Abstract
Since most human immunodeficiency virus (HIV) infections are initiated following mucosal exposure to the virus, the anatomic containment or abortion of an HIV infection is likely to require vaccine-elicited cellular immune responses in those mucosal sites. Studying vaccine-elicited mucosal immune responses has been problematic because of the difficulties associated with sampling T lymphocytes from those anatomic compartments. In the present study, we demonstrate that mucosal cytotoxic T lymphocytes (CTL) specific for simian immunodeficiency virus (SIV) and simian HIV can be reproducibly sampled from intestinal mucosal tissue of rhesus monkeys obtained under endoscopic guidance. These lymphocytes recognize peptide-major histocompatibility complex class I complexes and express gamma interferon on exposure to peptide antigen. Interestingly, systemic immunization of monkeys with plasmid DNA immunogens followed by live recombinant attenuated poxviruses or adenoviruses with genes deleted elicits high-frequency SIV-specific CTL responses in these mucosal tissues. These studies therefore suggest that systemic delivery of potent HIV immunogens may suffice to elicit substantial mucosal CTL responses.
Events at mucosal surfaces play a central role in human immunodeficiency virus (HIV) pathogenesis. Most HIV infections occur as a result of genital or rectal mucosal exposure to the virus (9, 10, 29). If an infection can be anatomically contained or aborted during an early phase of the infection process, this control must occur in mucosal tissue (8). Considerable interest, therefore, exists in the development of vaccines that can elicit HIV-specific immunity in mucosal sites.
There is a growing appreciation for the importance of CD8+ cytotoxic T lymphocytes (CTL) in containing HIV replication. CD8+ lymphocytes can inhibit HIV replication in autologous CD4+ T lymphocytes in vitro (30). A temporal correlation has been demonstrated in infected individuals between the partial containment of HIV replication during the period of primary infection and the emergence of HIV-specific CD8+ CTL (13). Monkeys depleted of CD8+ lymphocytes by monoclonal anti-CD8 antibody infusion never control an AIDS virus infection and die with a rapidly progressive clinical disease course (23). Finally, recent studies have shown that vaccine-elicited CD8+ CTL can contribute to the partial containment of subsequent AIDS virus infections in monkeys (2, 3, 4, 22, 26). Taken together, these findings argue that an HIV vaccine should be capable of eliciting a virus-specific CTL response. Moreover, there is reason to presume that such vaccine-elicited cell populations should exist in mucosal sites.
Nonhuman primate models have been crucial in AIDS vaccine development efforts. Asian macaques infected with various simian immunodeficiency virus (SIV) and chimeric simian HIV (SHIV) isolates have provided important models in which to test prototype HIV vaccines (16). Studies of these nonhuman primate models have been facilitated by the genetic characterization of Indian-origin rhesus monkeys and the definition of specific dominant CTL epitopes and the major histocompatibility complex (MHC) class I molecules that present those peptide epitopes to CD8+ T lymphocytes. In particular, the rhesus monkey MHC class I molecule Mamu-A*01 has been shown to present a nine-amino-acid fragment of SIV Gag, called p11C, as a dominant epitope to CD8+ T lymphocytes (1, 18). The quantitation of SIV- and SHIV-specific CTL populations that recognize this dominant epitope has provided a valuable tool for comparing various immunization modalities that are being considered for human vaccine trials (4, 26).
The present studies were initiated to explore approaches to sampling mucosal lymphocyte populations in SIV- and SHIV-infected and vaccinated rhesus monkeys for evaluating virus-specific CTL. In the course of these studies, we found that distal colonic mucosal T-lymphocyte populations in infected monkeys contain an extremely high frequency of virus-specific CTL. Moreover, pilot studies indicate that CTL populations are readily elicited in mucosal sites by systemic vaccination strategies.
MATERIALS AND METHODS
Animals, viruses, and mucosal biopsies.
All rhesus monkeys (Macaca mulatta) evaluated in this study were screened for the presence of the Mamu-A*01 allele using a PCR-based technique described previously (12). For distal-colon biopsies, the monkeys were anesthetized with ketamine (10 to 20 mg/kg of body weight). A commercially available, disposable, sterile 2-mm3-pinch biopsy forceps was inserted approximately 3 to 4 cm into the rectums of the monkeys, and 10 to 12 biopsy specimens were obtained from the distal colonic segment. For duodenal biopsies, the monkeys fasted for 12 h and were anesthetized with telazol (5 mg/kg) and intubated. An Olympus GIFxP10 or P20 pediatric gastroscope was used. With the animal in left lateral recumbency, the scope was inserted through the oral cavity and advanced through the stomach and gastric duodenal sphincter to the duodenum. Six to eight mucosal biopsies were obtained using Olympus 1.9-mm endoscopic-biopsy forceps. The biopsy specimens were placed in RPMI 1640 medium supplemented with 12% fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and l-glutamine (R12 medium) and transported on ice for immediate processing.
