Abstract
As most human immunodeficiency virus (HIV) infection occurs via mucosal surfaces, an important goal of vaccination may be the induction of virus-specific immune responses at mucosal sites to contain viral infection early on. Here we designed a study in macaques carrying the major histocompatibility complex class I Mamu-A∗01 molecule to assess the capacity of the highly attenuated poxvirus NYVAC/simian immunodeficiency virus (SIV) SIVgpe vaccine candidate administered by the intranasal, intramuscular, or intrarectal route to induce mucosal immunity. All macaques, including one naive macaque, were exposed to SIVmac251 by the intrarectal route and sacrificed 48 h after infection. The kinetics of immune response at various time points following immunization with NYVAC/SIVgpe and the anamnestic response to SIVmac251 at 48 h after challenge were assessed in blood, in serial rectal and vaginal biopsy samples, and in tissues at euthanasia with an SIVmac Gag-specific tetramer. In addition, at euthanasia, antigen-specific cells producing gamma interferon or tumor necrosis factor alpha from the jejunum lamina propria were quantified in all macaques. Surprisingly, antigen-specific CD8+ T cells were found in the mucosal tissues of all immunized macaques regardless of whether the vaccine was administered by a mucosal route (intranasal or intrarectal) or systemically. In addition, following mucosal SIVmac251 challenge, antigen-specific responses were mainly confined to mucosal tissues, again regardless of the route of immunization. We conclude that immunization with a live vector vaccine results in the appearance of CD8+ T-cell responses at mucosal sites even when the vaccine is delivered by nonmucosal routes.
Transmission of human immunodeficiency virus (HIV) occurs predominantly via mucosal surfaces. Effective neutralization of the virus at this anatomic site depends on several components. Preexisting virus-specific immunoglobulin A (IgA) antibodies in rectal secretions are associated with decreased viral burden but are not sufficient to prevent infection (52). HIV-1/simian immunodeficiency virus (SIV)-specific CD8+ cytotoxic T lymphocytes are another such component and have been shown to be present during the early weeks following infection, before a neutralizing antibody response is demonstrable (8, 26, 27). Such cell-mediated immunity (40) and direct killing by cytotoxic lymphocytes from the vagina and colon lamina propria may be an important factor in containing viral infection at the site of primary infection (35), and it may explain the fact that, despite the rapid heterosexual dissemination of HIV-1 in the world, mucosal transmission of HIV-1 appears to be relatively inefficient and is estimated to occur only once in 300 or more high-risk exposures (47).
While many studies have investigated the effect of the route of immunization on antibody production at the local or systemic level, few have directly investigated the induction of cell-mediated responses at mucosal sites. Studies in mice immunized with synthetic, multideterminant HIV peptides plus cholera toxin adjuvant have shown that intrarectal immunization induces cytotoxic T lymphocyte responses in Peyer's patches, the spleen, and lamina propria of the intestine (4) and that these responses were able to reduce the viral titer in the ovaries and the colorectal tissue upon intrarectal recombinant vaccinia virus challenge. In contrast, subcutaneous immunization induced cytotoxic T lymphocytes only in the spleen and not in the Peyer's patches or lamina propria of the intestinal tract and was not able to reduce the viral titers (4).
Similar results were obtained with an modified vaccinia virus Ankara-based recombinant expressing the simian-human immunodeficiency virus (SHIV) strain SHIV89.6 gp160 (6), and more recently, it has been demonstrated that mucosal immunization of macaques afforded better protection than subcutaneous immunization following intrarectal exposure to SHIVku2 (5). These studies suggest that cutaneous or intramuscular immunization generates antigen-specific cells that may not travel to mucosal sites (3, 11, 22, 32, 49).
A somewhat different conclusion was suggested by studies in which macaques were immunized intramuscularly with the highly attenuated poxvirus vector NYVAC encoding the SIVmak6w Gag, Pol, and Env products (7, 19). In this case immunization resulted in long-term viremia containment following intravenous or intrarectal challenge with SIVmac251 (7, 18a). Since, in these studies, all macaques were immunized by the intramuscular route, the results suggested that even in the absence of priming at mucosal sites, systemic immunization can lead to virological control following SIVmac mucosal challenge exposure (7, 18a, 19).
To further explore these results, we designed a study to directly assess the extent of mucosal immune responses to a Gag immunodominant SIVmac251 epitope following immunization of macaques by either the intramuscular, intranasal, or intrarectal route. Accordingly, two female macaques expressing the Mamu-A∗01 major histocompatibility complex class I molecule were used for each route of immunization, and the extent and kinetics of the immune response following both immunization and SIV challenge were measured in the blood and in serial mucosal biopsy samples with Gag-specific tetramer-staining reagents (1). Importantly, the size and specificity of the anamnestic virus-specific CD8+ T-cell responses to the virus were investigated within 48 h of a SIVmac251 intrarectal challenge exposure to determine whether it differed in various anatomical compartments according to the route of immunization.
MATERIALS AND METHODS
Animals and procedures.
Seven Mamu-A∗01-positive macaques were involved in this study. Animals 536 and 815 were immunized with three inoculations of 108 PFU of NYVAC/SIVgpe by the intramuscular route. Animals 582 and 816 were immunized intranasally and animals 814 and 818 were immunized intrarectally with three inoculations of 5 × 108 PFU of NYVAC/SIVgpe each. The first boost was given 1 month after the first immunization, and the second boost was given 3 months after the second immunization. All six immunized macaques and a control naive macaque (no. 654) were challenged intrarectally with 30 mucosal infectious doses of the SIVmac251(561) isolate (37) and sacrificed 48 h after infection.
Blood samples were collected at several time points during the immunization schedule as well as on the day of SIV exposure and sacrifice. Similarly, multiple vaginal and rectal pinch biopsy samples were taken prior to and during the immunization course.
At the time of sacrifice, blood, spleen, mesenteric, iliac, and pararectal lymph nodes, vagina, cervix, jejunum, colon, and rectum samples were collected. The tissues were placed in RPMI supplemented with 10% bovine serum and penicillin/streptomycin. Rectal tissue was also placed in phosphate-buffered saline (PBS)-bovine serum albumin solution and used for in situ tetramer staining.
Isolation of tissue lymphocytes.
Mononuclear cells were isolated from peripheral blood mononuclear cells, lymph nodes, spleen, intestines, vagina, and cervix. Mononuclear cells from spleen and lymph nodes were isolated by mechanical dissociation of the tissue and consecutive Ficoll gradient centrifugation. Tissues from jejunum, colon, rectum, vagina, and cervix were isolated by a modification of a previously published method (9, 21). Tissues were treated with 1 mM dithiothreitol (ICN Biomedicals Inc., Aurora, Ohio) for 30 min, followed by incubation in calcium- and magnesium-free Hanks' balanced salt solution (Life Technologies, Baltimore, Md.) three (intestines) to four (vagina and cervix) times for 1 h with stirring at room temperature to remove the epithelial layer. At this stage, pieces of tissue were fixed in 10% neutral formalin and embedded in paraffin, and sections were cut and stained with hematoxylin and eosin. Microscopic examination was performed to ensure that all of the epithelium was removed and the lamina propria was intact.
Intraepithelial lymphocytes were then purified by Percoll gradient density centrifugation.
Tissue sections were cut into smaller pieces and incubated at 37°C in Iscove's medium supplemented with 10% fetal calf serum and penicillin/streptomycin containing 400 U of collagenase D (Boehringer GmbH, Mannheim, Germany) and 25 U of DNase (Worthington Biochemical Corporation, Lakewood, N.J.) per ml for 2 to 3 h. Vaginal and cervical tissues were digested with 0.5 mg of collagenase type IV (Sigma Chemical, St. Louis, Mo.) (50). The mononuclear cells were isolated from the supernatant containing dissociated cells by Percoll gradient centrifugation.
Flow cytometry.
Fresh cells were directly stained with phycoerythrin-conjugated tetrameric complexes folded with the immunodominant Gag181-189 CM9 (p11c) peptide. Fluorescein isothiocyanate-conjugated anti-CD3Ε (Pharmingen, San Diego, Calif.) and peridinin chlorophyll protein (PerCP)-conjugated anti-CD8 (Becton Dickinson, San Jose, Calif.) were used in conjunction with the tetrameric complex.
As confirmation of results obtained from freshly isolated lymphocytes, lymphocytes were also cultured at a concentration of 3 × 106/ml in RPMI enriched with 10% human serum, with addition of 1 μg of the appropriate peptide and 20 U of interleukin-2 per ml for 7 days (data not shown). Staining with tetrameric complexes was done afterward as described above. Staining with unrelated tetramer and of cells isolated from Mamu-A∗01-negative or naive animals was used as a negative control. Staining with phycoerythrin-CD4 (Becton Dickinson) was used as a positive control.
