Abstract
Infection of human epithelial cells with adenoviruses induces an apoptosis paradigm that is efficiently suppressed by the expression of viral E1B-19K protein, which is a functional homolog of the cellular antiapoptosis protein BCL-2. The mechanisms of adenovirus (Ad)-induced apoptosis appear to involve the cellular BCL-2 family proapoptotic proteins. Recent genetic studies with fibroblasts derived from mutant mouse embryos indicate that a class of the BCL-2 family proapoptotic proteins (designated BH-123 or multidomain proteins) such as BAX and BAK constitutes an essential component of the core apoptosis machinery in animal cells. We have examined the role of BAX in Ad-induced apoptosis in human epithelial cells using two colon cancer cell lines, HCT116Bax (Bax+/−) and HCT116BaxKO (Bax−/−) (L. Zhang, J. Yu, B. H. Park, K. W. Kinzler, and B. Vogelstein, Science 290:989-992, 2000). Infection of Bax+/− cells with an Ad type 2 mutant (dl250) defective in expression of the E1B-19K protein resulted in enhanced cytopathic effect, large plaques on cell monolayers, fragmentation of cellular DNA, and enhanced cell death. These mutant phenotypes were not efficiently expressed in Bax−/− cells, suggesting that BAX is essential for Ad-induced apoptosis. Infection of Bax+/− cells with dl250 induced increased levels of an N-terminally processed form of BAX. Cells infected with the 19K mutant also contained enhanced levels of truncated BAX in membrane-inserted form. Our results suggest that at least a part of the mechanism utilized by E1B-19K to suppress apoptosis during Ad infection may involve modulation of the activities of BAX.
Apoptosis is a physiological process of cell death that is essential during the development of multicellular organisms for selective cell elimination. This process functions as a cellular defense mechanism to restrict viral replication and pathogenesis in cells infected with a number of viruses (reviewed in reference 28). In cells infected with human adenovirus (Ad), the apoptosis paradigm is initiated by the E1A proteins (reviewed in reference 8). The apoptosis-inducing activity of E1A is intimately linked to its ability to induce cellular DNA synthesis (reviewed in reference 1). The E1A region also amplifies cell death by activating the expression of other viral early proteins encoded within the gene regions E4 (4) and E3 (39), particularly during late phases of viral infection. Apoptosis induced during Ad infection can be efficiently suppressed by the E1B-19K protein (26, 32, 38). Although other viral proteins are also implicated in suppression of Ad-induced apoptosis, their relative contribution appears to be limited, since elimination of only the E1B-19K coding region results in characteristic manifestation of apoptosis in infected human epithelial cells. Human cells infected with Ad mutants defective in E1B-19K exhibit the enhanced cytopathic effect (Cyt phenotype) characterized by severe cellular destruction, with features that resemble apoptosis (7, 30, 33, 34). As a consequence of the enhanced cytopathic effect, the infected cell monolayers contain large plaques (Lp phenotype) (7, 34). Another distinguishing feature of cells infected with the 19K mutants is that the viral and cellular DNA is fragmented (Deg phenotype), as in other instances of apoptotic death (13, 26, 29, 38).
The mechanism that underlies apoptosis in Ad-infected cells remains to be fully clarified. Since Ad-induced apoptosis can be efficiently suppressed by E1B-19K, at least, some of the underlying mechanisms should involve components that engage the cellular BCL-2 family proteins. E1B-19K appears to be a functional homolog of BCL-2, since BCL-2 can efficiently substitute for E1B-19K during viral replication (9, 31, 35). Further, both proteins interact with a common set of BCL-2 family proapoptotic proteins (2, 3, 6, 14, 19, 20). The 19K-interacting BCL-2 family proapoptotic proteins include those that contain only a single conserved BCL-2 homology (BH) domain, only a BH3 domain (BH3-only proteins), and those that contain BH1, BH2, and BH3 domains (BH-123 proteins, also called multidomain proapoptotic proteins). In the canonical apoptosis paradigm of animal cells, the various BH3-only proteins appear to link different apoptotic stimuli to the core apoptosis machinery through the BH-123 proteins, resulting in the release of apoptogenic factors from the mitochondria and in general mitochondrial dysfunction (reviewed by Reed and Green [27]). Recent studies with rodent fibroblasts that are nullizygous for two different BH-123 proteins, BAX and BAK, suggest that both proteins are essential for manifestation of apoptosis induced by diverse stimuli (37, 44). E1B-19K has been reported to complex with both BAK (14) and BAX (19). Interestingly, according to a recent report BAX appears to complex with 19K only in cells treated with the cytokine tumor necrosis factor alpha while BAK appears to complex in untreated Ad-infected cells (32). A genetic link between the BH-123 proteins and Ad-induced apoptosis remains to be established. We have recently observed that a human epithelial Bax knockout cell line, HCT116BaxKO (43), is defective in manifestation of apoptosis induced by diverse stimuli (36). Here, we have examined the requirement of BAX for apoptosis induced by Ad type 2 (Ad2) in human epithelial cancer cells and report that BAX is essential for manifestation of apoptosis during viral infection.
MATERIALS AND METHODS
Cells and viruses.