The monkeys were infected by intravenous inoculation with SIVmac251; the J5 clone of the 32H strain of SIVmac251, a virus with limited pathogenic potential; or SHIV 89.6P (Table 1). The rhesus monkeys used in this study were maintained at the New England Regional Primate Research Center and at Bioqual Inc. (Rockville, Md.). These animal facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care International and meet National Institutes of Health standards as set forth in the Guidelines for Care and Use of Laboratory Animals.
TABLE 1.
Clinical profiles of chronically SIVmac251-infected, SHIV 89.6P-infected, and vaccinated Mamu-A*01+ rhesus monkeys
| Monkey | Virus or vaccine | No. of CD4+ T lympho- cytes (μl) | Plasma viral load (RNA copies/ml) | Duration of infection (days) |
|---|---|---|---|---|
| 138-88 | SIVmac251 | 135 | <500 | 2,860 |
| 93-98 | SIVmac251 | 695 | <500 | 850 |
| 191-96 | SIVmac251 | 450 | <500 | 1,275 |
| 94-98 | SIVmac251 | 206 | <4,091 | 850 |
| 154-97 | SIVmac251 | 244 | 1,368 | 1,550 |
| 403-91 | SIVmac251(J5) | 479 | <500 | 3,315 |
| 448-98 | SIVmac251 | 590 | <500 | NAc |
| 253-96 | SIVmac251 | 637 | 839 | 1,315 |
| 88-98 | SIVmac251 | 84 | 1,030,655 | 1,215 |
| H547 | SHIV 89.6P | 213 | 55,023 | 580 |
| H544 | SHIV 89.6P | 804 | <500 | 580 |
| AW2P | DNA/rAd5a | 1,328 | ||
| AW28 | DNA/rAd5 | 2,401 | ||
| 90-98 | DNA/rMVAb | 1,190 | ||
| 95-98 | DNA/rMVA | 1,114 | ||
| 128-97 | DNA/rMVA | 1,889 | ||
| 135-97 | DNA/rMVA | 1,349 |
Immunized with plasmid DNA containing the SIVmac239 gag-pol-nef and HIV-1 89.6P env gene and boosted with recombinant adenovirus serotype 5 constructs expressing the SIV gag-pol-nef and HIV-1 89.6P env genes.
Immunized with plasmid DNAs containing the SIVmac239 gag, pol, and nef and the HIV-1 89.6 env epitope genes and boosted with recombinant MVA vectors expressing SIV gag-pol and HIV-1 89.6 env.
NA, data not available.
T-lymphocyte subsets and plasma viral RNA assays.
Peripheral blood CD4+- and CD8+-T-lymphocyte absolute counts were derived by multiplying the total lymphocyte counts by the percentages of CD3+ CD4+ and CD3+ CD8+ T cells as determined by cell staining and flow cytometric analysis. Plasma viral RNA levels were determined by a quantitative branched-chain DNA assay (Bayer Diagnostics, Berkeley, Calif.). The results of this assay were expressed in copies per milliliter of plasma with a detection limit of 500 copies per ml (21).
Isolation of lymphocytes from gastrointestinal mucosal biopsies.
Duodenal and colonic biopsy specimens were transported in R12 medium. Samples were then transferred into RPMI medium containing collagenase type IV (300 IU/ml) and DNase (30 IU/ml) (Sigma Chemicals) and incubated in a water bath at 37°C for 1 h to release the lymphocytes. The biopsy tissues were then filtered through a thin mesh, pelleted at 450 × g for 7 min, placed in a Percoll gradient (65 and 30%), and subjected to centrifugation for 25 min at 1,000 × g. The lymphocytes were collected at the interface between 65 and 30% of the Percoll gradient and washed twice in R12 medium. There was a consistent yield of 1 × 106 to 3 × 106 lymphocytes from 6 to 8 duodenal and 5 × 105 to 1 × 106 lymphocytes from ∼10 distal-colonic 2-mm3 biopsy specimens.
Mamu-A*01/p11C tetramer complex staining.
The Mamu-A*01/p11C tetramer was produced as previously described (14) by mixing biotinylated Mamu-A*01/p11C complex with phycoerythrin (PE)-labeled ExtrAvidin (Sigma, St. Louis, Mo.) at a 4:1 molar ratio. The monoclonal antibodies (MAbs) utilized in this study were directly coupled to fluorescein isothiocyanate (FITC), PE-Texas Red (ECD), or allophycocyanin (APC). These MAbs included anti-CD8α-ECD (7pt3F9; Beckman Coulter Inc., Fullerton, Calif.), anti-CD3-FITC (SP34; BD Pharmingen, San Diego, Calif.), and anti-gamma interferon (IFN-γ)-APC (B27; BD Pharmingen). All cells referred to as CD8+ T lymphocytes were CD8α+ CD3+ cells, and tetramer staining of CD8α+ cells was performed on gated CD3+ cells. Samples were analyzed on a FACSCalibur instrument using CELLQuest software.