In addition, cells were stained with fluorescein isothiocyanate-conjugated activation markers CD25, CD69, and HLA-DR. Briefly, 5 × 105 lymphocytes isolated by Ficoll diatrizoate or Percoll gradient centrifugation were incubated with 2 μg of tetrameric complexes and/or selected antibodies for 30 min at room temperature. After washing the cells twice in Dulbecco's phosphate-buffered saline supplemented with 2% fetal calf serum and fixation in 1% paraformaldehyde (pH 7.4), samples were analyzed by flow cytometry with CellQuest and the FACScalibur (Becton Dickinson) instrument.
Tetramer staining in situ.
In situ tetramer staining was performed on fresh tissues as previously described with some modifications (17, 48). Briefly, tissues were collected by pinch biopsy, washed in cold PBS, and cut into small strips. The resulting sections (n = 4 to 6) were then incubated with 10 μl of antigen-specific tetramer labeled with indocarbocyanine (Amersham, Piscataway, N.J.) per section and gently agitated at 37°C for 15 min. The tissue was then rinsed repeatedly at 37°C with PBS and then twice with ice-cold PBS prior to fixation with cold 2% paraformaldehyde for 20 min.
After additional washes, antibodies to CD3 (rabbit polyclonal; Dako) and CD8 (directly conjugated to fluorescein isothiocyanate; Becton Dickinson) were applied singly or together for 1 h. The tissues were then washed and incubated with anti-rabbit immunoglobulin-Alexa 488 (Molecular Probes) when using only CD3 or anti-rabbit immunoglobulin-Alexa 568 when using both CD3 and CD8. Tissues were then washed, mounted on a glass slide with antifading medium (Vectashield; Vector Laboratories, Inc., Burlingame, Calif.), and examined by confocal microscopy.
Confocal microscopy was performed with a Leica TCS SP laser scanning microscope equipped with three lasers (Leica Microsystems, Exton, Pa.). Individual optical slices represent 0.2 μm, and 20 to 60 optical slices were collected at a 512- by 512-pixel resolution. The fluorescence of individual fluorochromes was captured separately in sequential mode after optimization to reduce bleedthrough between channels (photomultiplier tubes) with Leica software. NIH Image version 1.62 and Adobe Photoshop version 6 software were used to assign colors to each fluorochrome and the differential interference contrast image (gray scale). Colocalization of antigens was indicated by the addition of colors as indicated in the figure legends.
Intracellular cytokine staining.
Intracellular cytokine staining was done on jejunal lamina propria lymphocytes isolated at necropsy by a modified previously published method (16, 24, 53). Isolated and previously frozen lamina propria lymphocytes were thawed and washed. Viability was assessed by trypan blue dye exclusion, and viable cells were counted. Cells were incubated for 1 h at 37°C in 5% CO2 at a concentration of 106/ml in the presence of 1 μl of anti-CD28 (Becton Dickinson), 1 μl of anti-CD49d (Becton Dickinson), and 2 μg of the SIV Gag181-189 CM9 peptide per ml. Stimulation with 25 ng of phorbol myristate acetate (Sigma-Aldrich Corp., St. Louis, Mo.) and 1 μg of ionomycin (Sigma-Aldrich Corp.) per ml for gamma interferon (IFN-γ) or staphylococcal enterotoxin B (Toxin Technology, Inc.) for tumor necrosis factor alpha (TNF-α) was used as a positive control.
After 1 h of incubation, 10 μg of brefeldin A (Sigma-Aldrich Corp.) was added and cells were incubated for an additional 5 h. Cells were then washed twice with PBS-2% fetal calf serum and stained for surface antigens with fluorescein isothiocyanate-labeled anti-CD3 and PerCP-labeled anti-CD8. After incubating them at room temperature for 30 min and washing them twice with PBS-2% fetal calf serum, cells were fixed in Cytofix/Cytoperm (Pharmingen) for 20 min at 4°C. Following two washes with Wash/Perm buffer (Pharmingen), cells were incubated with phycoerythrin-conjugated anti-IFN-γ or allophycocyanin-conjugated anti-TNF-α antibodies for 30 min at 4°C. This was followed with two washes in Wash/Perm buffer and direct reading of the samples on the FACScalibur flow cytometer.
Cytotoxic T-lymphocyte assay.
Cytotoxic T-lymphocyte assays were performed as previously described (7). Fresh cells were cultured overnight in the presence of 300 IU of interleukin-2 per ml and then incubated at different effector-to-target cell ratios for 6 h with Mamu-A∗01-positive, 51Cr-labeled autologous transformed B cells pulsed overnight with 1 μg of a specific peptide per ml, without peptide, or with an unrelated peptide.
RESULTS
Induction of CD8+ T-cell response to Gag181-189 CM9 immunodominant peptide in blood and at mucosal sites by vaccination.
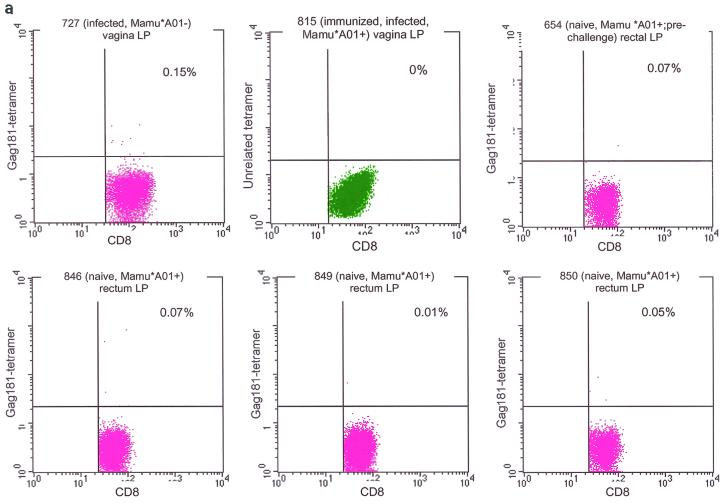
Two Mamu-A∗01-positive female macaques each received three immunizations with NYVAC/SIVgpe by either the intramuscular, intrarectal, or intranasal route. Virus-specific CD8+ T-cell response to the immunodominant Gag181-189 CM9 peptide in the blood and tissues of the immunized macaques was quantitated by staining CD3+ CD8+ T cells with the specific Mamu-A∗01/Gag181-189 CM9 tetramer after each immunization. The specificity of tetramer staining was assessed following each immunization in isolated lymphocytes from biopsy samples of both Mamu-A∗01-positive uninfected macaques and Mamu-A∗01-negative infected macaques. Staining with unrelated tetramer was also performed (Fig. 1a).
FIG. 1.
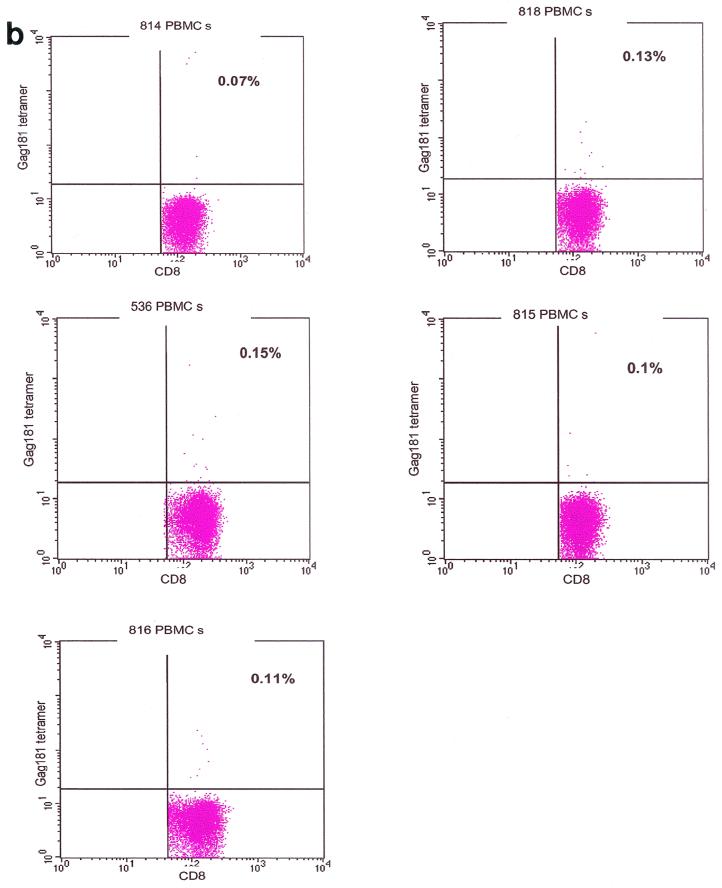
Control staining for tetramers. Staining of isolated lamina propria lymphocytes from vaginal and rectal biopsy samples obtained from infected Mamu-A∗01-negative and naive Mamu-A∗01-positive macaques and macaque 654 before viral challenge, and staining with unrelated tetramer on isolated vaginal and rectal lamina propria lymphocytes from immunized, infected Mamu-A∗01-positive macaques (a) was used to control for the specificity of the staining. Nonspecific binding accounted for up to 0.13% of the CD3+ CD8+ lymphocytes. Therefore, values above this percentage could be considered positive. Tetramer staining of peripheral blood mononuclear cells (PBMCs) isolated prior to immunization was negative in the five macaques examined (b).