Human colon carcinoma cell lines HCT116Bax (Bax+/−) and HCT116BaxKO (Bax−/−) were gifts from B. Vogelstein (43) and were grown in McCoy 5A medium supplemented with 10% fetal bovine serum. Ad2 mutant dl250 defective in coding the E1B-19K protein has been described previously (29, 30). Ad2 wild-type (wt) and dl250 were propagated in human 293 cells, and their titers were determined by the standard plaque assay on human A549 cells.
Immunoprecipitation.
Cells in 100-mm-diameter dishes (107 cells/dish) were either mock infected or infected with Ad2 wt or dl250 at 100 PFU/cell. At indicated times, cells were lysed in 1 ml of buffer containing 50 mM Tris (pH 7.4), 50 mM NaCl, 10% glycerol, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 2 mM Na-EDTA, and a cocktail of protease inhibitors (Roche) for 1 h on ice. Insoluble debris was removed by centrifugation at 13,000 × g for 15 min at 4°C. The resulting supernatants were precleared by incubation with 50 μl of protein A-Sepharose beads (Sigma Chemicals, St. Louis, Mo.) for 1 h at 4°C on a rocker shaker. Protein A beads were removed by centrifugation at 13,000 × g at 4°C for 1 min. The resulting supernatants containing equal amounts of total protein were mixed with the 19K antiserum and gently rocked overnight at 4°C on a rocker shaker. The immunocomplexes were captured by adding 100 μl of protein A-Sepharose beads and gentle rocking for 2 h at 4°C on a rocker shaker. Sepharose beads were collected by pulse centrifugation, washed three times with ice-cold buffer containing 50 mM Tris (pH 7.5)-50 mM NaCl, resuspended in sample buffer, mixed, and boiled for 3 min to dissociate immunocomplexes from the beads. The beads were collected by centrifugation, and the resulting supernatants were analyzed by Western blot analysis.
Preparation of HM fraction.
HCT116Bax and HCT116BaxKO cells were either mock infected or infected with Ad2 wt or dl250. Infected cells were harvested at indicated times by gentle scraping with a rubber policeman, collected by centrifugation at 200 × g for 5 min at 4°C, and washed twice with ice-cold phosphate-buffered saline, pH 7.4. Cells were suspended (107 cells/ml) in buffer A (250 mM sucrose, 20 mM HEPES-KOH [pH 7.4], 10 mM KCl, 1.5 mM Na-EGTA, 1.5 mM Na-EDTA, 1 mM MgCl2, 1 mM dithiothreitol) and a cocktail of protease inhibitors (Roche) and were incubated on ice for 30 min. Cells were disrupted by 50 strokes in a glass Dounce homogenizer with a tight-fitting pestle (type B). The cell homogenates were centrifuged at 600 × g for 8 min at 4°C to remove nuclei and unbroken cells. Supernatants were further centrifuged at 13,000 × g for 15 min at 4°C. The resulting pellet was washed with buffer A and centrifuged at 13,000 × g for 15 min at 4°C to separate the heavy membrane (HM) fraction enriched in mitochondria. The HM fractions were lysed for 30 min on ice in buffer B (50 mM HEPES [pH 7.4], 1% [vol/vol] NP-40, 10% [vol/vol] glycerol, 1 mM EDTA, 2 mM dithiothreitol) and a cocktail of protease inhibitors. Samples were clarified by centrifugation at 13,000 × g for 15 min at 4°C. The supernatants containing mitochondrial proteins were analyzed by Western blot analysis. To prepare the whole-cell lysate (WCL), cells were harvested; washed twice in phosphate-buffered saline (pH 7.4); and lysed for 1 h on ice in a buffer containing 50 mM Tris (pH 8), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS), supplemented with a cocktail of protease inhibitors. The lysates were clarified by centrifugation at 13,000 × g for 15 min at 4°C. Supernatants were collected and analyzed by Western blotting or stored at −70°C until further analysis. The amount of total protein in each fraction was quantified by detergent-compatible protein assay (Bio-Rad, Richmond, Calif.).
To analyze insertion of proteins into membranes, the HM fraction enriched in mitochondria was subjected to alkali extraction (12, 24). The HM fraction was uniformly resuspended in buffer A. Aliquots containing equal amounts of proteins were centrifuged at 13,000 × g for 15 min at 4°C. The pellets were either left untreated or resuspended in 0.1 M Na2CO3, pH 11.5, and incubated on ice for 30 min. Alkali-insoluble membrane protein was then recovered by centrifugation for 30 min at 13,000 × g at 4°C and washed with buffer A. The pellets of untreated and alkali-treated HM were lysed in 2× SDS sample buffer by boiling for 10 min and subjected to Western blot analysis.
Western blot analysis.
Samples containing equal amounts of total protein (30 to 100 μg) were mixed with Laemmli's loading buffer, boiled for 5 min, and subjected to SDS-15% polyacrylamide gel electrophoresis followed by electroblotting onto nitrocellulose membranes. Membranes were blocked for 1 h with 5% (wt/vol) nonfat milk in Tris-buffered saline, pH 7.6, containing 0.1% Tween 20 at room temperature and subsequently probed overnight at 4°C with various monoclonal or polyclonal antibodies. The membranes were rinsed and incubated with a horseradish peroxidase-conjugated secondary anti-mouse or anti-rabbit antibody (Upstate, Lake Placid, N.Y.). Following the secondary antibody incubation, the membranes were rinsed, and protein bands were visualized with ECL Western blotting detection reagent (Amersham) according to the manufacturer's instructions.