Intracellular cytokine staining.
To determine the cytokine expression capacity of SIVmac- and SHIV-specific CTL, we stained lymphocytes with the anti-IFN-γ antibody B27 (BD Pharmingen) after stimulating them with either phorbol myristate acetate (PMA) plus ionomycin or the optimal nine-amino-acid Mamu-A*01-restricted Gag CTL epitope peptide p11C (17). Approximately 2 × 105 lymphocytes from mucosal tissue or 150 μl of whole blood was suspended in 350 μl of R12 medium. These cells were stimulated with either PMA (200 ng/ml) and ionomycin (1 μg/ml) or the peptide p11C (CTPYDINQM) (5 μg/ml) in the presence of 2 μg of anti-CD28 and anti-CD49d MAbs/ml. Cells in a third tube contained R12 medium with no lectin or peptide. All tubes contained 10 μg of brefeldin A (BD Pharmingen)/ml, an inhibitor of intracellular transport, to prevent cellular secretion of any cytokines produced. Each cell population was then incubated at 37°C for 6 h in a water bath, washed twice with phosphate-buffered saline-2% fetal calf serum, and incubated at room temperature for 15 min in the dark with the Mamu-A*01/p11C tetramer, anti-CD3-FITC, and anti-CD8-ECD. When whole blood was analyzed, red blood cells were lysed and the specimens were fixed (with the Immunoprep reagent system and a Q-prep workstation [Beckman Coulter, Inc.]). The cells were washed once with phosphate-buffered saline-2% fetal calf serum and permeabilized with 500 μl of Cytofix/Cytoperm (BD Pharmingen). The cells were then washed twice with 1× Perm/Wash buffer (BD Pharmingen), stained with 1 μg of anti-cytokine antibody (anti-IFN-γ-APC; BD Pharmingen), and incubated for 45 min. The cells were washed twice with 1× Perm/Wash buffer, fixed with 1.5% formaldehyde, and analyzed by flow cytometry. The proportion of cytokine producing CD8+ CD3+ p11C tetramer+ T cells in each sample was determined. Samples were analyzed on a FACSCalibur utilizing CELLQuest software.
Immunohistochemistry.
Immunohistochemical staining for CD8+ T cells was performed on 5-μm-thick sections of cryopreserved jejunum and distal colon as previously described (20). Briefly, formalin-fixed, paraffin-embedded sections were deparaffinized and unmasked using Trilogy reagent solution (Cell Marque Corp., Hot Springs, Ark.) according to the manufacturer's instructions. The sections were then blocked for 5 min with 0.3% hydrogen peroxide, washed for 5 min in phosphate-buffered saline containing 0.1% fish skin gelatin (Sigma), incubated for 10 min with Protein Block (Dako, Carpinteria, Calif.), washed, and incubated with anti-CD8 (Novacastra clone 1A5) for 1 h at room temperature. The sections were then washed and incubated for 30 min with horseradish peroxidase-labeled polymer (Dako) and developed with diaminobenzidine (Dako).
Vaccination protocols.
Two groups of vaccinated monkeys were evaluated in this study. Group I monkeys (128-97, 135-97, 90-98, and 95-98) were immunized four times by the intramuscular route with 8 mg of plasmid DNA containing nef, env, pol, and gag CTL epitope genes; rested for 10 months; and boosted by intramuscular inoculation with 108 PFU each of two recombinant modified vaccinia virus Ankara (MVA) isolates, one expressing full-length sequence of the SIVmac239 gag-pol and the second expressing the HIV type 1 (HIV-1) 89.6 env gene (7). Colonic mucosal biopsies were performed on these monkeys 3 weeks after the final recombinant MVA inoculations. Group II monkeys (AW2P and AW28) were injected three times (weeks 0, 4, and 8) intramuscularly with 5 mg of a plasmid DNA vaccine containing HIV-1 89.6P env (KB9) and 5 mg of a plasmid DNA vaccine containing SIVmac239 gag-pol-nef and boosted by intramuscular inoculation with recombinant adenovirus serotype 5 constructs with the E1 gene deleted and the E3 gene inactivated. These recombinant adenoviruses carried the same viral env and gag-pol-nef gene inserts as the plasmid DNA vaccine constructs. Colonic mucosal biopsies were performed on these monkeys 4 weeks following the recombinant adenovirus inoculations.
RESULTS
Gastrointestinal mucosal CD8+ lymphocytes in SIVmac- and SHIV-infected rhesus monkeys.
