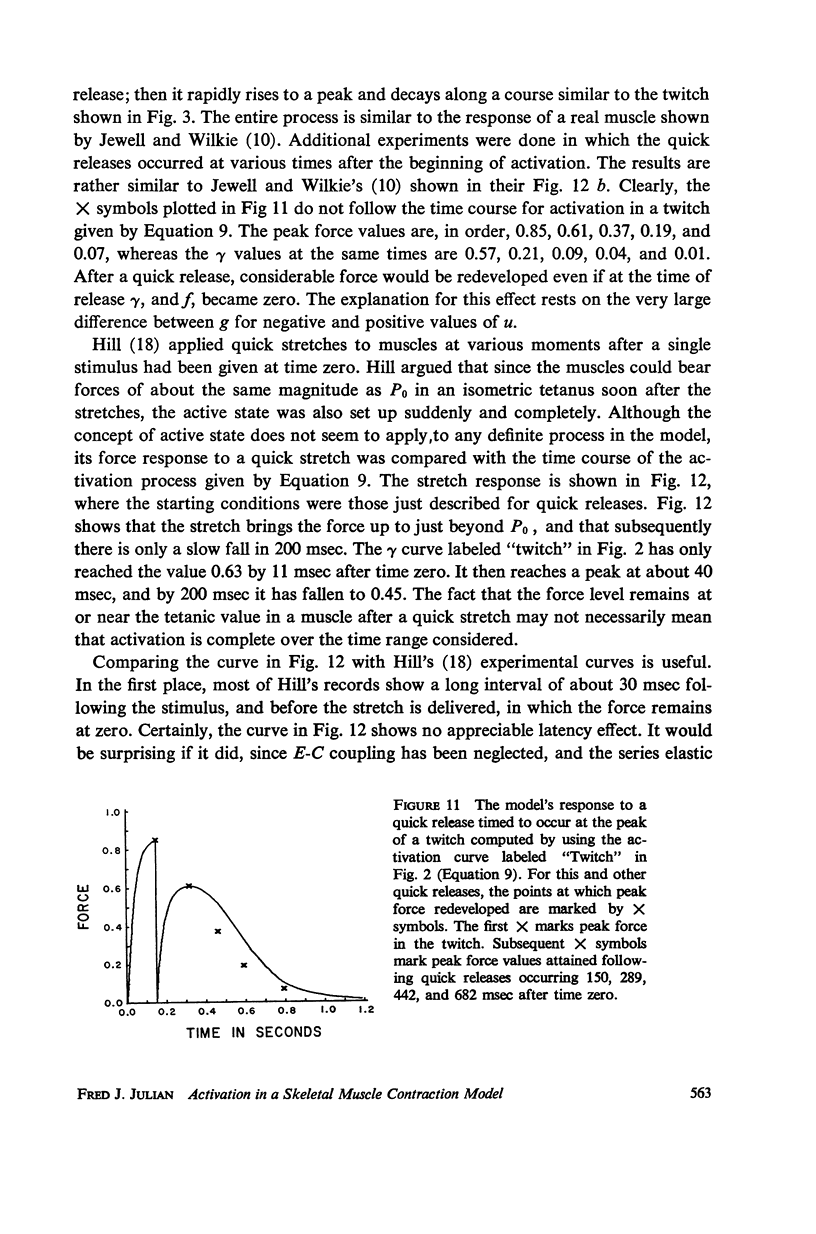
- FENN W. O., GILBERT D. L. Calcium equilibrium in muscle. J Gen Physiol. 1957 Jan 20;40(3):393–408. doi: 10.1085/jgp.40.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
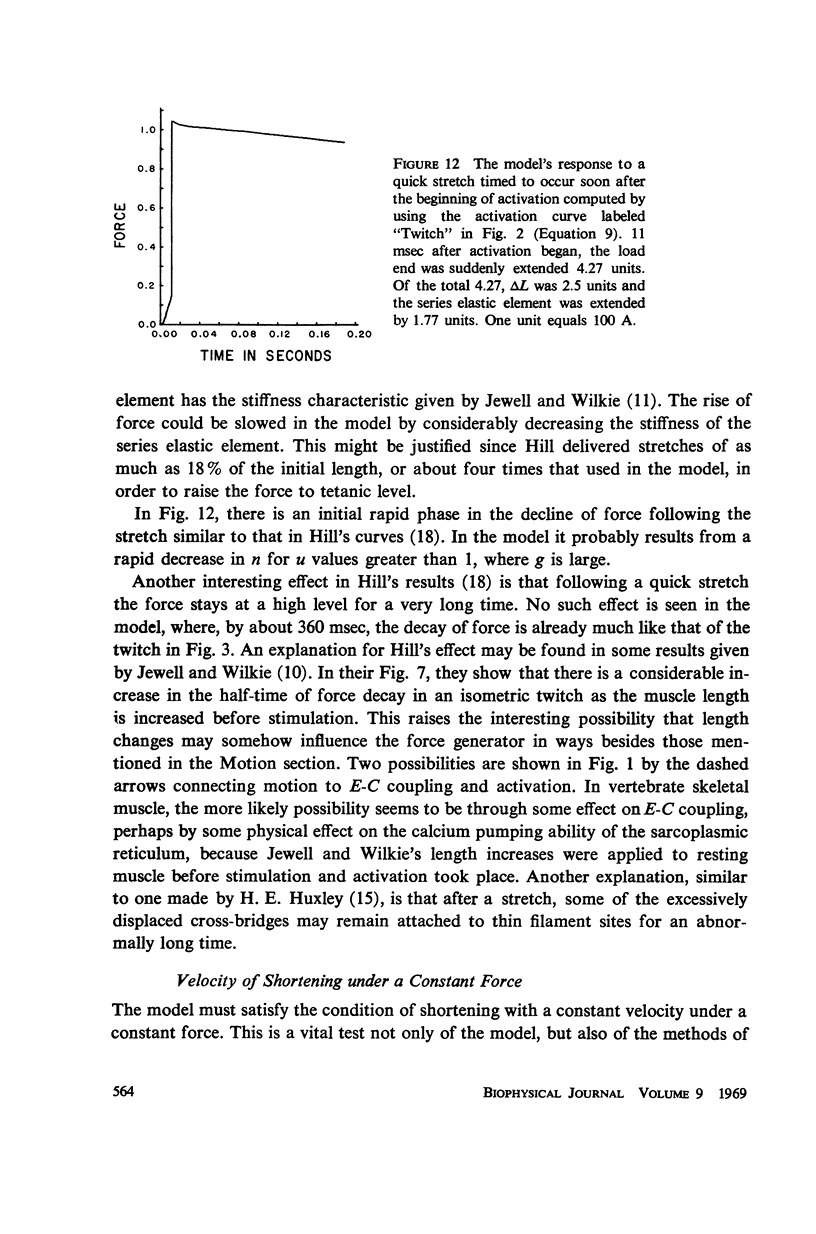
- Fuchs F., Briggs F. N. The site of calcium binding in relation to the activation of myofibrillar contraction. J Gen Physiol. 1968 May;51(5):655–676. doi: 10.1085/jgp.51.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
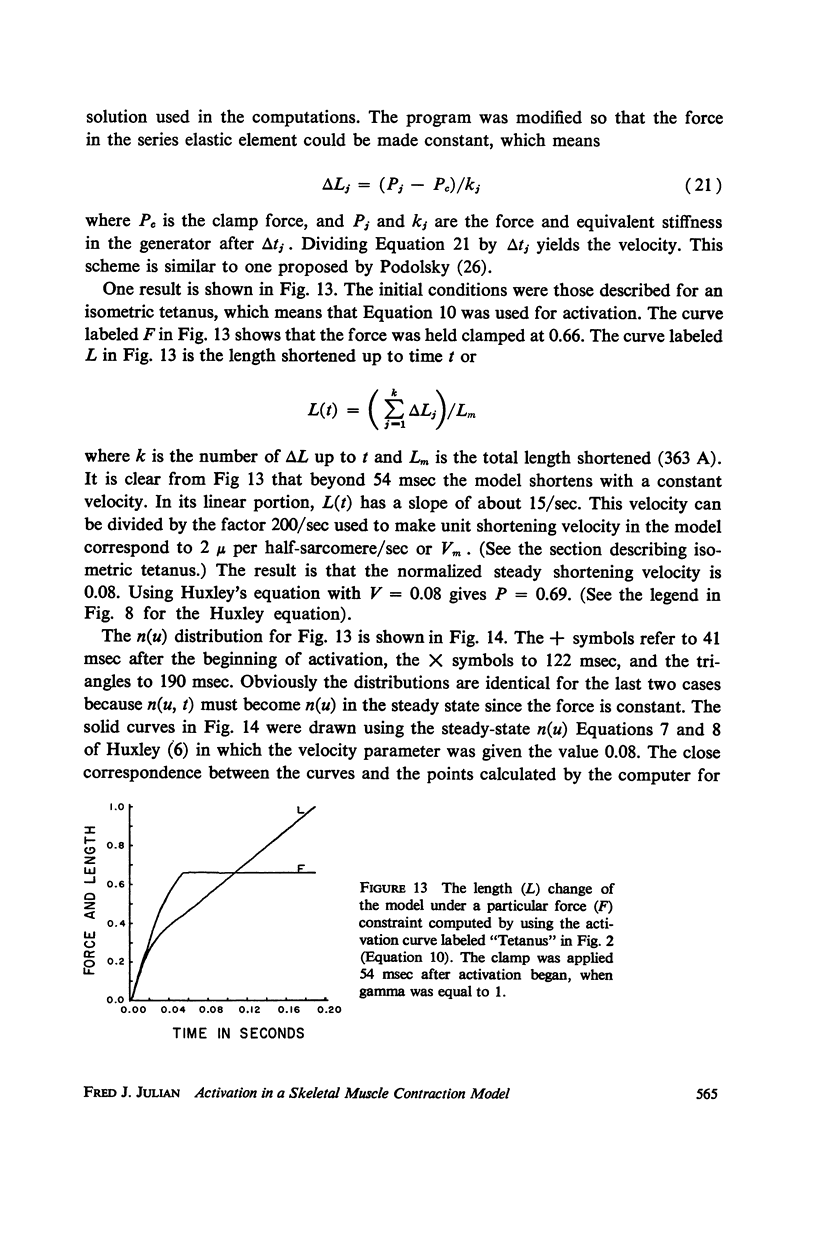