Antibodies.
The following antibodies were used: anti-Bax (residues 43 to 61) polyclonal antibody (PharMingen, San Diego, Calif.), anti-Bax NT (residues 1 to 21) polyclonal antibody (Upstate), anti-Bak polyclonal antibody (Upstate), and 19K antiserum generated against the synthetic peptide H-CQEQSPWNPRAGLDPRE-OH (17).
RESULTS
Effect of BAX on E1B-19K mutant phenotypes.
To determine the role of BAX in Ad-induced apoptosis, we utilized two epithelial colon cancer cell lines developed by Vogelstein and colleagues (43). One of these cell lines (HCT116Bax) is heterozygous for Bax (Bax+/−) and expresses functional BAX protein. A knockout derivative of this cell line (HCT116BaxKO) is null for BAX expression (Bax−/−). We and others have shown that both cell lines express functional BAK protein (23, 36). While Bax-deficient mouse embryo fibroblasts are not defective in manifestation of apoptosis by various stimuli, the HCT116BaxKO cells are strongly defective for apoptosis induced by a number of stimuli (10, 23, 36), suggesting that the relative requirement of the BH-123 proapoptotic proteins, BAX and BAK, for apoptosis may be cell type dependent and that BAX may be crucial for apoptosis in human epithelial cells. Since human epithelial cells are natural hosts for group C Ads, the HCT116Bax and HCT116BaxKO cell lines provide ideal systems to investigate the role of BAX in Ad-induced apoptosis.
Adenovirus-induced apoptosis is readily discernible in cells infected with viral mutants defective in the E1B-19K protein. Three distinguishing features characterize infection of permissive human cells by these mutants. The 19K mutants produce large clear plaques (Lp phenotype) on infected cell monolayers, while the wt virus produces smaller fuzzy-edged plaques (7, 34). Cells infected with the 19K mutants exhibit enhanced cytopathic effect (Cyt phenotype) characterized by detached, rounded, and disintegrating cells compared to the adherent cells with Ad cytopathic effect, which display a clumped “grape bunch” morphology (34). In cells infected with 19K mutants, cellular and viral DNAs are significantly degraded (Deg phenotype) compared to cells infected with the wt virus (13, 26, 29, 38).
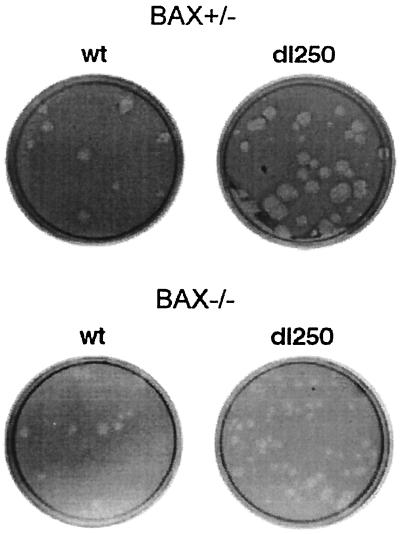
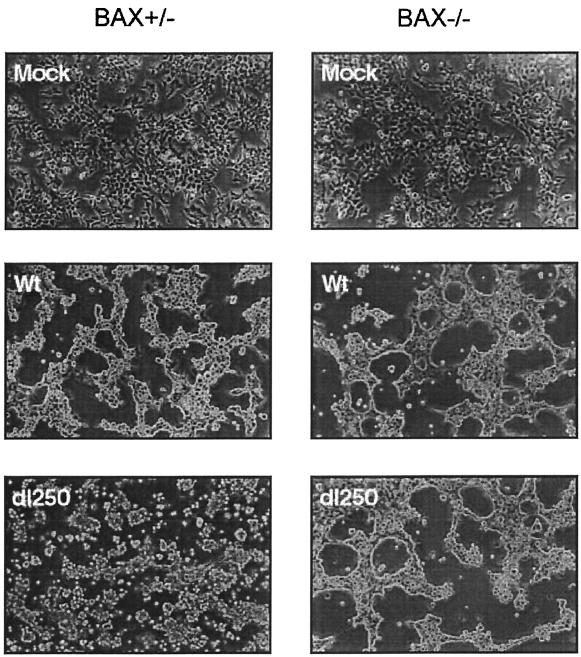
To determine the effect of BAX on the manifestation of these mutant phenotypes, the HCT116Bax (Bax+/−) and the HCT116BaxKO (Bax−/−) cell lines were infected with Ad2 wt or an E1B-19K deletion mutant (dl250) (30). The infected cells were assayed for plaque formation, development of cytopathic effect, and fragmentation of cellular and viral DNA. In BAX-expressing (Bax+/−) cells, dl250 formed relatively large plaques compared to Ad2 wt (Fig. 1). In contrast, in the BAX-deficient (Bax−/−) cells, the plaques of dl250 resembled the wt plaques, suggesting that BAX expression is important for manifestation of the Lp phenotype. Similarly, infection of BAX-expressing cells with dl250 induced extensive cytopathic effect characterized by large amount of detached round cells compared to BAX-deficient cells infected with dl250 (Fig. 2). The cytopathic effect observed in BAX-deficient cells infected with dl250 resembled that observed in cells infected with Ad2 wt. These results suggest that BAX may be essential for induction of the Cyt phenotype by the 19K mutant.