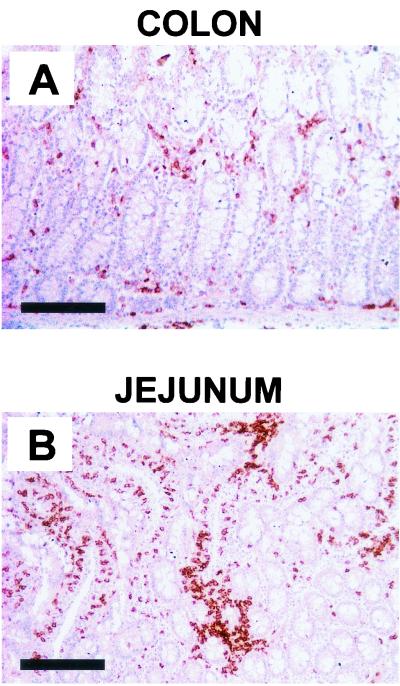
In initiating studies of mucosal CTL populations in infected and vaccinated rhesus monkeys, we sought to determine the distribution of CD8+ lymphocytes in accessible mucosal sites in these animals. Since T-lymphocyte populations can be obtained from the gastrointestinal mucosa of monkeys through biopsies, we concentrated our attention on this anatomic compartment. Histologic sections of duodenal and distal-colonic mucosal tissues of chronically SIVmac-infected monkeys were first obtained at necropsy and evaluated for the presence of CD8+ lymphocytes by using immunohistochemical staining techniques (Fig. 1). These studies demonstrated the presence of CD8+ cells diffusely distributed throughout the mucosa and submucosa of the tissues. Interestingly, CD8+ lymphocytes were present at a much higher density in the duodenal than in the distal-colonic mucosal tissues.
FIG. 1.
Immunohistochemically stained serial sections from distal colon (A) and jejunum (B) of a SIVmac-infected rhesus monkey demonstrating distribution of CD8+ lymphocytes. Bar, 200 μm.
Studies were then initiated to sample lymphocyte populations from these mucosal sites in chronically SIVmac-infected monkeys. Pinch biopsy specimens were obtained from the duodenal and distal colons of the monkeys. We found that substantial numbers of lymphocytes could be harvested from these gastrointestinal compartments. Moreover, consistent with the histologic findings, greater numbers of cells were obtained from the duodenal than from the distal colonic biopsy specimens. Consistently, 1 × 106 to 3 × 106 duodenal lymphocytes and 5 × 105 to 1 × 106 distal-colonic lymphocytes were obtained from 10 2-mm3 biopsy specimens.
Tetramer+ CD8+ T lymphocytes are present in the gastrointestinal mucosa of SIVmac- and SHIV 89.6P-infected monkeys.
To assess the distribution of virus-specific CTL in mucosal compartments of chronically infected monkeys, studies were done with monkeys expressing the MHC class I allele Mamu-A*01. By using these genetically selected monkeys, CTL that recognize the Mamu-A*01-restricted dominant Gag epitope p11C could be monitored using the tetramer technology. We thus quantitated Mamu-A*01/p11C tetramer-binding CD8+ T lymphocytes in selected tissues of a cohort of SIVmac- and SHIV 89.6P-infected, Mamu-A*01+ rhesus monkeys (Tables 1 and 2). The distribution of tetramer-binding CD8+ T lymphocytes was evaluated in the peripheral blood, lymph node, duodenum, and distal colon of the monkeys. Interestingly, a significantly higher proportion of CD8+ T lymphocytes were tetramer+ in the distal-colonic mucosa than in the other evaluated anatomic compartments. The percentage of tetramer+ cells in the colonic mucosal compartment ranged from 0.80 to 28.14% of the CD8+ T cells, while in the peripheral blood the tetramer+ cells ranged from 0.31 to 6.79%. The representation of tetramer+ cells in the CD8+ T lymphocytes in the duodenal mucosal compartment was comparable to that in the peripheral blood of the monkeys, while the proportion of CD8+ T lymphocytes that were tetramer+ cells in the lymph nodes was higher than in the peripheral blood or duodenal mucosa.
TABLE 2.
Distribution of p11C tetramer-binding CD3+ CD8+ T lymphocytes in systemic and mucosal lymphoid compartments of chronically SIVmac- and SHIV 89.6P-infected Mamu-A*01+ rhesus monkeys
| Monkey | Virus | % p11C tetramer-binding CD3+ CD8+ T lymphocytesa
|
|||
|---|---|---|---|---|---|
| PBLb | Lymph nodec | Duodenumd | Colone | ||
| 138-88 | SIVmac251 | 4.85 | NAf | 2.83 | 28.14 |
| 93-98 | SIVmac251 | 6.79 | 12.69 | 5.69 | 14.74 |
| 191-96 | SIVmac251 | 1.70 | 22.10 | 10.18 | 26.87 |
| 154-97 | SIVmac251 | 1.36 | 12.06 | 11.09 | 20.46 |
| 94-98 | SIVmac251 | 0.38 | 0.54 | 1.27 | 2.84 |
| 403-91 | SIVmac251 | 1.79 | 0.95 | 0.81 | 0.80 |
| 448-98 | SIVmac251 | 3.39 | NA | NA | 15.31 |
| 88-98 | SIVmac251 | 0.16 | 1.03 | 0.54 | 1.48 |
| H-544 | SHIV 89.6P | 1.65 | NA | NA | 11.19 |
| H-547 | SHIV 89.6P | 0.31 | NA | NA | 6.24 |
Staining with PE-coupled tetrameric Mamu-A*01/p11C complex and gated on CD8+ T lymphocytes
PBL, peripheral blood lymphocyte.