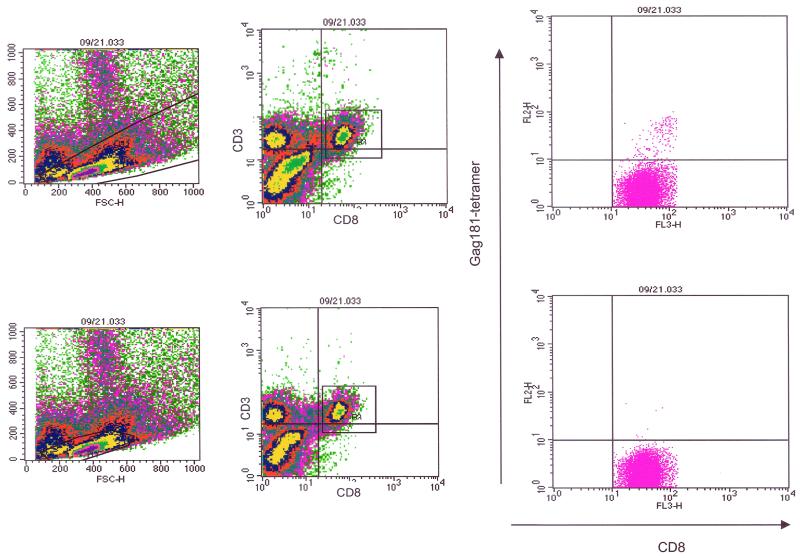
Staining of cells from tissue biopsy samples of a Mamu-A∗01-positive, SIVmac251-infected macaque revealed the presence of a population of tetramer-positive CD3+ CD8+ cells. Many, if not most, of those tetramer-positive cells were found to be larger (that is, to have a higher forward and side profile) than those which would normally be included in more conventional lymphocyte gating (Fig. 2) (2, 13, 30, 45). To explore the possibility that this population represents activated CD8 T cells, the same sample was stained with the activation markers CD69 and CD25. By gating on cells of larger size, we demonstrated that, in the tissues of this macaque, these large cells expressed both the CD8 and CD69 markers, suggesting that they might have been recently activated (data not shown). Therefore, a slightly enlarged gate as shown in Fig. 2, upper left panel, that included these cells was used for all further flow cytometric analyses of tetramer-positive cells in the tissues and blood of the immunized and control macaques.
FIG. 2.
Gating of tissue lymphocytes for flow cytometry analysis. Extending the lymphocyte gate to include larger cells revealed a population of CD3+ CD8+ tetramer-positive cells that was concealed when the conventional lymphocyte gate was used.
With these gates, the percentage of CD3+ CD8+ T cells staining with the Gag181-189 CM9 tetramer in the blood of the macaques immunized by the intranasal, intramuscular, and intrarectal routes was determined (top panels of Fig. 3a to d). These studies demonstrated the presence of tetramer-positive cells in the blood of all immunized animals and showed no significant difference among immunization routes. At the end of the immunization period, the percentage of CD3+ CD8+ T cells that were specifically