FIG. 1.
Plaque formation by Ad2 wt and dl250 on Bax+/− and Bax−/− cells. Infected cell monolayers were maintained under agarose overlay containing growth medium and 2% fetal bovine serum. Cells were stained with neutral red 8 days after infection and photographed on the next day.
FIG. 2.
Cytopathic effect of Ad2 wt or dl250 on Bax+/− and Bax−/− cells. Cells were infected at 50 PFU/cell and photographed with a phase-contrast microscope 36 h after infection.
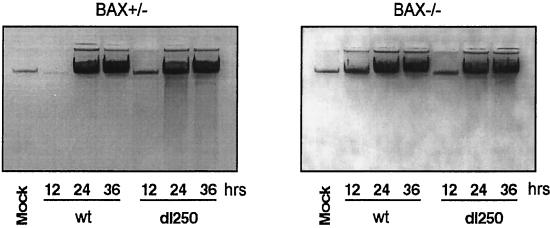
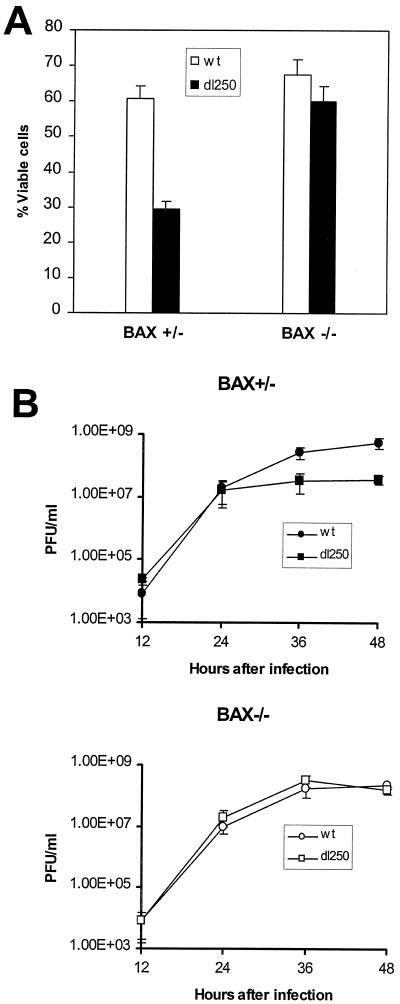
The HCT116Bax and BaxKO cells infected either with wt or dl250 were also analyzed to determine the effect on the Deg phenotype. The low-molecular-weight DNA from infected cells was prepared by Hirt extraction and analyzed by electrophoresis on agarose gels (Fig. 3). In the BAX-expressing cells, infection with dl250 induced significant DNA fragmentation compared to cells infected with the wt virus during late stages of infection (24 and 36 h after infection). The extent of DNA fragmentation in the BaxKO cells infected with dl250 is much reduced. A semiquantitative estimation based on analysis of the photographic images has indicated about a two- to threefold reduction in the extent of DNA fragmentation in BaxKO cells compared to Bax+/− cells. These results suggest that BAX is required for efficient manifestation of the Deg phenotype in cells infected with dl250. Consistent with enhanced cytopathic effect and DNA fragmentation observed in the BAX-expressing cells infected with dl250, the cell viability (as shown by trypan blue exclusion) was also reduced compared to that of cells infected with wt (Fig. 4A). In contrast, BAX-deficient cells infected either with wt or dl250 exhibited more or less similar viability. The effect of BAX on viral replication was measured by single-step growth curve analysis (Fig. 4B). In Bax+/− cells, replication of dl250 was retarded compared to that of Ad2 wt, while both viruses replicated to similar levels in BaxKO cells. This suggests that enhanced apoptosis observed in Bax+/− cells infected with dl250 restricts viral replication. Taken together, the results presented in Fig. 1 through 4 suggest that the BCL-2 family BH-123 (multidomain) proapoptotic protein BAX is required for efficient manifestation of apoptosis induced by Ad2 in human epithelial cells.
FIG. 3.
Fragmentation of cellular DNA in Bax+/− and Bax−/− cells infected with Ad2 wt or dl250. Low-molecular-weight DNA was extracted from infected cells (106) at various times after infection by the Hirt method, precipitated with ethanol, treated with RNase, and analyzed by electrophoresis in a 1% agarose gel.
FIG. 4.