Lymphocytes from femoral lymph node obtained by incisional biopsy.
Lymphocytes isolated from duodenal mucosal specimens obtained by endoscopic pinch biopsy.
Lymphocytes isolated from distal-colonic mucosal biopsy specimens obtained by pinch biopsy.
NA, not available.
Mucosal tetramer+ CD8+ T lymphocytes secrete IFN-γ.
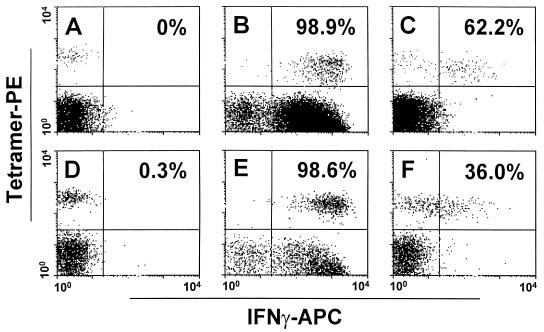
To assess the functional capability of the Mamu-A*01/p11C-binding mucosal CD8+ T cells, we evaluated the abilities of these cells from five chronically SIVmac-infected Mamu-A*01+ rhesus monkeys to secrete IFN-γ (Fig. 2). Cytokine secretion was assessed in the isolated lymphocyte populations following either nonspecific (PMA plus ionomycin) or peptide antigenic (p11C) stimulation (Table 3). On stimulation with PMA plus ionomycin, the proportion of IFN-γ-secreting tetramer+ CD8+ T cells in the blood ranged from 92 to 99% (mean, 96.4%), while IFN-γ-secreting tetramer+ cells from the colonic mucosa ranged from 95 to 99.5% (mean, 97.5%). Similarly, a mean of 38.9% (range, 26.4 to 62.2%) of the tetramer+ CD8+ T cells secreted IFN-γ following stimulation by Gag epitope peptide (p11C) in the blood compared to a mean of 49.2% (range, 36.5 to 70.2%) in the colonic mucosa. Thus, the proportions of p11C tetramer+ CD8+ T cells secreting IFN-γ were comparable in the blood and the colonic mucosa.
FIG. 2.
IFN-γ expression by p11C tetramer+ CD3+ CD8+ T lymphocytes from peripheral blood and distal-colonic mucosal lymphocytes of a SHIV 89.6P-infected rhesus monkey. Tetramer-binding peripheral blood (A to C) and distal-colonic mucosal (D to F) CD8+ T lymphocytes stain with monoclonal anti-IFN-γ antibody after exposure in vitro to PMA plus ionomycin (B and E) or the Gag epitope peptide p11C (C and F). Percentages of p11C tetramer+ CD3+ CD8+ T lymphocytes positive for IFN-γ are shown in each panel.
TABLE 3.
IFN-γ production by peripheral blood and colonic mucosal tetramer+ CD8+ T lymphocytes of chronically infected Mamu-A*01+ rhesus monkeys
| Monkey | % IFN-γ+ p11C tetramer+ CD8+ T lymphocytes
|
|||||
|---|---|---|---|---|---|---|
| Peripheral blood lymphocytes
|
Distal-colonic lymphocytes
|
|||||
| Unstimu- lated | PMA + Ionoa | p11C | Unstimu- lated | PMA + Iono | p11C | |
| 138-88 | 1.50 | 97.60 | 37.80 | 0.60 | 95.30 | 60.00 |
| 191-96 | 2.50 | 94.60 | 41.30 | 4.00 | 95.50 | 36.50 |
| 448-98 | 0.19 | 98.80 | 27.22 | 2.67 | 98.60 | 70.20 |
| 93-98 | 0.80 | 92.11 | 26.40 | 1.6 | 99.50 | 43.70 |
| H-544 | 0.00 | 98.90 | 62.20 | 0.26 | 98.60 | 36.00 |
Iono, ionomycin.
Mucosal CTL responses can be elicited by systemic priming with plasmid DNA vaccines and boosting with either recombinant MVA or recombinant adenoviruses.