recognized by the Gag-specific tetramer in blood was in the range expected with this vaccine modality (19). The cytotoxic T lymphocyte assay performed 2 weeks after the second immunization revealed specific lysis of Gag181-189 CM9-pulsed, Mamu-A∗01-positive, 51Cr-labeled autologously transformed B cells in some of the macaques (816 and 815) (data not shown).
FIG. 3.
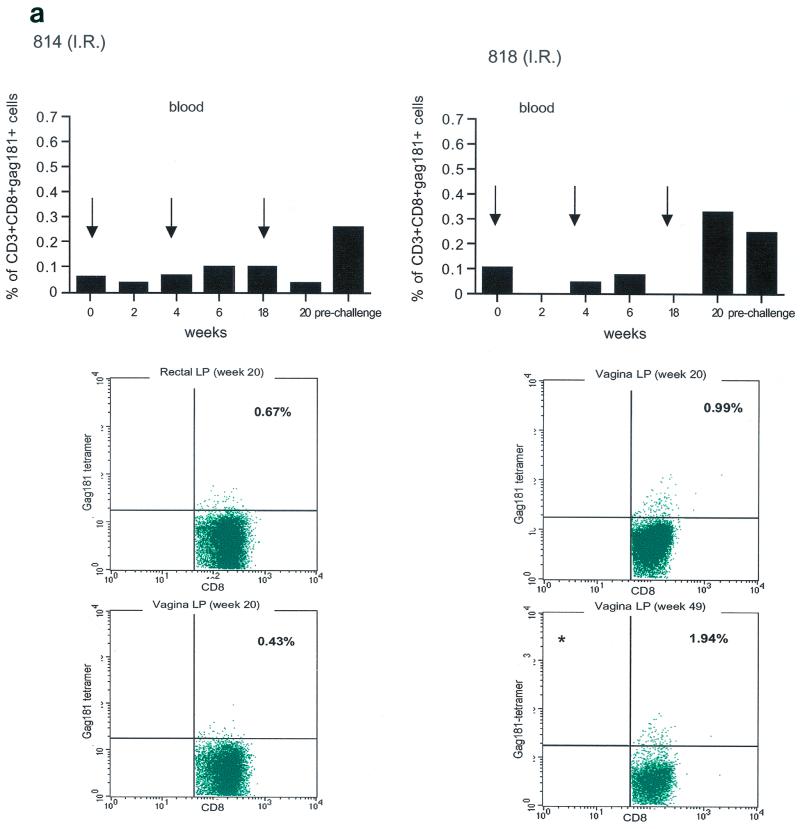
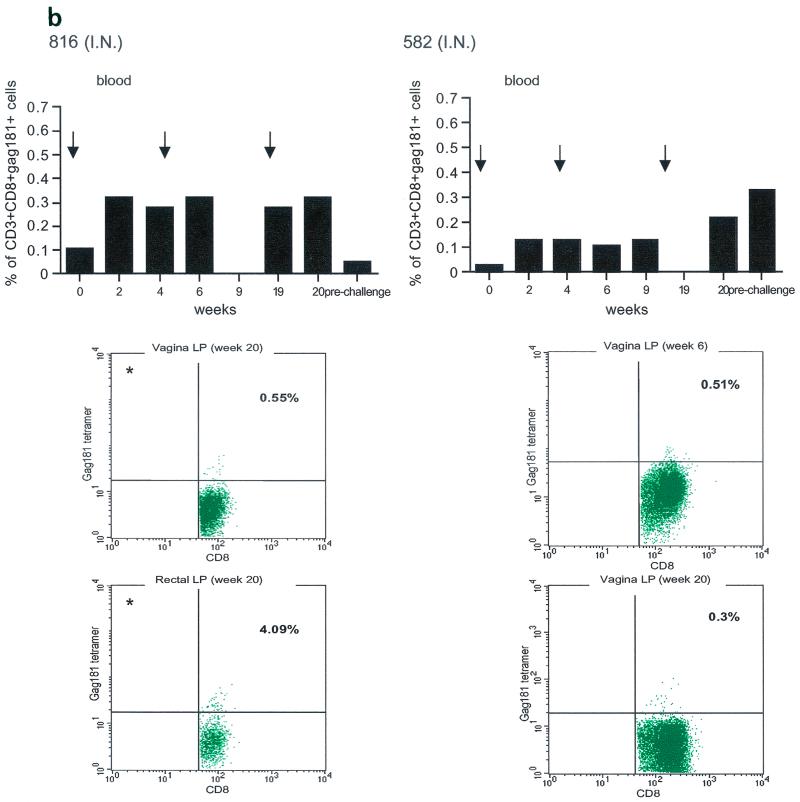
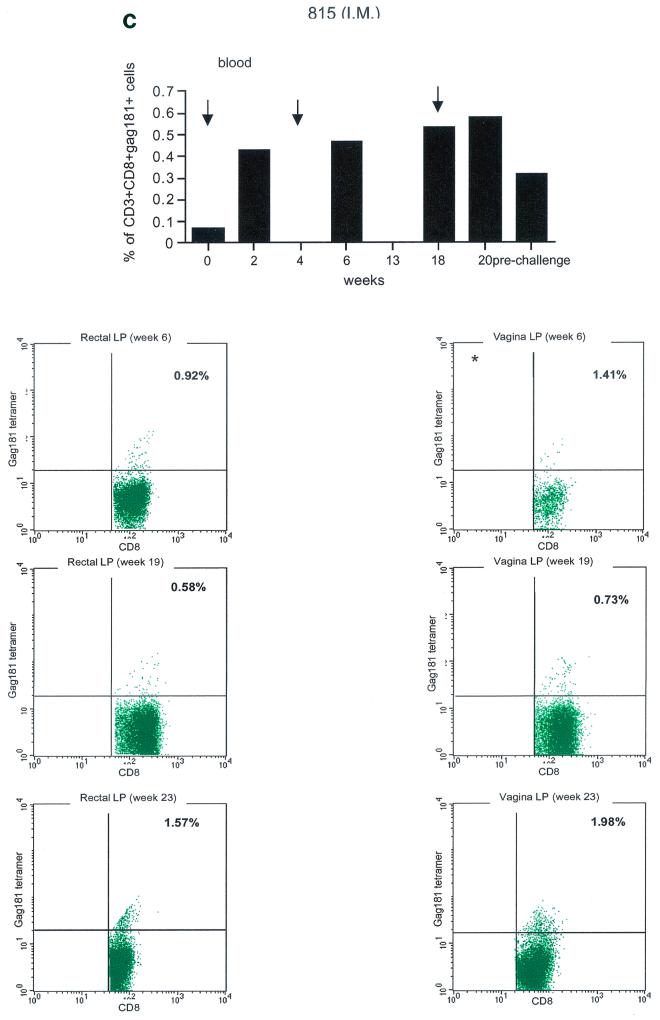
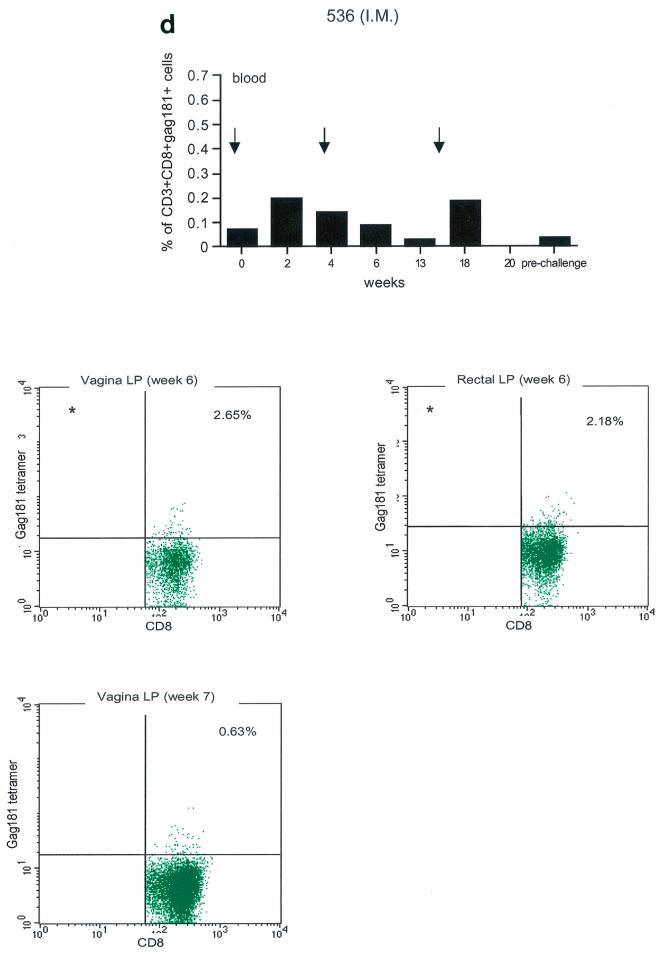
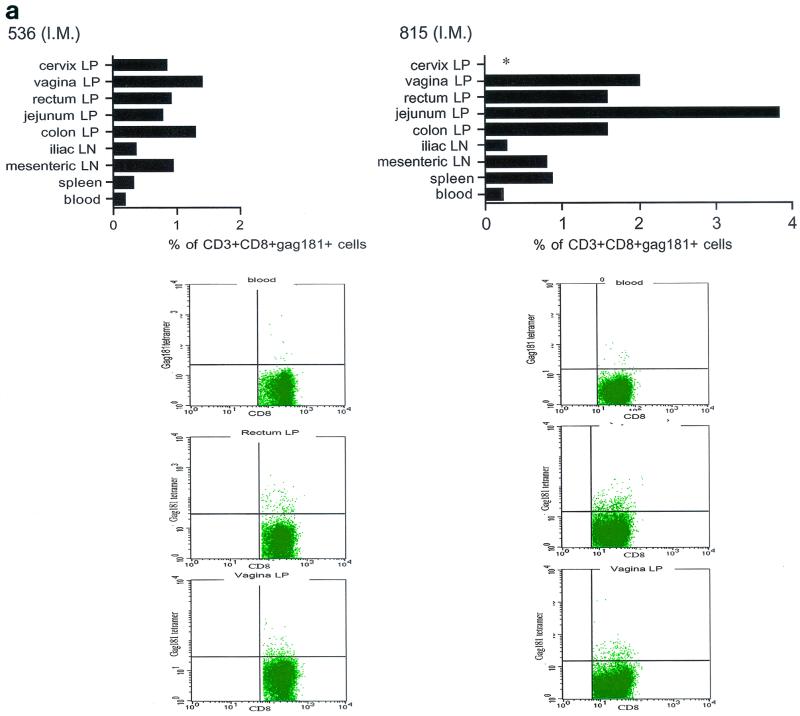
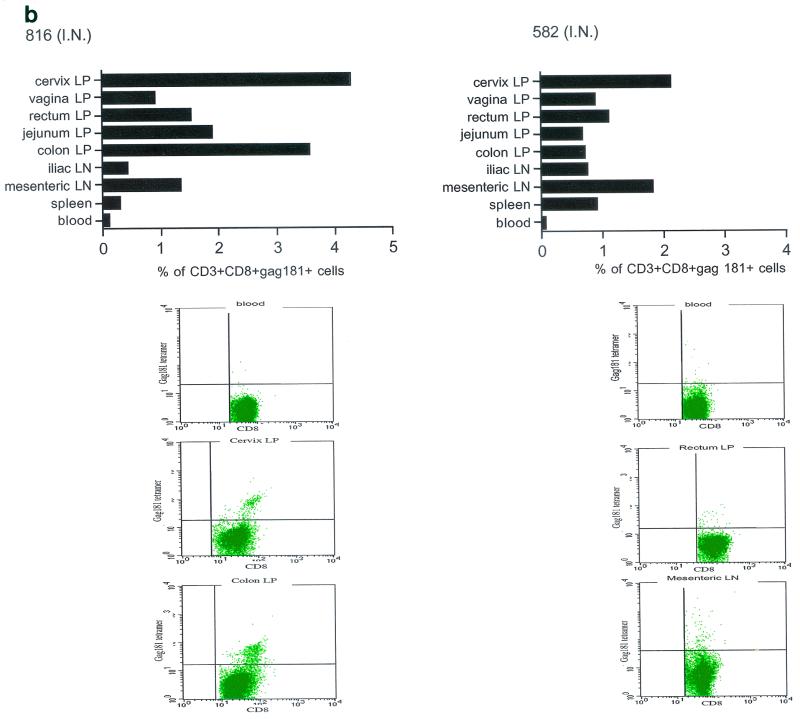
Tetramer staining during immunization. Immune responses during the immunization procedure were assessed by measuring the percentage of CD3+ CD8+ Gag181-189 CM9-positive cells in blood (upper graphs in panels a, b, c, and d; arrows indicate time of immunization) and in isolated lymphocytes from rectal and vaginal biopsy samples (flow charts in panels a, b, c, and d). Cells were gated through the CD3+ CD8+ gate, and the percentage of Gag181-189 CM9-positive cells was determined by histogram analysis. The flow charts are shown here as dot blots rather than histograms in order to better demonstrate the population of cells. An equal number of CD3+ CD8+ cells (104) was acquired for each sample analyzed. Samples in which fewer than 5 × 103 CD3+ CD8+ cells were acquired are indicated (∗). Times of immunization are indicated on the graphs with arrows. (a) Findings in intrarectally (I.R.) immunized macaques 814 and 818 shown as a graph for blood and as flow charts for biopsy samples underneath the graph. (b) Findings in intranasally (I.N.) immunized animals 816 and 582. (c) Findings in intramuscularly (I.M.) immunized macaque 815. (d) Findings in intramuscularly immunized macaque 536. LP, lamina propria.