Effect of apoptosis on viral replication. (A) Viability of Bax+/− and Bax−/− cells infected with Ad2 wt or dl250. Cells were infected at 50 PFU/cell. Forty-eight hours after infection, adherent cells were collected by trypsinization and mixed with floating cells. The cell suspension was mixed with an equal volume of trypan blue (Sigma Chemicals), and the dye-excluded cells were quantified. (B) Viral replication in Bax+/− and Bax−/− cells. Cells were infected with Ad2 wt or dl250 at 5 PFU/cell, and at various times after infection, cells were collected and viruses were released by freeze-thawing and sonication. The viruses were titrated on A549 cells. Error bars, standard deviations.
Interaction of E1B-19K with multidomain proapoptotic proteins.
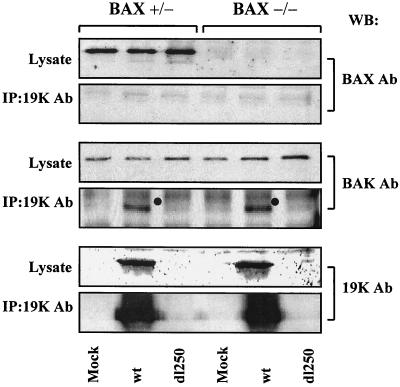
Considering the requirement of BAX for efficient Ad-induced apoptosis (Fig. 1 to 4), it is of interest to determine if E1B-19K suppresses apoptosis during viral infection by complexing with BAX. BAX-expressing and BAX-deficient human cells were infected with Ad2 wt or dl250, and the cell lysates prepared at a late time (36 h) after infection were immunoprecipitated with the 19K antibody and probed with the BAX (residues 43 to 61) antibody. Under our experimental conditions, we did not observe detectable levels of BAX interaction with E1B-19K in BAX-expressing cells (Fig. 5). In contrast, we observed readily detectable levels of interaction of BAK in both Bax+/− and Bax−/− cells. Therefore, it appears that E1B-19K may suppress apoptosis in Bax+/− cells without directly interacting with BAX.
FIG. 5.
Interaction of BAX and BAK with E1B-19K. WCLs prepared from infected cells were immunoprecipitated with E1B-19K antiserum, and the blots were probed with BAX (residues 43 to 61) or BAK or 19K antibodies (Ab). WB, Western blotting; IP, immunoprecipitation.
Effect of Ad infection on BAX expression.
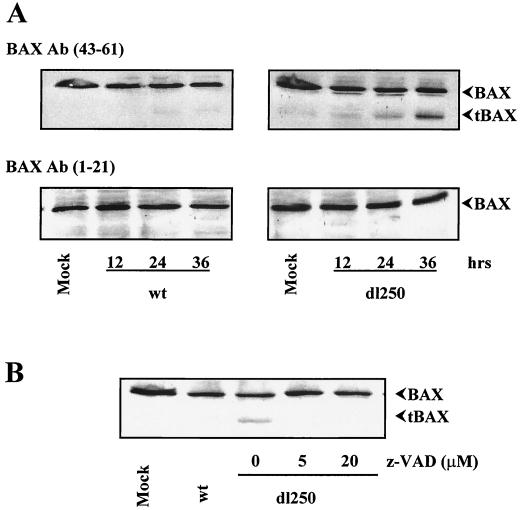
In order to determine if Ad infection alters other aspects of BAX, we analyzed the effect on BAX expression. HCT116Bax cells were infected with Ad2 wt or dl250, and the whole-cell extracts were prepared at different times after infection and analyzed by Western blot analysis using a BAX antibody targeted against amino acids 43 to 61 (Fig. 6A, top panels). In cells infected with Ad2 wt or dl250, the overall level of BAX expression was not significantly altered during the course of infection. However, in cells infected with dl250 in addition to the full-length BAX, a prominent polypeptide form of about 18 kDa was also detected with the BAX (residues 43 to 61) antibody. There was a progressive increase in the level of the 18-kDa band during late stages of infection. The 18-kDa band was not detected when the blots were probed with an antibody specific for the N-terminal region (amino acids 1 to 21) of BAX (Fig. 6A, bottom panels). It appears that the 18-kDa band may be an N-terminally processed form of BAX (hereafter designated tBAX). A similar N-terminally truncated version of BAX has been observed by other investigators during drug-induced apoptosis (15, 41, 42). Although tBAX was also detectable in HCT116Bax cells infected with Ad2 wt, the relative level was much lower than that detected in cells infected with dl250. These results indicate that infection of human epithelial cancer cells with Ad2 dl250 may result in increased levels of tBAX formation compared to that observed in cells infected with Ad2 wt, suggesting that E1B-19K protein may retard the events that result in generation of tBAX during Ad-induced apoptosis.
FIG. 6.
Expression of BAX in Bax+/− cells infected with Ad2 wt or dl250. (A) At various times after infection, WCLs were prepared and analyzed by Western blotting. Blots were probed with indicated BAX antibodies (Ab). (B) Infected cells were treated with indicated concentrations of z-VAD-fmk for 30 h. WCLs were prepared and analyzed by Western blotting using the BAX (residues 43 to 61) antibody.