To assess the efficiency of systemic vaccination strategies in inducing cellular immune responses in mucosal sites, we evaluated six monkeys that were vaccinated according to two different vaccine regimens (Table 4). Group I consisted of four monkeys (135-97, 128-97, 90-98, and 95-98) that were immunized by the intramuscular route with a plasmid DNA construct encoding Mamu-A*01-restricted epitopes derived from nef, env, pol, and gag and boosted with two recombinant modified vaccinia viruses (MVA), one expressing full-length sequences of SIV gag-pol and the other expressing full-length sequence of the HIV-1 89.6 env gene. Group II consisted of two monkeys (AW2P and AW28) immunized by the intramuscular route with plasmid DNA vaccines containing HIV-1 89.6P env (KB9) and SIVmac239 gag-pol-nef and boosted with a recombinant adenovirus type 5 with genes deleted expressing these same genes.
TABLE 4.
Mucosal p11C tetramer-binding CD3+ CD8+ lymphocytes in systemically vaccinated Mamu-A*01+ rhesus monkeys
| Vaccine | Monkey | % p11C tetramer-binding CD3+ CD8+ T lymphocytes
|
|
|---|---|---|---|
| Peripheral blood | Distal colon | ||
| DNA/rMVAa | 135-97 | 1.39 | 0.66 |
| DNA/rMVA | 128-97 | 1.04 | 0.68 |
| DNA/rMVA | 90-98 | 0.34 | 0.34 |
| DNA/rMVA | 95-98 | 0.33 | 0.15 |
| DNA/rAd5b | AW2P | 2.30 | 1.00 |
| DNA/rAd5 | AW28 | 2.46 | 1.75 |
Immunized with plasmid DNAs containing the SIVmac239 gag, pol, and nef and the HIV-1 89.6 env epitope genes and boosted with recombinant MVA vectors expressing SIV gag-pol and HIV-1 89.6 env.
Immunized with plasmid DNA containing the SIVmac239 gag-pol-nef and HIV-1 89.6P env gene and boosted with recombinant adenovirus serotype 5 constructs expressing the SIV gag-pol-nef and HIV-1 89.6P env genes.
A population of tetramer-binding CD8+ T lymphocytes was detectable in the colon and peripheral blood of all the monkeys in both groups of vaccinated animals. Group I vaccinated animals had a mean of 0.45% (range, 0.15 to 0.68%) tetramer+ CD8+ T cells in the colonic mucosa and a mean of 0.75% (range, 0.33 to 1.39%) tetramer+ CD8+ T cells in the peripheral blood, while group II animals had a mean of 1.37% (range, 1.00 to 1.75%) tetramer+ CD8+ T cells in the colonic mucosa and a mean of 2.38% (range, 2.30 to 2.46%) in the peripheral blood.
A pilot study was then done to evaluate the functional capabilities of such vaccine-elicited mucosal CTL (Table 5). The tetramer+ CD8+ T cells from two of the vaccinated monkeys were assessed for the capacity to secrete IFN-γ. The potent polyclonal stimulation by PMA plus ionomycin induced >94% of both the peripheral blood and colonic mucosa tetramer+ CD8+ T cells to produce IFN-γ. Therefore, these vaccine-elicited mucosal tetramer+ CD8+ T lymphocytes were functional.
TABLE 5.
Intracellular cytokine staining in peripheral blood and mucosal (distal-colon) tetramer+ CD8+ lymphocytes from DNA-primed and recombinant-adenovirus-boosted rhesus monkeys following in vitro stimulation
| Monkey | % p11C tetramer+ IFN-γ+ CD8+ T cells
|
|||
|---|---|---|---|---|
| Peripheral blood lymphocytes
|
Distal-colonic lymphocytes
|
|||
| Unstimulateda | PMA + Ionob | Unstimulated | PMA + Iono | |
| AW28 | 0.40 | 98.91 | 3.40 | 97.93 |
| AW2P | 0.00 | 98.37 | 3.00 | 94.28 |
Peripheral blood and colonic lymphocytes containing brefeldin A (10μg/ml) were cultured for 6 h at 37°C suspended in RPMI containing 12% fetal calf serum.
Iono, ionomycin.
DISCUSSION
The limitations in our understanding of the biology of mucosal T lymphocytes in nonhuman primates and humans can be attributed at least in part to the difficulties associated with accessing these cell populations for study. Murphey-Corb et al. were forced to use tissues obtained at necropsy to evaluate intestinal T-cell responses in rhesus monkeys that had been exposed to SIV by the colonic route (19). Belyakov et al. resorted to laparotomy and colonic wedge resection in monkeys to sample mucosal T lymphocytes in their evaluation of the efficacy of a vaccine strategy specifically devised to elicit mucosal immunity (6). Duodenal lymphocytes from SIV-infected monkeys have recently been accessed by endoscopy-guided biopsy, a procedure that is also relatively noninvasive (24). Mucosal lymphocytes sampled from the seminal fluid of monkeys obtained by electroejaculation, a strategy for accessing cells that is not uniformly successful, have also been studied (11). The procedure employed for harvesting mucosal lymphocytes in the present study, isolating them from proctoscopy-guided pinch biopsy specimens, represents a more reproducibly successful and less invasive approach to sampling mucosal lymphocytes from monkeys than the strategies that have previously been used.
