Abstract
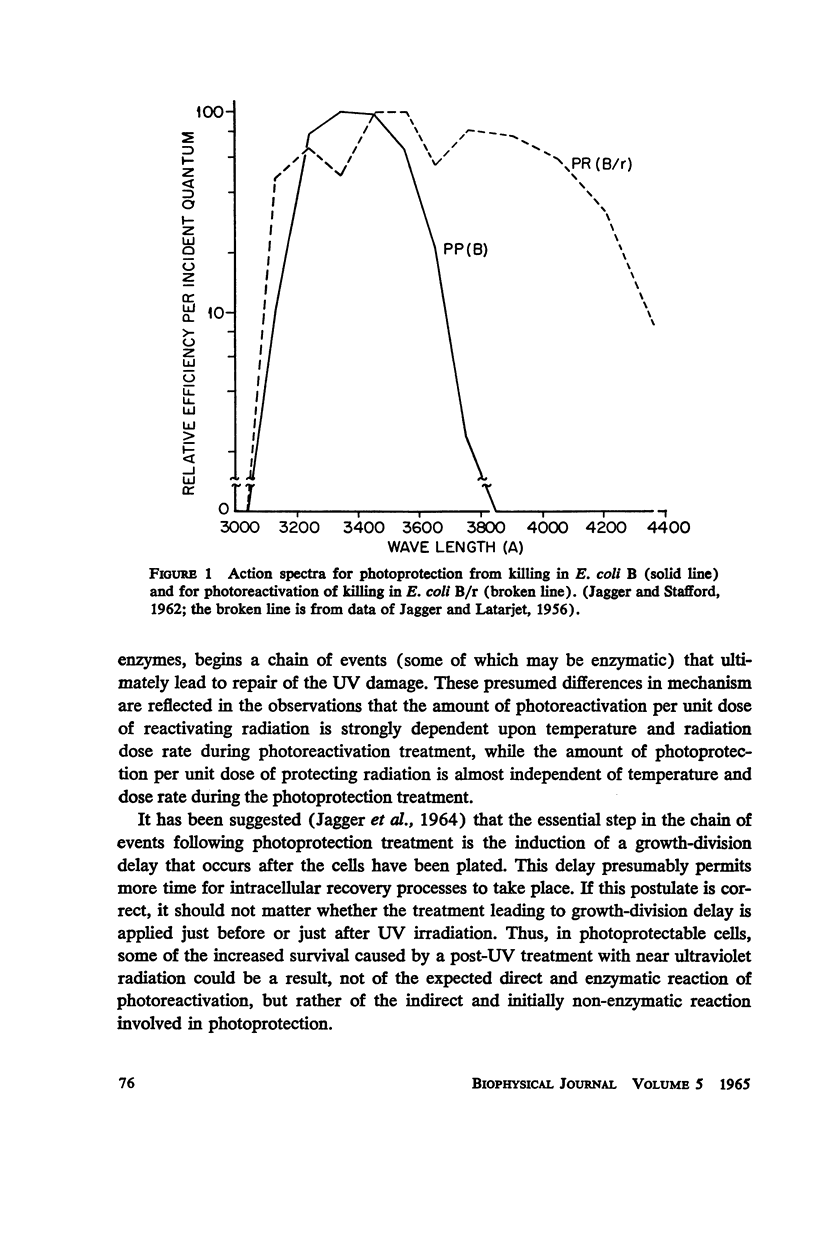
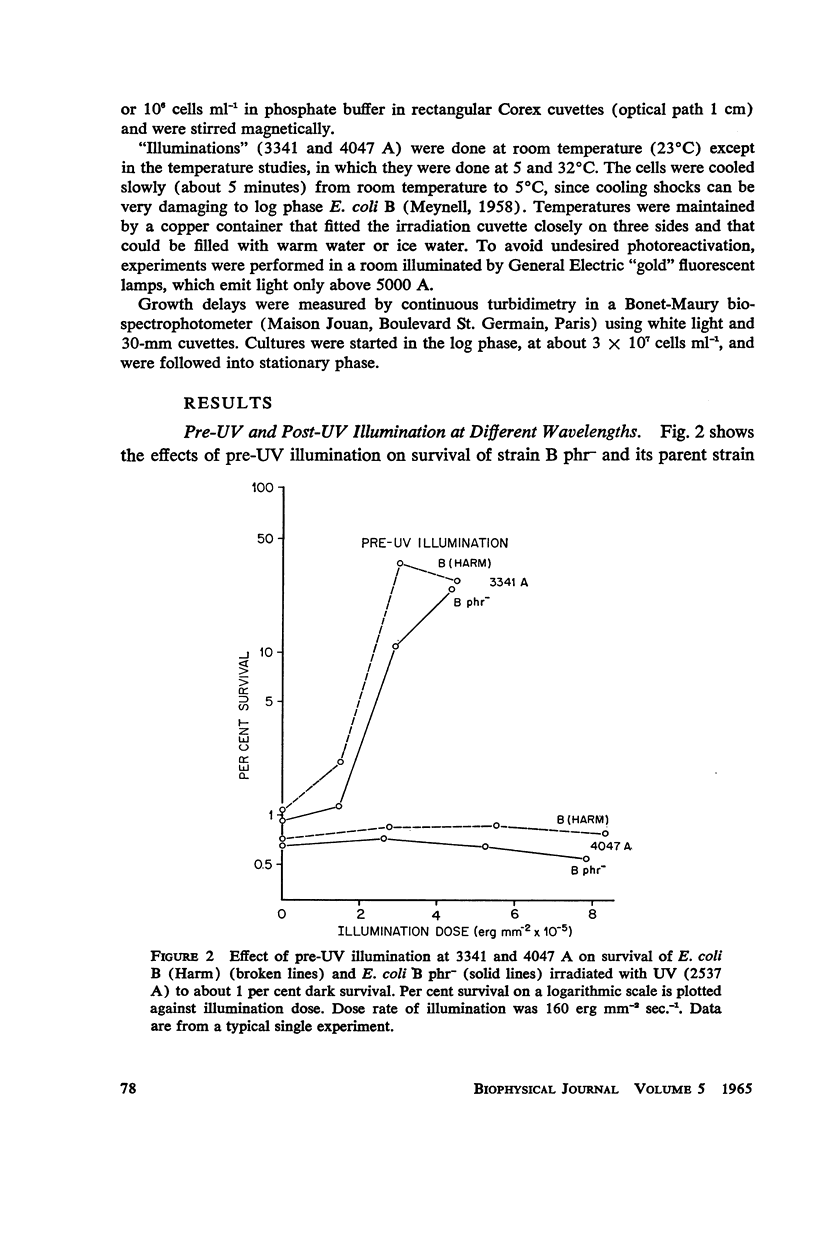
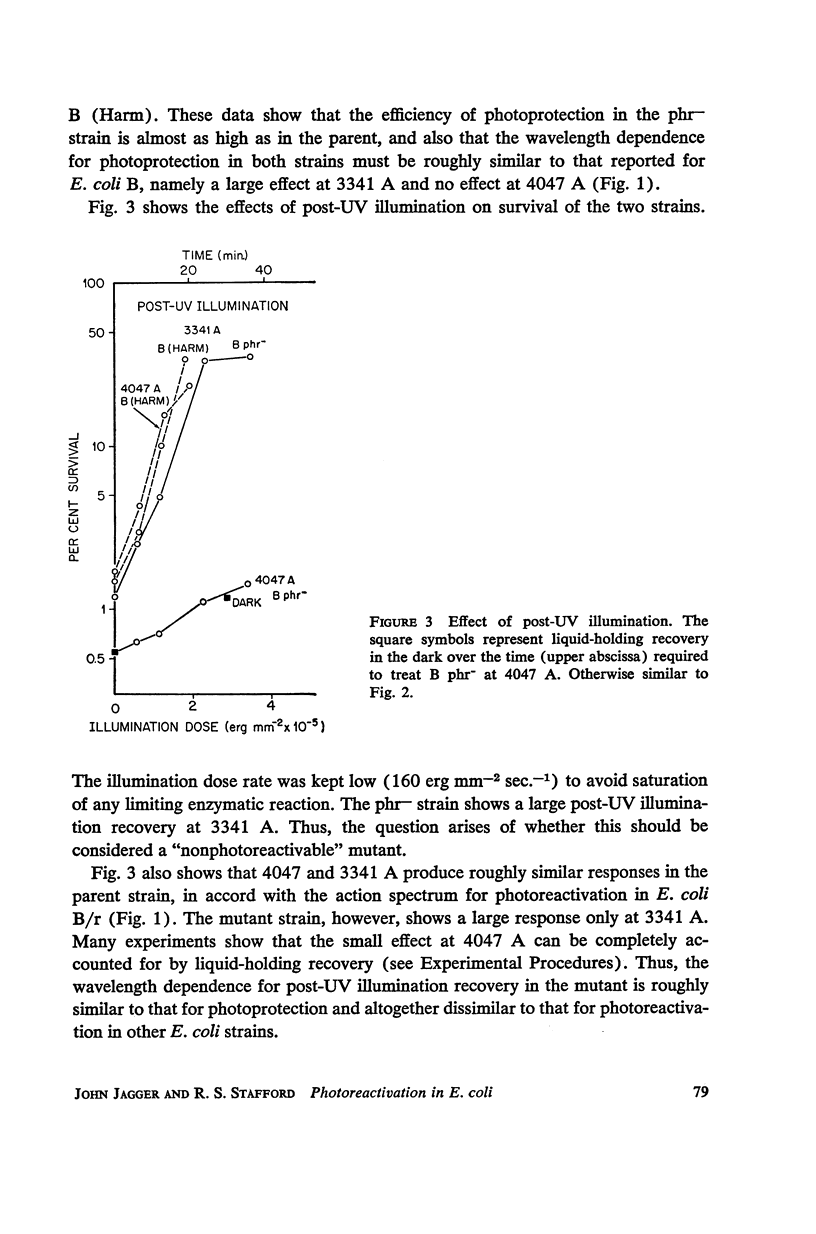
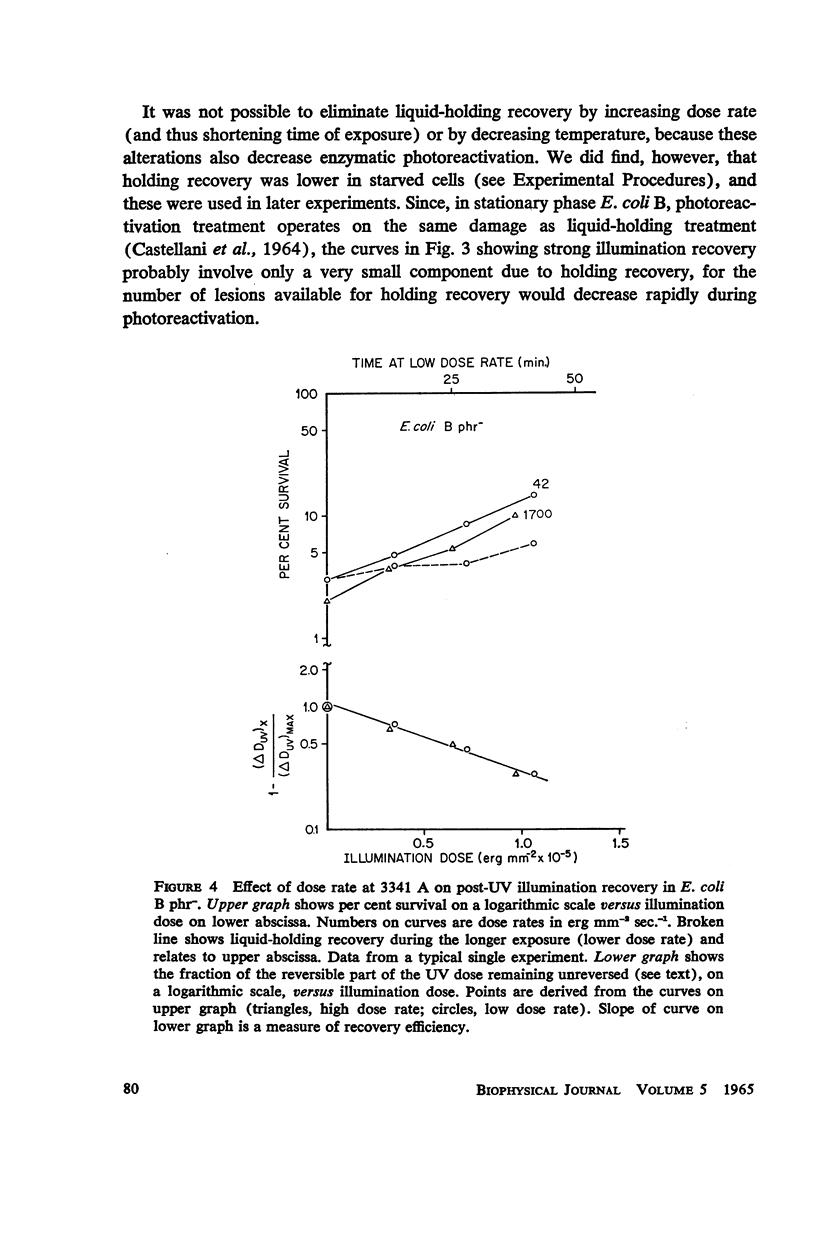
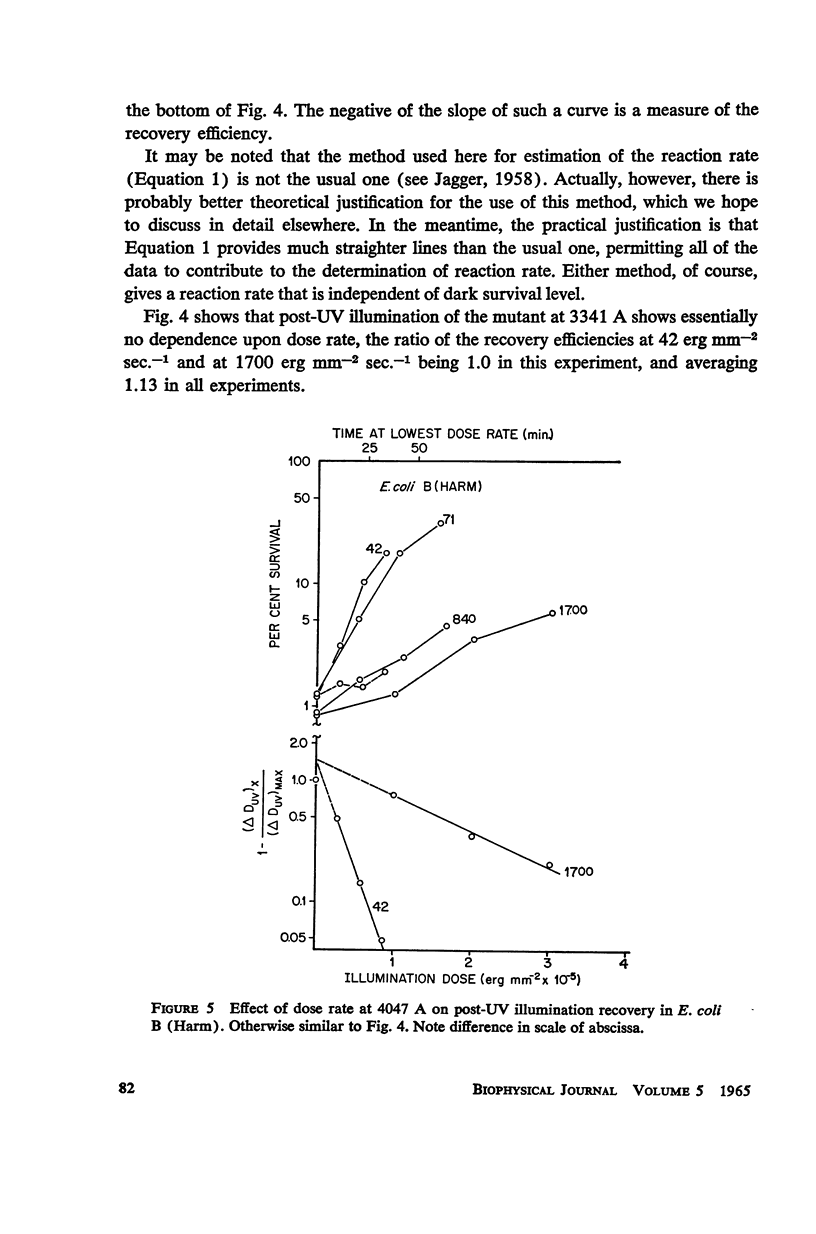
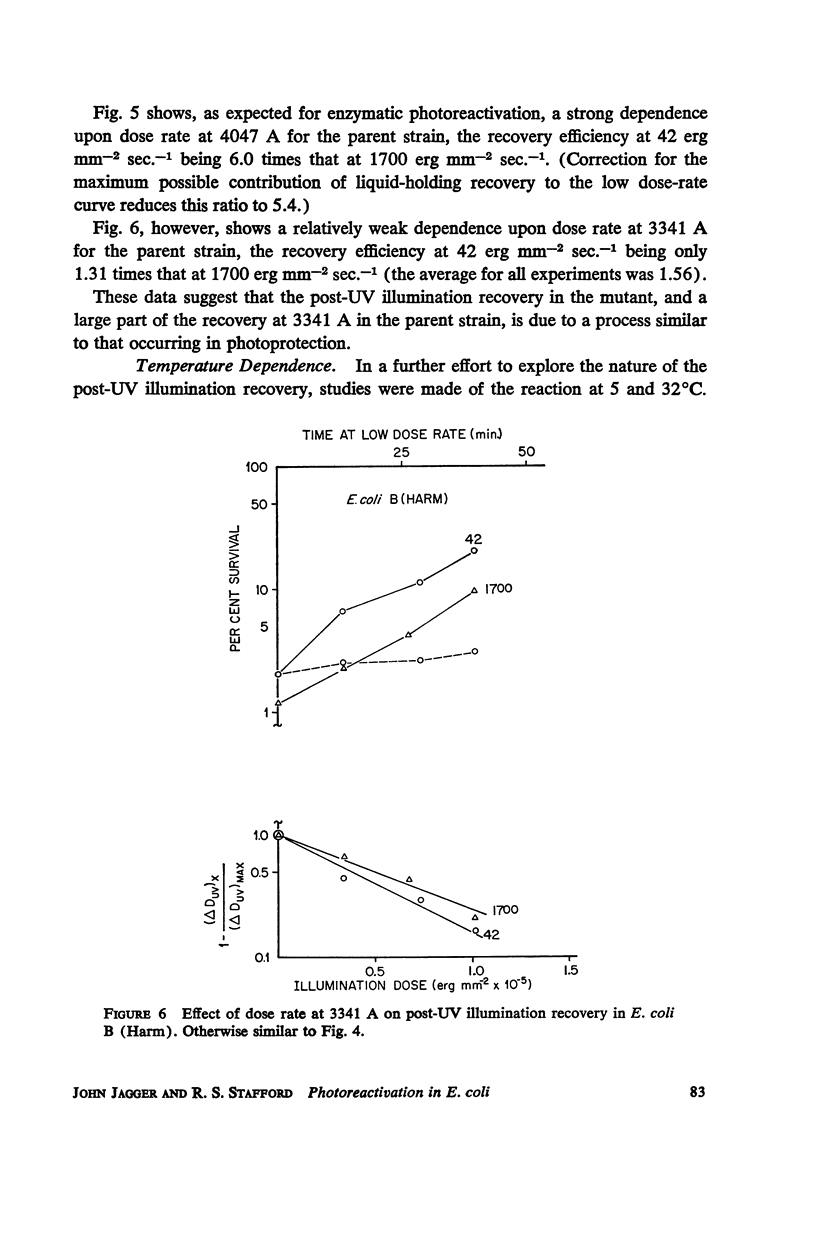
Escherichia coli B phr-, which is not photoreactivable under certain conditions, has been shown to exhibit photoreactivation of killing in the logarithmic growth phase at 3341 A. Dependence of the reaction upon (a) wavelength, (b) dose, and (c) dose rate of the reactivating radiation, as well as upon (d) temperature during reactivation treatment, is very similar to