The presence of Gag181-189 CM9-specific CD3+ CD8+ T cells at mucosal sites was assessed on isolated lamina propria lymphocytes from rectal and vaginal biopsy samples (bottom panels of Fig. 3a, b, c, and d). We did not attempt to quantify these cells because often the number of cells obtained from the biopsy samples was insufficient to reliably compare frequencies. In both macaques 814 and 818 immunized by the intrarectal route, a discrete population of cells staining the Gag181-189 CM9 tetramer was detected in serial biopsy samples (Fig. 3a, bottom panels). Similarly, in animals 816 and 582 immunized by the intranasal route, a convincing staining for Gag181-189 CM9 tetramer-positive cells in vaginal lamina propria was detected at week 19 and/or week 20 in both macaques (bottom panels of Fig. 3b). At week 20, a discrete population was also found in the rectal lamina propria of macaque 816 (Fig. 3b).
Unexpectedly, in both macaques immunized by the intramuscular routes (815, bottom panels of Fig. 3c, and 536, bottom panels of Fig. 3d), Gag181-189 CM9-positive CD3+ CD8+ T cells were detected as early as week 6 from immunization in both the rectal and vaginal lamina propria. Thus, Gag181-189 CM9-specific CD3+ CD8+ cell populations in the gastrointestinal lamina propria and the cervicovaginal compartment were induced by NYVAC/SIVgpe in all of the immunized animals regardless of the route of immunization.
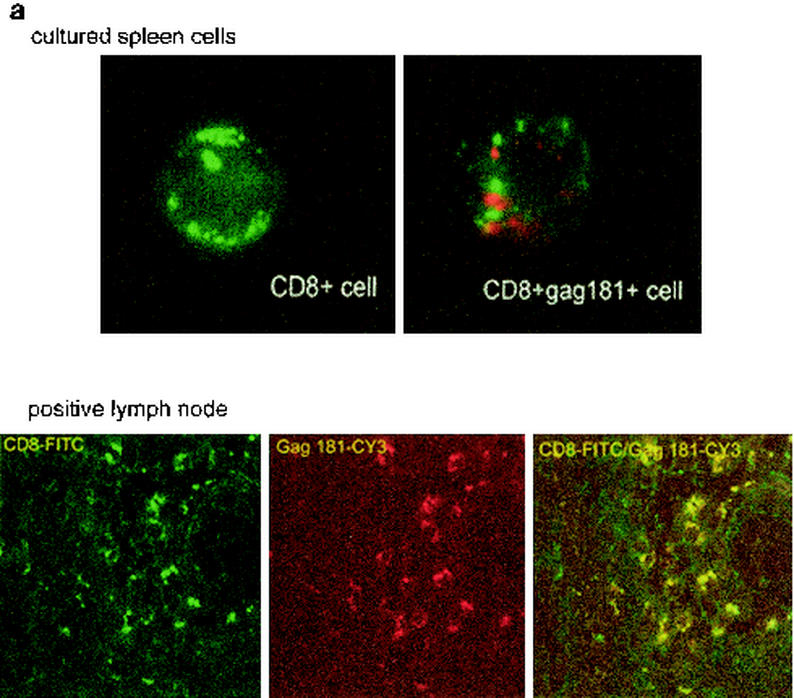
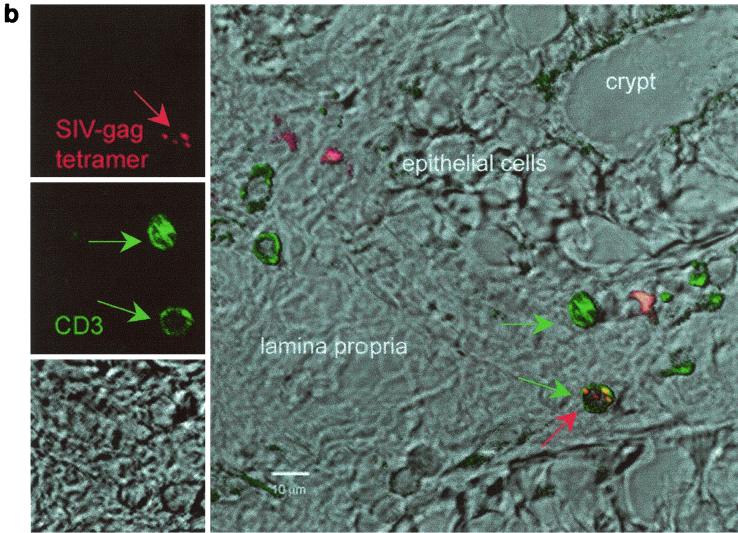
To confirm these results with an independent approach, tetramer-positive cells were visualized by in situ tetramer staining of rectal biopsy samples from a few macaques. Tissues from a lymph node of a chronically infected macaque known to have an extremely high percentage of CD3+ CD8+ Gag181-189 CM9-positive cells as well as cultured spleen cells from the same animal were used as a positive control, and biopsy samples from uninfected, nonimmunized macaques were used as a negative control. Spleen culture cells or lymph node tissue (Fig. 4a) of chronically infected macaques had clusters of double positive cells for Gag181-189 CM9 and CD8. Single cells with similar staining patterns were detected in vaginal and rectal biopsy samples of the immunized macaques. Figure 4b shows tetramer-positive cells in the colonic lamina propria of animal 816 2 weeks after the second immunization.
FIG. 4.
In situ tetramer staining. Double immunofluorescent staining and image analysis were initially done on positive samples from cultured spleen cells and from lymph nodes taken from a known Gag181-189 CM9-positive animal (a). In immunized macaques, positive staining is shown in the colonic tissue from animal 816 2 weeks after the boost (b). Images for individual channels (CD3, green; SIV-Gag tetramer, red; differential interference contrast, gray scale) are shown on the left side, and a larger merged image containing all three channels is shown on the right (b). Several CD3+ cells are present, and one is also labeled with the SIV-Gag tetramer. Bar, 10 μm (b). FITC, fluorescein isothiocyanate; CY3, indocarbocyanine.
Influence of route of immunization on anamnestic immune response in systemic and mucosal compartments following SIVmac251 challenge exposure.
All seven macaques were exposed intrarectally to SIV251 and euthanized 48 h postexposure. The time frame of 48 h postexposure was chosen because, at the time of euthanasia, we wanted to capture and quantitate the local secondary response to Gag181-189 CM9 before the systemic spread of infection. The choice of a time frame of 48 h postexposure was based on the information that mucosal exposure to SIVmac251 results in colonization of local lymph nodes by day 2 to 3 postinfection (20; Chris Miller, personal communication). Since the commitment to proliferation of primed T cells upon encounter with the antigen occurs quickly (0.5 to 2 h) (28) and a cycle of viral replication requires approximately 12 to 18 h, it is therefore reasonable to assume that Gag-specific local memory T cells might be able to undergo mitoses within 48 h and/or be recruited to this locale.