While the techniques employed in the present study should lend themselves to routine use in monitoring mucosal T-cell populations in nonhuman primates, they are still far from ideal for studying T-lymphocyte biology. Relatively small numbers of lymphocytes can be obtained from an animal at any one time, usually only ∼106 cells. This drastically limits the diversity of studies that can be carried out on a sampled lymphocyte population. Moreover, in that these studies are dependent on the use of tetramer staining for defining antigen-specific T lymphocytes, this work can only be done with monkeys with a defined, shared MHC class I haplotype and known CTL epitopes (24). The number of monkeys that can be used for any single study at the present time is therefore also limited.
The reason that a particularly high concentration of Gag-specific CD8+ CTL exists in the distal-colonic mucosal tissue of SIV- and SHIV-infected monkeys is not readily apparent. The kinetics of the evolution of duodenal and peripheral blood CTL during primary SIV infection have been shown to be comparable (27). However, previous studies have demonstrated that SIV-specific CTL are variably localized in different anatomic compartments in the chronically infected monkey. Thus, these CTL populations are present at higher frequencies in the bone marrow, spleen, and liver than in lymph nodes and peripheral blood (15). It is possible that the CTL localize to the distal-colonic tissue because of a particularly high frequency of virus-infected cells in that anatomic location. It has certainly been well documented that the gastrointestinal tissue is a major site of SIV replication in the infected animal (28). It is also possible that subpopulations of CTL express cell surface molecules that favor the homing of these cells to the distal-colonic mucosa (27). Precedent exists for this as well, since it has been previously shown that the CTL localized to the liver in SIV-infected monkeys express CD62L, a molecule that mediates adhesion of lymphocytes to high endothelial venules (25). Finally, it is possible that the absolute numbers of CD8+ CTL are comparable in the duodenum and distal colon of the infected monkeys. The differences in percent tetramer-binding CD8+ T lymphocytes in these two regions of the gastrointestinal mucosa may simply reflect differences in numbers of resident CD8+ lymphocytes that are not virus-specific CTL. The substantial differences in absolute numbers of tetramer+ CD8+ lymphocytes in the duodenum and distal colon of infected monkeys shown in the present study are consistent with this possibility.
A definition of the functional repertoire of gut-associated virus-specific CTL in SIV-infected monkeys has been incomplete. In previous studies of jejunal CTL in infected animals, it was shown that these lymphocytes express CD11a, CD49d, and CD95, suggesting that they are highly activated (24). It was also shown that lymphocytes obtained from duodenal biopsy specimens mediate SIV-specific lytic activity (24). Our demonstration in the present experiments that the CD8+ tetramer-binding lymphocytes in the distal colon of infected monkeys produce IFN-γ on exposure to a dominant epitope peptide provides further evidence that these are normally functioning CTL populations.
A number of factors make it unlikely that the cells evaluated in the mucosal biopsy specimens of the vaccinated monkeys in these studies represent contaminating blood lymphocyte populations. First, the sampled tissue specimens were washed extensively prior to lymphocyte isolation. Second, the finding that tetramer-binding CD8+ CD3+ lymphocyte populations were present in much higher numbers in distal-colonic mucosa than in other anatomic compartments of infected monkeys (Table 2) argues that the technique employed for isolating these cell populations was valid. Finally, the consistency of finding larger tetramer-binding CD8+ CD3+ lymphocyte populations in the blood than in the colonic tissue—an observation made in five of the six evaluated vaccinated monkeys—argues for its validity. Nevertheless, future studies will be done to assess tetramer-binding lymphocyte populations in mucosal tissues using in situ staining techniques to address the possibility formally.
Since an extremely high percentage of new HIV infections are initiated through mucosal exposure to the virus, there is a consensus among investigators that an HIV vaccine should elicit a potent mucosal immune response. Therefore, a variety of targeted vaccine strategies are being pursued to elicit mucosal immunity to the virus through the use of novel adjuvants and localized mucosal application of antigen. Many of these strategies are being evaluated in nonhuman primate models. For example, Belyakov et al. have recently reported the mucosal administration of peptide antigen formulated with an Escherichia coli toxin to elicit mucosal T-cell immune responses (5, 6). In the present study, we have shown that certain systemic immunization strategies can elicit high-frequency CTL responses in a mucosal tissue compartment. This finding raises the possibility that it may not be necessary to employ specific mucosally targeted vaccination strategies to elicit mucosal HIV-specific CTL. Nevertheless, mucosal routes of immunization may prove even more efficient at eliciting mucosal cellular immunity than systemic immunization.
Acknowledgments
Jamal Baig and Daniel B. Levy contributed equally to this work.
We acknowledge the animal technicians at the New England Primate Research Center and Marisa St. Claire and the animal technicians at the Bioqual animal facility for animal care and assistance with biopsy procedures.