- GOODALL M. C. Kinetics of muscular contraction. I. Yale J Biol Med. 1957 Dec;30(3):224–243. [PMC free article] [PubMed] [Google Scholar]
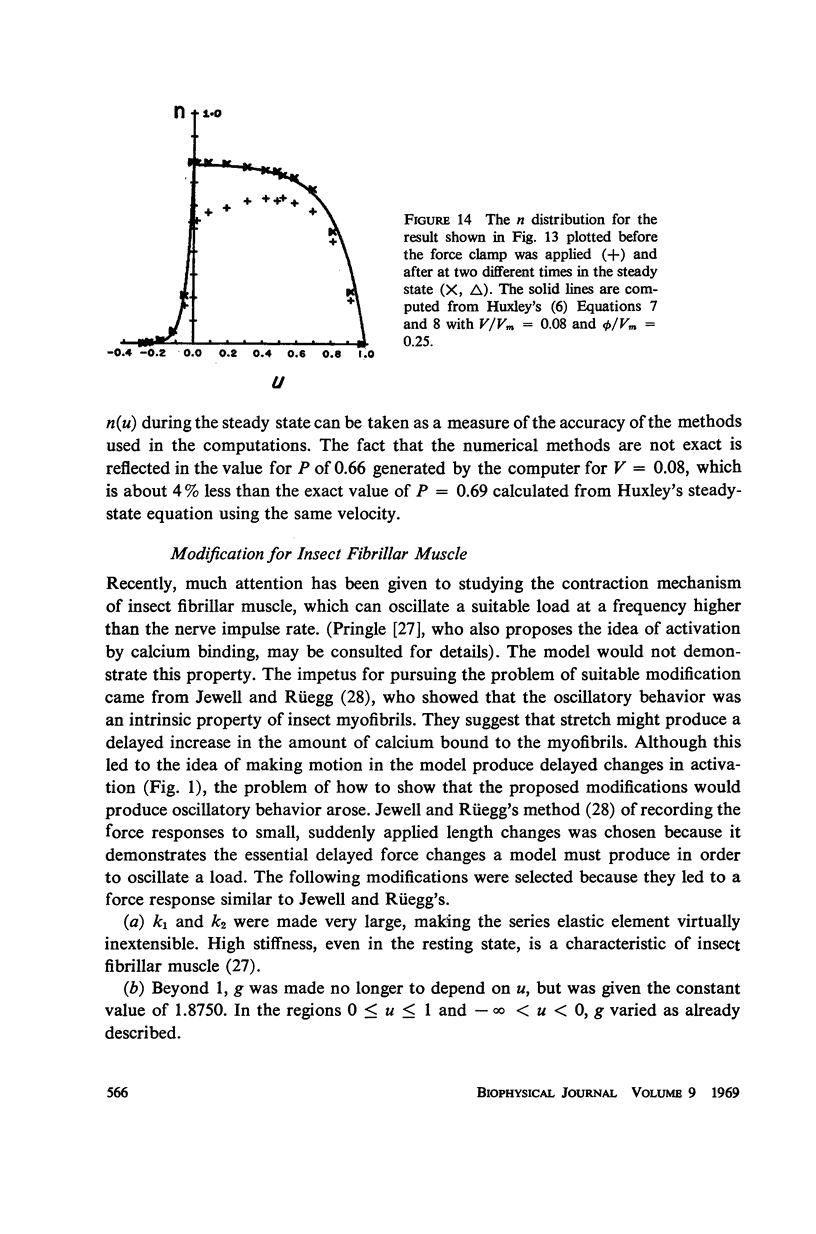
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V. The mechanics of active muscle. Proc R Soc Lond B Biol Sci. 1953 Mar 11;141(902):104–117. doi: 10.1098/rspb.1953.0027. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. The mechanical properties of relaxing muscle. J Physiol. 1960 Jun;152:30–47. doi: 10.1113/jphysiol.1960.sp006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PODOLSKY R. J. Kinetics of muscular contraction: the approach to the steady state. Nature. 1960 Nov 19;188:666–668. doi: 10.1038/188666a0. [DOI] [PubMed] [Google Scholar]
- PODOLSKY R. J. Mechanochemical basis of muscular contraction. Fed Proc. 1962 Nov-Dec;21:964–974. [PubMed] [Google Scholar]
- PRINGLE J. W. Models of muscle. Symp Soc Exp Biol. 1960;14:41–68. [PubMed] [Google Scholar]
- Pringle J. W. The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol. 1967;17:1–60. doi: 10.1016/0079-6107(67)90003-x. [DOI] [PubMed] [Google Scholar]
- RITCHE J. M. The duration of the plateau of full activity in frog muscle. J Physiol. 1954 Jun 28;124(3):605–612. doi: 10.1113/jphysiol.1954.sp005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E. B., Ashley C. C. Calcium transients in single muscle fibers. Biochem Biophys Res Commun. 1967 Oct 26;29(2):229–234. doi: 10.1016/0006-291x(67)90592-x. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]