In drug-treated cells, tBAX has been reported to be generated by a protease sensitive to the broad spectrum caspase/calpain peptidyl inhibitor z-VAD-fmk (15, 42). Recently, a novel transcript that encodes an N-terminally truncated (deletion of exon 1) version of BAX has also been identified (5). To determine if the tBAX observed in cells infected with dl250 is generated by caspase-like proteases activated during viral infection, the infected cells were treated with 5 or 20 μM z-VAD-fmk and the cell extracts were analyzed by Western blot analysis with BAX (residues 43 to 61) antibody (Fig. 6B). The formation of tBAX was strongly inhibited by z-VAD-fmk, suggesting that tBAX observed in dl250-infected cells was generated by a caspase-like activity.
Effect of Ad infection on insertion of BAX into membrane.
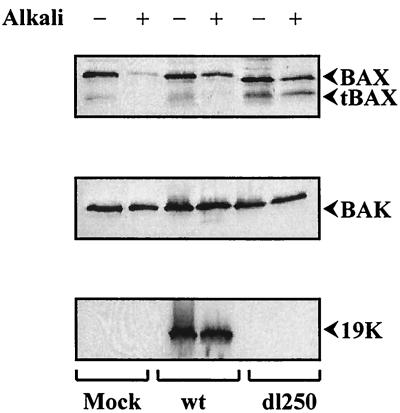
A number of apoptotic stimuli have been shown to induce conformational change in BAX (11, 21, 40), possibly resulting in oligomerization (18, 21) and insertion into the outer mitochondrial membrane (12, 16). In order to determine the effect of Ad infection on insertion of BAX in the membrane components, we infected HCT116Bax cells with Ad2 wt or dl250, prepared the HM fractions of the infected cells, and performed alkali extraction of proteins from these fractions (12, 16, 24). Western blot analysis of these proteins revealed that a significant amount of BAX from mock-infected cells was lost during alkali extraction, while a significant amount of full-length BAX was resistant to alkali treatment of samples prepared from Ad-infected cells (Fig. 7). Although reduced amounts of tBAX were observed in the samples prepared from cells infected with Ad2 wt, significant amounts of it were not resistant to alkali treatment. In contrast, near-equal amounts of both full-length BAX and tBAX were resistant to alkali treatment in samples prepared from cells infected with dl250. In comparison to the patterns observed with BAX, BAK was resistant to alkali treatment in all samples, indicating constitutive BAK insertion into membrane. In cells infected with Ad2 wt, most of E1B-19K was also inserted into HM fractions.
FIG. 7.
Insertion of BAX into membranes. The HM fractions prepared from Bax+/− cells infected with Ad2 wt or dl250 were either untreated (−) or treated (+) with alkali. The samples were analyzed by Western blotting and probed with BAX (residues 43 to 61) or BAK or E1B-19K antibodies.
DISCUSSION
We have shown that the BH-123 (multidomain) BCL-2 family proapoptotic protein BAX is required for efficient manifestation of apoptosis induced by Ad2 in human epithelial cancer cells. This conclusion is based on our observation that the infection of a colon cancer cell line (HCT116BaxKO) deficient in BAX expression (Bax−/−) with the Ad2 mutant dl250 results in reduced levels of manifestation of three distinguishing phenotypes (Lp, Cyt, and Deg) associated with infection by Ad mutants defective in E1B-19K expression. However, our studies do not rule out the requirement of other components of the human apoptosis machinery for Ad-induced apoptosis. Studies with mouse embryo fibroblasts deficient for BAX and BAK have revealed that both proteins are essential for apoptosis induced by multiple stimuli, suggesting that these proapoptotic proteins are functionally overlapping in these cells (37, 44). We have shown elsewhere that the HCT116BaxKO cell line expresses functional BAK protein (36). Although the possibility that BAK may also be required for efficient Ad-induced apoptosis cannot be ruled out, our studies show that BAX plays an important role in human epithelial cancer cells.
Our results suggest that modulation of apoptosis by E1B-19K in Ad-infected colon cancer cells may not involve direct BAX-19K complex formation. Our observation agrees with a recent report by Sundararajan et al. (32), who observed interaction between E1B-19K and BAX only in Ad-infected cells that were exposed to tumor necrosis factor alpha and not in untreated infected cells. Our results suggest that E1B-19K may suppress Ad-induced apoptosis by modulating the activities of BAX. We have made two interesting observations that may be important in this regard. First, we have observed in Bax+/− cells that infection with the 19K mutant (dl250) causes enhanced levels of tBAX. Second, cells infected with the mutant virus also contain increased levels of tBAX inserted into the membrane. tBAX has been previously observed in certain cells during drug-induced apoptosis (15, 41, 42). Recently, a novel Bax transcript (possibly generated by alternate promoter usage) that deletes exon 1 of Bax has been identified (5). The truncated versions of BAX that lack the N-terminal 32 residues (corresponding to a product generated by calpain) (15, 41) as well as the truncated BAX (lacking exon 1) encoded by the newly discovered Bax transcript (5) have been shown to exhibit enhanced cell death activity. A number of studies have reported that BAX is activated during apoptosis by modifications at the N terminus. The nature of the N-terminal modification is unknown. It has been postulated that the N-terminal modification of BAX may free the C-terminal hydrophobic membrane-anchoring domain (22, 25). In addition to other potential sequence-specific modifications of the N terminus, BAX may also be activated by N-terminal truncation that may enhance the cell death activity of BAX. This step may be a target for E1B-19K.