This work was supported in part by National Institutes of Health grants AI28147, AI85343, AI20729, CA50139, and AI48394 (J.E.S.) and RR00168 and by the Center for AIDS Research (CFAR) grant AI28691.
REFERENCES
- 1.Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95:1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 7.Earl, P. L., L. S. Wyatt, D. C. Montefiori, M. Bilska, R. Woodward, P. D. Markham, J. D. Malley, T. U. Vogel, T. M. Allen, D. I. Watkins, N. Miller, and B. Moss. 2002. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric env protein boost in the SHIV rhesus macaque model. Virology 294:270-281. [DOI] [PubMed] [Google Scholar]
- 8.Haase, A. T. 2001. The pathogenesis of sexual mucosal transmission and early stages of infection: obstacles and a narrow window of opportunity for prevention. AIDS 15(Suppl. 1):10-11. [DOI] [PubMed] [Google Scholar]
- 9.Holmberg, S. D. 1997. Risk factors for sexual transmission of human immunodeficiency virus, p. 569-575. In V. DeVita, S. Hellman, and S. A. Rosenberg (ed.), AIDS, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 10.Joint United Nations Program on HIV/AIDS. 1996. The HIV/AIDS situation in mid 1996: global and regional highlights. UNAIDS fact sheet. United Nations, New York, N.Y.
- 11.Jordan, H. L., M. J. Kuroda, J. E. Schmitz, T. Steenbeke, M. A. Forman, and N. L. Letvin. 1999. Detection of simian immunodeficiency virus Gag-specific CD8+ T lymphocytes in semen of chronically infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Virol. 73:4508-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 13.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroda, M. J., J. E. Schmitz, A. Seth, R. S. Veazey, C. E. Nickerson, M. A. Lifton, P. J. Dailey, M. A. Forman, P. Racz, K. Tenner-Racz, and N. L. Letvin. 2000. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac-infected rhesus monkeys. Blood 96:1474-1479. [PubMed] [Google Scholar]
- 16.Letvin, N. L., D. H. Barouch, and D. C. Montefiori. 2002. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu. Rev. Immunol. 20:73-99. [DOI] [PubMed] [Google Scholar]
- 17.McKay, P. F., J. E. Schmitz, M. J. Kuroda, D. H. Barouch, M. A. Lifton, D. A. Gorgone, and N. L. Letvin. 2001. Vaccine protection against functional CTL abnormalities in simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 168:332-337. [DOI] [PubMed] [Google Scholar]
- 18.Miller, M. D., H. Yamamoto, A. L. Hughes, D. I. Watkins, and N. L. Letvin. 1991. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J. Immunol. 147:320-329. [PubMed] [Google Scholar]
- 19.Murphey-Corb, M., L. A. Wilson, A. M. Trichel, D. E. Roberts, K. Xu, B. Woodson, and J. Blanchard. 1999. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 162:540-549. [PubMed] [Google Scholar]
- 20.Norton, A. J., S. Jordan, and P. Yeomans. 1994. Brief, high-temparature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J. Pathol. 173:371-379. [DOI] [PubMed] [Google Scholar]
- 21.Pachl, C., J. A. Todd, D. G. Kern, P. J. Sheridan, S. J. Fong, M. Stempien, B. Hoo, D. Besemer, T. Yeghiazarian, B. Irvine, J. Kolberg, R. Kokka, P. Neuwald, and M. S. Urdea. 1995. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:446-454. [DOI] [PubMed] [Google Scholar]
- 22.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. C. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz, J. E., R. S. Veazey, M. J. Kuroda, D. B. Levy, A. Seth, K. G. Mansfield, C. E. Nickerson, M. A. Lifton, X. Alvarez, A. A. Lackner, and N. L. Letvin. 2001. Simian immunodeficiency virus (SIV)-specific cytotoxic T lymphocytes in gastrointestinal tissues of chronically SIV-infected rhesus monkeys. Blood 98:3757-3761. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz, J. E., M. J. Kuroda, R. S. Veazey, A. Seth, W. M. Taylor, C. E. Nickerson, M. A. Lifton, P. J. Dailey, M. A. Forman, P. Racz, K. Tenner-Racz, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV)-specific CTL are present in large numbers in livers of SIV-infected rhesus monkeys. J. Immunol. 164:6015-6019. [DOI] [PubMed] [Google Scholar]
- 26.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 27.Veazey, R. S., M. C. Gauduin, K. G. Mansfield, I. C. Tham, J. D. Altman, J. D. Lifson, A. A. Lackner, and R. P. Johnson. 2001. Emergence and kinetics of simian immunodeficiency virus-specific CD8+ T cells in the intestines of macaques during primary infection. J. Virol. 75:10515-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 29.Vermund, S. H. 1997. Transmission of HIV, p. 147-165. In V. De Vita, S. Hellman, and S. A. Rosenberg (ed.), AIDS, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 30.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 234:1563-1566. [DOI] [PubMed] [Google Scholar]