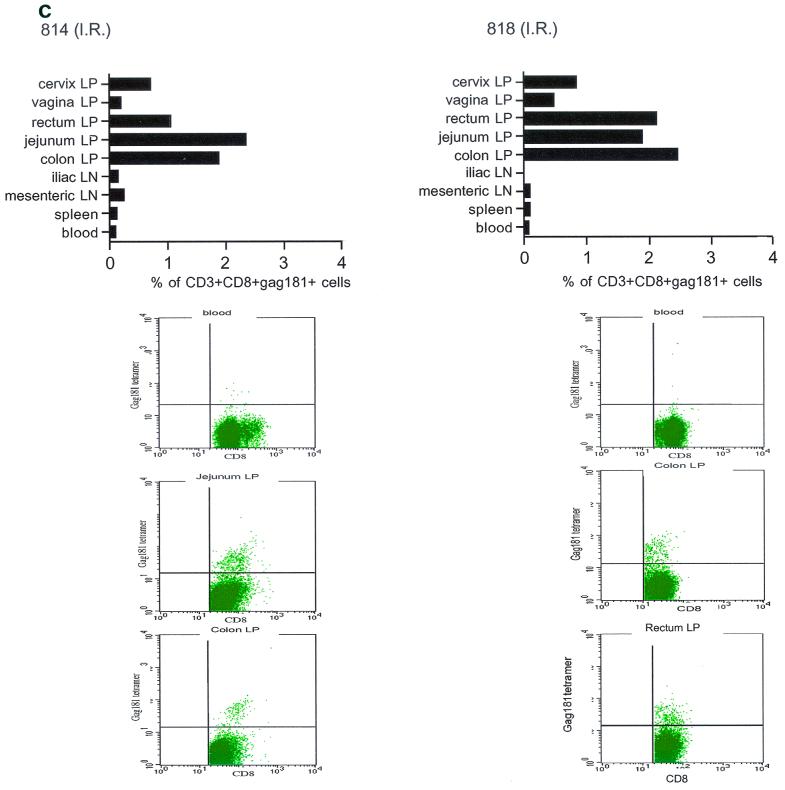
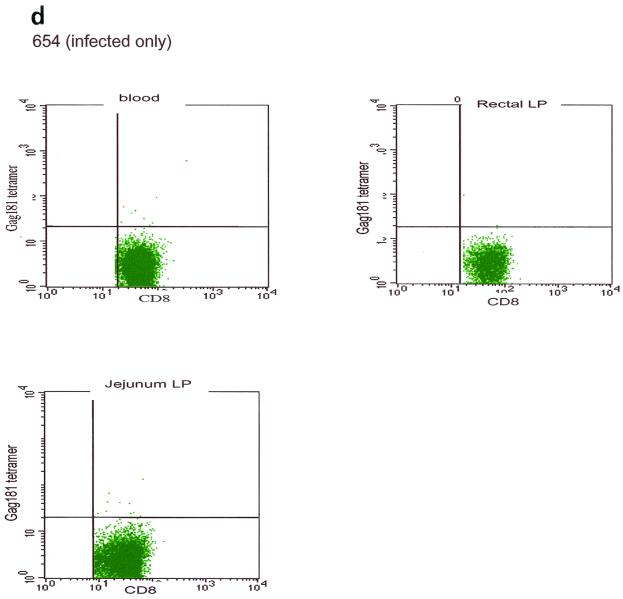
At necropsy, an SIV-specific response, higher than that found after the last immunization in blood to the Gag181-189 CM9 epitope, was found in macaques 815 and 536, immunized intramuscularly, both in some systemic and most mucosal compartments, and appeared to be higher in the latter (Fig. 5a). In macaques immunized intranasally (macaques 816 and 582), the response to the Gag181-189 CM9 epitope following challenge exposure was present in most compartments and was generally higher in mucosal compartments (Fig. 5b). However, in the two macaques immunized intrarectally (macaques 814 and 818), this response appeared to be mainly confined to mucosal sites (Fig. 5c). In animal 654, naive at the time of challenge exposure, CD3+ CD8+ T cells staining the Gag181-189 CM9 tetramer were not found in any tissues (Fig. 5d).
FIG. 5.
Tetramer staining after challenge. Macaques were challenged with SIVmac251 and sacrificed 48 h later. SIV-specific cells were determined as a percentage of Gag181-189 CM9-positive cells of the CD3+ CD8+ population in lymphocytes isolated from each tissue. An equal number of CD3+ CD8+ cells (104) was acquired for each sample analyzed. SIV-specific responses in intramuscularly (I.M.) immunized macaques 536 and 815 (a) are shown as a graph (∗, not done). Samples of blood lymphocytes at the time of necropsy and highly positive mucosal tissue samples are shown as flowcharts beneath the graph for each macaque. Tetramer-positive cells in intranasally (I.N.) immunized animals 816 and 582 (only 1.152 × 103 CD3+ CD8+ cervical lamina propria cells were analyzed for animal 582) (b) and in intrarectally (I.R.) immunized macaques 818 and 814 (c) are shown. Animal 654, which was not immunized but was challenged, is shown (d). LP, lamina propria; LN, lymph node.
These findings suggested that early viral replication following rectal challenge may have preferentially expanded virus-specific immune responses at the mucosal sites, since at 48 h the virus may not be amplified sufficiently to induce a sizable immune response at systemic sites. In fact, in the macaques immunized by the intrarectal route, an expansion of virus-specific responses was evident only at mucosal sites (Fig. 5c).
To correlate the observed immune responses after viral challenge to the level of virus in tissues, we isolated RNA either from lymphocytes collected from tissues or from the entire tissue of all seven macaques and quantitated viral RNA by nucleic acid sequence-based amplification (NASBA) assay as previously described. With this assay we can detect as few as 500 copies of RNA per μg of total RNA (43). From our estimate, derived from the yield of RNA from a known number of cells cultured in vitro, 1 μg of RNA corresponds to a total of 104 to 105 cells. Analysis of RNAs from the spleen, blood, vagina, and rectum of each macaque by NASBA resulted in fewer than 500 copies of viral RNA in all samples (data not shown), suggesting that viral expression was still at too low a level to be detected by the technique. We do not believe that this lack of detection of viral RNA was related to an abortive infection, since we infected a total of 34 naive macaques with the same viral stock by the rectal route (37; our unpublished data).
Cytokine production in response to immunization.
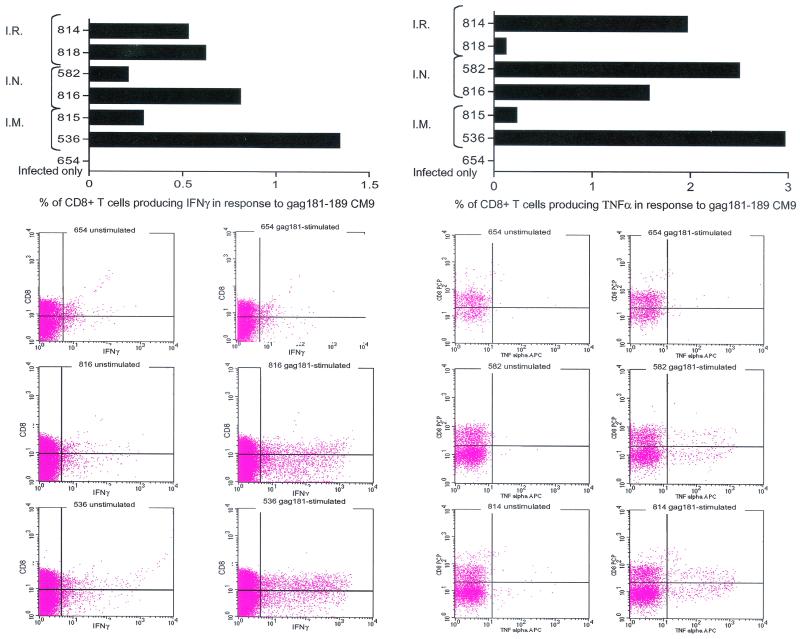
Gag181-189 CM9-specific functional responses in the jejunal lamina propria of all seven macaques were assessed by intracellular cytokine staining of isolated lymphocytes following in vitro stimulation with the specific peptide, using as a read-out both TNF-α and IFN-γ production. In all six immunized macaques, considerable numbers of CD8α/β+ cells producing both cytokines were found, and as expected, these cells were absent in macaque 654 (Fig. 6, top panel). Surprisingly, a number of CD8α/β− cells also appeared to be producing either IFN-γ or TNF-α (Fig. 6, flow charts). This finding could be related to a downregulation of the CD3 or the CD8α/β receptor on the cell surface during the procedure, as noted by others (29). The graphs on the top of Fig. 6 include only CD8α/β+ cells that produced IFN-γ (left top panel) or TNF-α (right top panel).
FIG. 6.
Intracellular cytokine staining. Isolated jejunum lamina propria lymphocytes at necropsy were incubated with or without Gag181-189 CM9 peptide and then stained for expression of IFN-γ and TNF-α. The percentage of CD8+ IFN-γ/TNF-α-positive cells was determined by quadrant analysis as shown on the flow charts. The graphs represent the net population of CD8+ cells stimulated to express these cytokines, i.e., these percentages were derived after the percentage of CD8+ cells spontaneously expressing the cytokines was deducted from the percentage of CD8+ cells expressing them after stimulation with Gag181-189 CM9. The flowcharts show the population of CD8+ IFN-γ/TNF-α-positive cells before and after stimulation in animal 654 (not immunized) and in some of the highest responders. I.R., intrarectal; I.N., intranasal; I.M., intramuscular; APC, allophycocyanin.
The percentage of Gag-specific CD8+ cells expressing IFN-γ or TNF-α was derived by subtracting the spontaneous secretion of cytokines in the absence of peptide stimulation (see raw data on the flow charts in Fig. 6).
The extent of this response, although variable among the animals, did not appear to be related to the route of immunization. Overall, the ability of lymphocytes at mucosal sites to express cytokines did not differ among macaques immunized by different routes.