that of photoprotection. We conclude that this photoreactivation is similar in mechanism to photoprotection, believed to be an indirect repair process, the initial step of which is non-enzymatic and leads to a growth-division delay. We therefore call the present phenomenon “indirect photoreactivation.” Similar studies suggest that indirect photoreactivation of killing occurs also in the parent strain, E. coli B (Harm). It has often been supposed that all photoreactivation results from a photoenzymatic reaction similar to that found to operate in vitro on transforming DNA. Our data provide the first evidence for two distinct types of photoreactivation of cell killing, one of which appears not to involve photoenzymes. These experiments also show that photoprotection results from intracellular events that can be induced by treatment after, as well as before, far ultraviolet irradiation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOWEN G. Kinetic studies on the mechanism of photoreactivation of bacteriophage T2 inactivated by ultraviolet light. Ann Inst Pasteur (Paris) 1953 Jan;84(1):218–221. [PubMed] [Google Scholar]
- CASTELLANI A., JAGGER J., SETLOW R. B. OVERLAP OF PHOTOREACTIVATION AND LIQUID HOLDING RECOVERY IN ESCHERICHIA COLI B. Science. 1964 Mar 13;143(3611):1170–1171. doi: 10.1126/science.143.3611.1170. [DOI] [PubMed] [Google Scholar]
- JAGGER J., LATARJET R. Spectres d'action de la photo-restauration chez E. coli B/r. Ann Inst Pasteur (Paris) 1956 Dec;91(6):858–873. [PubMed] [Google Scholar]
- JAGGER J. Photoprotection from ultraviolet killing in Escherichia coli B. Radiat Res. 1960 Oct;13:521–539. [PubMed] [Google Scholar]
- JAGGER J. Photoreactivation. Bacteriol Rev. 1958 Jun;22(2):99–142. doi: 10.1128/br.22.2.99-142.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASHKET E. R., BRODIE A. F. Effects of near-ultraviolet irradiation on growth and oxidative metabolism of bacteria. J Bacteriol. 1962 May;83:1094–1100. doi: 10.1128/jb.83.5.1094-1100.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL G. G. The effect of sudden chilling on Escherichia coli. J Gen Microbiol. 1958 Oct;19(2):380–389. doi: 10.1099/00221287-19-2-380. [DOI] [PubMed] [Google Scholar]
- RUPERT C. S., GOODGAL S. H., HERRIOTT R. M. Photoreactivation in vitro of ultraviolet-inactivated Hemophilus influenzae transforming factor. J Gen Physiol. 1958 Jan 20;41(3):451–471. doi: 10.1085/jgp.41.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPERT C. S. Photoenzymatic repair of ultraviolet damage in DNA. II. Formation of an enzyme-substrate complex. J Gen Physiol. 1962 Mar;45:725–741. doi: 10.1085/jgp.45.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B., Aldous E. RECOVERY FROM ULTRAVIOLET IRRADIATION IN ESCHERICHIA COLI. J Bacteriol. 1949 Mar;57(3):363–375. doi: 10.1128/jb.57.3.363-375.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLOW R. B., SETLOW J. K. Evidence that ultraviolet-induced thymine dimers in DNA cause biological damage. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1250–1257. doi: 10.1073/pnas.48.7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]