We have observed reduced levels of tBAX in cells infected with Ad2 wt. It appears that the tBAX observed in wt-infected cells may not be competent for insertion into the membrane (Fig. 7), while a significant amount of tBAX observed in dl250-infected cells is inserted into the membrane. It has previously been reported that membrane insertion of an N-terminally truncated version of BAX requires an apoptotic stimulus, and this step could be inhibited by a broad-spectrum caspase inhibitor, z-VAD-fmk (16). A chimeric BAX protein that contains an N-terminal truncation and the BCL-2 transmembrane domain has been previously shown to be constitutively targeted to mitochondrial membrane and induced enhanced cell death (16). It is possible that a z-VAD-sensitive protease activity may be required for directing insertion of the C-terminal hydrophobic domain into the membrane. It is possible that 19K may also interfere with the activation of such a protease.
Generally, it is believed that insertion of activated BAX into the outer mitochondrial membrane leads to release of apoptogenic factors and, in general, mitochondrial dysfunction (reviewed in reference 27). We postulate that E1B-19K may suppress Ad-induced apoptosis by interfering with early checkpoints that may regulate BAX activity rather than direct complex formation. We envision that this could be accomplished at a stage when the Ad apoptosis paradigm may be activated by a potential BH3-only protein. Further experiments are essential to test this hypothesis.
Acknowledgments
Elena Lomonosova and T. Subramanian contributed equally to this study.
We thank B. Vogelstein for the kind gift of HCT116Bax and BaxKO cell lines.
This study was supported by research grants CA-33616 and CA-73803.
REFERENCES
- 1.Bayley, S. T., and J. S. Mymryk. 1994. Adenovirus E1A proteins and transformation. Int. J. Oncol. 5:425-444. [DOI] [PubMed] [Google Scholar]
- 2.Boyd, J. M., G. J. Gallo, B. Elangovan, A. B. Houghton, S. Malstrom, B. J. Avery, R. G. Ebb, T. Subramanian, T. Chittenden, R. J. Lutz, and G. Chinnadurai. 1995. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene 11:1921-1928. [PubMed] [Google Scholar]
- 3.Boyd, J. M., S. Malstrom, T. Subramanian, L. K. Venkatesh, U. Schaeper, B. Elangovan, C. D'Sa-Eipper, and G. Chinnadurai. 1994. Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell 79:341-351. [DOI] [PubMed] [Google Scholar]
- 4.Branton, P. E., and D. E. Roopchand. 2001. The role of adenovirus E4orf4 protein in viral replication and cell killing. Oncogene 20:7855-7865. [DOI] [PubMed] [Google Scholar]
- 5.Cartron, P. F., L. Oliver, S. Martin, C. Moreau, M. T. LeCabellec, P. Jezequel, K. Meflah, and F. M. Vallette. 2002. The expression of a new variant of the pro-apoptotic molecule Bax, Baxpsi, is correlated with an increased survival of glioblastoma multiforme patients. Hum. Mol. Genet. 11:675-687. [DOI] [PubMed] [Google Scholar]
- 6.Chen, G., P. E. Branton, E. Yang, S. J. Korsmeyer, and G. C. Shore. 1996. Adenovirus E1B 19-kDa death suppressor protein interacts with Bax but not with Bad. J. Biol. Chem. 271:24221-24225. [DOI] [PubMed] [Google Scholar]
- 7.Chinnadurai, G. 1983. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell 33:759-766. [DOI] [PubMed] [Google Scholar]
- 8.Chinnadurai, G. 1998. Control of apoptosis by human adenovirus genes. Semin. Virol. 8:399-408. [Google Scholar]
- 9.Chiou, S. K., C. C. Tseng, L. Rao, and E. White. 1994. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J. Virol. 68:6553-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng, Y., Y. Lin, and X. Wu. 2002. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 16:33-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desagher, S., A. Osen-Sand, A. Nichols, R. Eskes, S. Montessuit, S. Lauper, K. Maundrell, B. Antonsson, and J. C. Martinou. 1999. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144:891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskes, R., S. Desagher, B. Antonsson, and J. C. Martinou. 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20:929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezoe, H., R. B. Fatt, and S. Mak. 1981. Degradation of intracellular DNA in KB cells infected with cyt mutants of human adenovirus type 12. J. Virol. 40:20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrow, S. N., J. H. White, I. Martinou, T. Raven, K. T. Pun, C. J. Grinham, J. C. Martinou, and R. Brown. 1995. Cloning of a bcl-2 homologue by interaction with adenovirus E1B 19K. Nature 374:731-733. [DOI] [PubMed] [Google Scholar]
- 15.Gao, G., and Q. P. Dou. 2000. N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J. Cell. Biochem. 80:53-72. [DOI] [PubMed] [Google Scholar]