DISCUSSION
The main finding in this study is that immunization of macaques with a live attenuated vaccine, NYVAC/SIVgpe, by either a mucosal (intrarectal and intranasal) or systemic (intramuscular) route results in the appearance of Gag181-189 CM9 tetramer-positive CD8+ cells in mucosal tissues. Furthermore, intrarectal challenge of such immunized macaques with SIVmac251 followed by sacrifice of the animals at 48 h after challenge led to the expansion of tetramer-positive CD8+ cells in mucosal tissues in all macaques regardless of the route of immunization. This confirmed that a systemic (intramuscular) immunization led to mucosal CD8+ T-cell responses, as did mucosal immunization.
These results are somewhat at odds with studies reviewed above (4-6) that show that the route of induction determines whether or not there is a mucosal immune response and that, in fact, mucosal immunization is necessary for a mucosal response. Thus, intramuscular and intradermal immunization with naked DNA generates systemic immune responses but is unable to confer protection at mucosal sites (36). Similarly, subcutaneous immunization in mice with HIV-1 gp160 expressing recombinant vaccinia virus induced cytotoxic T lymphocytes only in the spleen but not in Peyer's patches or intestinal lamina propria (3).
However, it should be noted that systemic immunization with recombinant vaccinia virus vac-gp160 protected three of four macaques against intrarectal challenge with SIVmne virus 47 (39) or, in another model, cats against feline immunodeficiency virus (38). Similarly, such protection has been achieved with live attenuated virus (41, 42) and whole killed virus (10). Finally, in the case of the NYVAC/SIVgpe vaccine, intramuscular immunization was previously shown to be able to protect 50% of the macaques that were challenged intrarectally with SIVmac251 from high viremia ((7) and an even higher number of macaques when a DNA prime/NYVAC/SIVgpe boost was used (18a).
It should be noted that in the studies where systemic immunization led to at least some degree of mucosal immunity, live virus vaccines were used. Such vaccines, as opposed to peptide vaccines, may be more capable of leading to the appearance of immune cells at mucosal sites, either because of antigen circulation or because of the traffic of cells that take up antigen.
Several additional conclusions concerning the responses elicited by immunization by different routes could be drawn from this study. First, tetramer-positive CD8+ T cells were found in the blood in all macaques regardless of the route of immunization. This is in agreement with data obtained in animals infected by different routes (18, 34, 51). Second, intrarectal immunization induced CD8+ T cells mainly in mucosal tissue. Thus, while intramuscular and intranasal immunization led to some level of tetramer-positive CD8+ cells in systemic tissues (spleen, iliac lymph node), intrarectal immunization led to little or no tetramer-positive CD8+ cells at these locations. This finding is in agreement with previous studies showing that intrarectal immunization is less effective in inducing systemic responses (31). Since intrarectal immunization with a multicomponent-peptide HIV vaccine led to cytotoxic T lymphocyte responses in the spleen only when cholera toxin was used as an adjuvant (23), it may be that systemic responses following mucosal immunization require the use of adjuvants.
Intranasal immunization has been proven by numerous reports to be particularly effective in inducing immune responses in the female genital tract (14, 15, 25, 44, 46). Studies done in macaques with attenuated vaccinia virus that express Env and Gag proteins of SIV or recombinant live attenuated poliovirus expressing the SIV proteins p17gag and gp41env confirmed these findings regarding antibody responses (12, 33). Studies in macaques that looked for cellular responses in the cervicovaginal tract following intranasal immunization, to our knowledge, have not been performed. In this study, intranasal immunization induced T-cell responses in both mucosal and systemic compartments. In addition, cytokine production (IFN-γ or TNF-α) in response to stimulation with the specific peptide Gag181-189 CM9 was high in both animals immunized intranasally.
One caveat of the results of this study is that, while responses to immunization and challenge were measured, protection against infection was not. Thus, it may be that mucosal immunization is still necessary, even with live virus vaccines, to achieve optimum protection. In a previous study with ALVAC/SIV, another poxvirus-based vaccine, mucosal/systemic immunization was not shown to provide better protection than systemic immunization alone, but in this study, the mucosal and systemic routes of immunization alone were not directly compared (37). Clearly, studies in larger numbers of macaques investigating protection achieved with different routes of immunization are now warranted.
Acknowledgments
The base grants for the New England Regional Primate Research Center and the Tulane Regional Primate Research Center are RR00164 and RR00168.
We thank Tatiana Karpova from the Confocal Imaging Facility at the National Cancer Institute for help, Nancy Miller for helpful discussion, John D. Altman for the Gag181-189 CM9 tetramer, Ruth Woodward for animal care, and Steven Snodgrass for editorial assistance.
REFERENCES
- 1.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Arstila, T., T. P. Arstila, S. Calbo, F. Selz, M. Malassis-Seris, P. Vassalli, P. Kourilsky, and D. Guy-Grand. 2000. Identical T-cell clones are located within the mouse gut epithelium and lamina propia and circulate in the thoracic duct lymph. J. Exp. Med. 191:823-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belyakov, I. M., J. D. Ahlers, B. Y. Brandwein, P. Earl, B. L. Kelsall, B. Moss, W. Strober, and J. A. Berzofsky. 1998. The importance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of interleukin-12. J. Clin. Investig. 102:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95:1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., L. S. Wyatt, J. D. Ahlers, P. Earl, C. D. Pendleton, B. L. Kelsall, W. Strober, B. Moss, and J. A. Berzofsky. 1998. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J. Virol. 72:8264-8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull, D. M., and M. A. Bookman. 1977. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J. Clin. Investig. 59:966-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cranage, M. P., A. Baskerville, L. A. Ashworth, M. Dennis, N. Cook, S. Sharpe, G. Farrar, J. Rose, P. A. Kitchin, and P. J. Greenaway. 1992. Intrarectal challenge of macaques vaccinated with formalin-inactivated simian immunodeficiency virus. Lancet 339:273-274. [DOI] [PubMed] [Google Scholar]
- 11.Cromwell, M. A., R. S. Veazey, J. D. Altman, K. G. Mansfield, R. Glickman, T. M. Allen, D. I. Watkins, A. A. Lackner, and R. P. Johnson. 2000. Induction of mucosal homing virus-specific CD8+ T lymphocytes by attenuated simian immunodeficiency virus. J. Virol. 74:8762-8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crotty, S., B. L. Lohman, F. X. Lu, S. Tang, C. J. Miller, and R. Andino. 1999. Mucosal immunization of cynomolgus macaques with two serotypes of live poliovirus vectors expressing simian immunodeficiency virus antigens: stimulation of humoral, mucosal, and cellular immunity. J. Virol. 73:9485-9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson, A. 1977. Intraepithelial lymphocytes of the small intestine. Gut 18:921-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flanagan, B., C. R. Pringle, and K. N. Leppard. 1997. A recombinant human adenovirus expressing the simian immunodeficiency virus Gag antigen can induce long-lived immune responses in mice. J. Gen. Virol. 78:991-997. [DOI] [PubMed] [Google Scholar]
- 15.Gallichan, W. S., and K. L. Rosenthal. 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 184:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghanekar, S. A., L. E. Nomura, M. A. Suni, L. J. Picker, H. T. Maecker, and V. C. Maino. 2001. Gamma interferon expression in CD8+ T cells is a marker for circulating cytotoxic T lymphocytes that recognize an HLA A2-restricted epitope of human cytomegalovirus phosphoprotein pp65. Clin. Diagn. Lab. Immunol. 8:628-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haanen, J. B., M. G. van Oijen, F. Tirion, L. C. Oomen, A. M. Kruisbeek, F. A. Vyth-Dreese, and T. N. Schumacher. 2000. In situ detection of virus- and tumor-specific T-cell immunity. Nat. Med. 6:1056-1060. [DOI] [PubMed] [Google Scholar]
- 18.Hel, Z., J. Nacsa, B. Kelsall, W. P. Tsai, N. Letvin, R. W. Parks, E. Tryniszewska, L. Picker, M. G. Lewis, Y. Edghill-Smith, M. Moniuszko, R. Pal, L. Stevceva, J. D. Altman, T. M. Allen, D. Watkins, J. V. Torres, J. A. Berzofsky, I. M. Belyakov, W. Strober, and G. Franchini. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J. Virol. 75:11483-11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Hel, Z., J. Nacsa, E. Tryniszewska, W.-P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Granchini. Containment of SIV infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and post-challenge CD4+ and CD8+ T-cell responses. J. Immunol., in press. [DOI] [PubMed]
- 19.Hel, Z., W. P. Tsai, A. Thornton, J. Nacsa, L. Giuliani, E. Tryniszewska, M. Poudyal, D. Venzon, X. Wang, J. Altman, D. I. Watkins, W. Lu, A. von Gegerfelt, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2001. Potentiation of simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T-cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J. Immunol. 167:7180-7191. [DOI] [PubMed] [Google Scholar]
- 20.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James, S. P., C. Fiocchi, A. S. Graeff, and W. Strober. 1985. Immunoregulatory function of lamina propria T cells in Crohn's disease. Gastroenterology 88:1143-1150. [DOI] [PubMed] [Google Scholar]
- 22.Kantele, A., J. Zivny, M. Hakkinen, C. O. Elson, and J. Mestecky. 1999. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 162:5173-5177. [PubMed] [Google Scholar]
- 23.Kato, H., H. Bukawa, E. Hagiwara, K. Q. Xin, K. Hamajima, S. Kawamoto, M. Sugiyama, E. Noda, M. Nishizaki, and K. Okuda. 2000. Rectal and vaginal immunization with a macromolecular multicomponent peptide vaccine candidate for HIV-1 infection induces HIV-specific protective immune responses. Vaccine 18:1151-1160. [DOI] [PubMed] [Google Scholar]
- 24.Kern, F., I. P. Surel, C. Brock, B. Freistedt, H. Radtke, A. Scheffold, R. Blasczyk, P. Reinke, J. Schneider-Mergener, A. Radbruch, P. Walden, and H. D. Volk. 1998. T-cell epitope mapping by flow cytometry. Nat. Med. 4:975-978. [DOI] [PubMed] [Google Scholar]
- 25.Klavinskis, L. S., C. Barnfield, L. Gao, and S. Parker. 1999. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J. Immunol. 162:254-262. [PubMed] [Google Scholar]
- 26.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of cytotoxic T lymphocyte coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127-5133. [PubMed] [Google Scholar]
- 28.Lanzavecchia, A., and F. Sallusto. 2001. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat. Immunol. 2:487-492. [DOI] [PubMed] [Google Scholar]
- 29.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27-40. [DOI] [PubMed] [Google Scholar]
- 30.Mayrhofer, G. 1980. Thymus-dependent and thymus-independent subpopulations of intestinal intraepithelial lymphocytes: a granular subpopulation of probable bone marrow origin and relationship to mucosal mast cells. Blood 55:532-535. [PubMed] [Google Scholar]
- 31.McCluskie, M. J., C. L. Brazolot Millan, R. A. Gramzinski, H. L. Robinson, J. C. Santoro, J. T. Fuller, G. Widera, J. R. Haynes, R. H. Purcell, and H. L. Davis. 1999. Route and method of delivery of DNA vaccine influence immune responses in mice and non-human primates. Mol. Med. 5:287-300. [PMC free article] [PubMed] [Google Scholar]
- 32.McGhee, J. R., M. E. Lamm, and W. Strober. 1999. Mucosal immune responses. Academic Press, San Diego, Calif.
- 33.Moldoveanu, Z., A. N. Vzorov, W. Q. Huang, J. Mestecky, and R. W. Compans. 1999. Induction of immune responses to SIV antigens by mucosally administered vaccines. AIDS Res. Hum. Retrovir. 15:1469-1476. [DOI] [PubMed] [Google Scholar]
- 34.Mothe, B. R., H. Horton, D. K. Carter, T. M. Allen, M. E. Liebl, P. Skinner, T. U. Vogel, S. Fuenger, K. Vielhuber, W. Rehrauer, N. Wilson, G. Franchini, J. D. Altman, A. Haase, L. J. Picker, D. B. Allison, and D. I. Watkins. 2002. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphey-Corb, M., L. A. Wilson, A. M. Trichel, D. E. Roberts, K. Xu, S. Ohkawa, B. Woodson, R. Bohm, and J. Blanchard. 1999. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 162:540-549. [PubMed] [Google Scholar]
- 36.Noll, A., N. Bucheler, E. Bohn, R. Schirmbeck, J. Reimann, and I. B. Autenrieth. 1999. DNA immunization confers systemic, but not mucosal, protection against enteroinvasive bacteria. Eur. J. Immunol. 29:986-996. [DOI] [PubMed] [Google Scholar]
- 37.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A∗01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perkus, M. E., J. Tartaglia, and E. Paoletti. 1995. Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious diseases. J. Leukoc. Biol. 58:1-13. [DOI] [PubMed] [Google Scholar]
- 39.Polacino, P., V. Stallard, D. C. Montefiori, C. R. Brown, B. A. Richardson, W. R. Morton, R. E. Benveniste, and S.-L. Hu. 1999. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines. J. Virol. 73:3134-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. R. Bangham, and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 94:1890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Putkonen, P., B. Makitalo, D. Bottiger, G. Biberfeld, and R. Thorstensson. 1997. Protection of human immunodeficiency virus type 2-exposed seronegative macaques from mucosal simian immunodeficiency virus transmission. J. Virol. 71:4981-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quesada-Rolander, M., B. Makitalo, R. Thorstensson, Y. J. Zhang, E. Castanos-Velez, G. Biberfeld, and P. Putkonen. 1996. Protection against mucosal SIVsm challenge in macaques infected with a chimeric SIV that expresses HIV type 1 envelope. AIDS Res. Hum. Retrovir. 12:993-999. [DOI] [PubMed] [Google Scholar]
- 43.Romano, J. W., B. van Gemen, and T. Kievits. 1996. NASBA: a novel, isothermal detection technology for qualitative and quantitative HIV-1 RNA measurements. Clin. Lab. Med. 16:89-103. [PubMed] [Google Scholar]
- 44.Rosenthal, K. L., and W. S. Gallichan. 1997. Challenges for vaccination against sexually transmitted diseases: induction and long-term maintenance of mucosal immune responses in the female genital tract. Semin. Immunol. 9:303-314. [DOI] [PubMed] [Google Scholar]
- 45.Rudzik, O., and J. Bienenstock. 1974. Isolation and characteristics of gut mucosal lymphocytes. Lab. Investig. 30:260-266. [PubMed] [Google Scholar]
- 46.Simmons, C. P., T. Hussell, T. Sparer, G. Walzl, P. Openshaw, and G. Dougan. 2001. Mucosal delivery of a respiratory syncytial virus cytotoxic T lymphocyte peptide with enterotoxin-based adjuvants elicits protective, immunopathogenic, and immunoregulatory antiviral CD8+ T-cell responses. J. Immunol. 166:1106-1113. [DOI] [PubMed] [Google Scholar]
- 47.Simonsen, J. N., D. W. Cameron, M. N. Gakinya, J. O. Ndinya-Achola, L. J. D'Costa, P. Karasira, M. Cheang, A. R. Ronald, P. Piot, and F. A. Plummer. 1988. Human immunodeficiency virus infection among men with sexually transmitted diseases. Experience from a center in Africa. N. Engl. J. Med. 319:274-278. [DOI] [PubMed] [Google Scholar]
- 48.Skinner, P. J., M. A. Daniels, C. S. Schmidt, S. C. Jameson, and A. T. Haase. 2000. Cutting edge: in situ tetramer staining of antigen-specific T cells in tissues. J. Immunol. 165:613-617. [DOI] [PubMed] [Google Scholar]
- 49.Stevceva, L., A. G. Abimiku, and G. Franchini. 2000. Targeting the mucosa: genetically engineered vaccines and mucosal immune responses. Genes Immun. 1:308-315. [DOI] [PubMed] [Google Scholar]
- 50.Stevceva, L., B. Kelsall, J. Nacsa, M. Moniuszko, Z. Hel, E. Tryniszewska, and G. Franchini. 2002. Cervicovaginal lamina propria lymphocytes: phenotypic characterization and their importance in cytotoxic T-lymphocyte responses to simian immunodeficiency virus SIVmac251. J. Virol. 76:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevceva, L., E. Tryniszewska, Z. Hel, J. Nacsa, B. Kelsall, R. Washington Parks, and G. Franchini. 2001. Differences in time of virus appearance in the blood and virus-specific immune responses in intravenous and intrarectal primary SIVmac251 infection of rhesus macaques; a pilot study BMC. Infect. Dis. 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, S. W., P. A. Kozlowski, G. Schmelz, K. Manson, M. S. Wyand, R. Glickman, D. Montefiori, J. D. Lifson, R. P. Johnson, M. R. Neutra, and A. Aldovini. 2000. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J. Virol. 74:10514-10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weynants, V., K. Walravens, C. Didembourg, P. Flanagan, J. Godfroid, and J. J. Letesson. 1998. Quantitative assessment by flow cytometry of T-lymphocytes producing antigen-specific gamma-interferon in Brucella immune cattle. Vet. Immunol. Immunopathol. 66:309-320. [DOI] [PubMed] [Google Scholar]