- 16.Goping, I. S., A. Gross, J. N. Lavoie, M. Nguyen, R. Jemmerson, K. Roth, S. J. Korsmeyer, and G. C. Shore. 1998. Regulated targeting of BAX to mitochondria. J. Cell Biol. 143:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green, M., K. H. Brackmann, L. A. Lucher, J. S. Symington, and T. A. Kramer. 1983. Human adenovirus 2 E1B-19K and E1B-53K tumor antigens: antipeptide antibodies targeted to the NH2 and COOH termini. J. Virol. 48:604-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross, A., J. Jockel, M. C. Wei, and S. J. Korsmeyer. 1998. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 17:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han, J., P. Sabbatini, D. Perez, L. Rao, D. Modha, and E. White. 1996. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 10:461-477. [DOI] [PubMed] [Google Scholar]
- 20.Han, J., P. Sabbatini, and E. White. 1996. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol. Cell. Biol. 16:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu, Y. T., K. G. Wolter, and R. J. Youle. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 94:3668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaled, A. R., K. Kim, R. Hofmeister, K. Muegge, and S. K. Durum. 1999. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc. Natl. Acad. Sci. USA 96:14476-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBlanc, H., D. Lawrence, E. Varfolomeev, K. Totpal, J. Morlan, P. Schow, S. Fong, R. Schwall, D. Sinicropi, and A. Ashkenazi. 2002. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat. Med. 8:274-281. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, M., D. G. Millar, V. W. Yong, S. J. Korsmeyer, and G. C. Shore. 1993. Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J. Biol. Chem. 268:25265-25268. [PubMed] [Google Scholar]
- 25.Pawlowski, J., and A. S. Kraft. 2000. Bax-induced apoptotic cell death. Proc. Natl. Acad. Sci. USA 97:529-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilder, S., J. Logan, and T. Shenk. 1984. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J. Virol. 52:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed, J. C., and D. R. Green. 2002. Remodeling for demolition. Changes in mitochrondrial ultrastructure during apoptosis. Mol. Cell 9:1-3. [DOI] [PubMed] [Google Scholar]
- 28.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian, T., M. Kuppuswamy, J. Gysbers, S. Mak, and G. Chinnadurai. 1984. 19-kDa tumor antigen coded by early region E1b of adenovirus 2 is required for efficient synthesis and for protection of viral DNA. J. Biol. Chem. 259:11777-11783. [PubMed] [Google Scholar]
- 30.Subramanian, T., M. Kuppuswamy, S. Mak, and G. Chinnadurai. 1984. Adenovirus cyt+ locus, which controls cell transformation and tumorigenicity, is an allele of lp+ locus, which codes for a 19-kilodalton tumor antigen. J. Virol. 52:336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subramanian, T., B. Tarodi, and G. Chinnadurai. 1995. p53-independent apoptotic and necrotic cell deaths induced by adenovirus infection: suppression by E1B 19K and Bcl-2 proteins. Cell Growth Differ. 6:131-137. [PubMed] [Google Scholar]
- 32.Sundararajan, R., A. Cuconati, D. Nelson, and E. White. 2001. Tumor necrosis factor-alpha induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K. J. Biol. Chem. 276:45120-45127. [DOI] [PubMed] [Google Scholar]
- 33.Takemori, N., C. Cladaras, B. Bhat, A. J. Conley, and W. S. Wold. 1984. cyt gene of adenoviruses 2 and 5 is an oncogene for transforming function in early region E1B and encodes the E1B 19,000-molecular-weight polypeptide. J. Virol. 52:793-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemori, N., J. L. Riggs, and C. Aldrich. 1968. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology 36:575-586. [DOI] [PubMed] [Google Scholar]
- 35.Tarodi, B., T. Subramanian, and G. Chinnadurai. 1993. Functional similarity between adenovirus E1b 19k gene and Bcl2 oncogene—mutant complementation and suppression of cell death induced by DNA damaging agents. Int. J. Oncol. 3:467-472. [DOI] [PubMed] [Google Scholar]
- 36.Theodorakis, P., E. Lomonosova, and G. Chinnadurai. 2002. Critical requirement of BAX for manifestation of apoptosis induced by multiple stimuli in human epithelial cancer cells. Cancer Res. 62:3373-3376. [PubMed] [Google Scholar]
- 37.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White, E., T. Grodzicker, and B. W. Stillman. 1984. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J. Virol. 52:410-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wold, W. S. M., and A. E. Tollefson. 1998. Adenovirus E3 proteins: 14.7K, RID, and gp19K inhibit immune-induced cell death; Adenovirus death protein promotes cell death. Semin. Virol. 8:515-523. [Google Scholar]
- 40.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood, D. E., and E. W. Newcomb. 2000. Cleavage of Bax enhances its cell death function. Exp. Cell Res. 256:375-382. [DOI] [PubMed] [Google Scholar]
- 42.Wood, D. E., A. Thomas, L. A. Devi, Y. Berman, R. C. Beavis, J. C. Reed, and E. W. Newcomb. 1998. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17:1069-1078. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, L., J. Yu, B. H. Park, K. W. Kinzler, and B. Vogelstein. 2000. Role of BAX in the apoptotic response to anticancer agents. Science 290:989-992. [DOI] [PubMed] [Google Scholar]
- 44.Zong, W. X., T. Lindsten, A. J. Ross, G. R. MacGregor, and C. B. Thompson. 2001. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]