Abstract
In vivo evidence suggests that T-cell-derived gamma interferon (IFN-γ) can directly inhibit the replication of herpes simplex virus type 1 (HSV-1). However, IFN-γ is a weak inhibitor of HSV-1 replication in vitro. We have found that IFN-γ synergizes with the innate IFNs (IFN-α and -β) to potently inhibit HSV-1 replication in vitro and in vivo. Treatment of Vero cells with either IFN-β or IFN-γ inhibits HSV-1 replication by <20-fold, whereas treatment with both IFN-β and IFN-γ inhibits HSV-1 replication by ∼1,000-fold. Treatment with IFN-β and IFN-γ does not prevent HSV-1 entry into Vero cells, and the inhibitory effect can be overcome by increasing the multiplicity of HSV-1 infection. The capacity of IFN-β and IFN-γ to synergistically inhibit HSV-1 replication is not virus strain specific and has been observed in three different cell types. For two of the three virus strains tested, IFN-β and IFN-γ inhibit HSV-1 replication with a potency that approaches that achieved by a high dose of acyclovir. Pretreatment of mouse eyes with IFN-β and IFN-γ reduces HSV-1 replication to nearly undetectable levels, prevents the development of disease, and reduces the latent HSV-1 genome load per trigeminal ganglion by ∼200-fold. Thus, simultaneous activation of IFN-α/β receptors and IFN-γ receptors appears to render cells highly resistant to the replication of HSV-1. Because IFN-α or IFN-β is produced by most cells as an innate response to virus infection, the results imply that IFN-γ secreted by T cells may provide a critical second signal that potently inhibits HSV-1 replication in vivo.
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) establish lifelong, latent infections in the peripheral nervous system of their human hosts (3, 27, 50). Latent HSV genomes periodically reinitiate productive phase gene expression and the de novo synthesis of infectious virus; however, the majority of these reactivation events are intercepted by the host's immune system before the secondary infection can cause disease (11, 29, 62, 63). In experimental animals, T cells are prominent in HSV-1 latently infected ganglia and appear to be critical in the rapid suppression of HSV-1 reactivation events (5, 7, 17, 35, 51, 52). In humans, the arrival of CD8+ T cells in recurrent genital lesions correlates with clearance of HSV-2 (28, 44). In contrast, suppression of T-cell function allows recurrent HSV infections to present as severe, disseminated lesions of the skin and mucosa (56, 57). Although T cells are essential, the mechanism(s) by which T cells terminate HSV-1 and HSV-2 infections in vivo are not well defined.
IFN-α, IFN-β, and IFN-γ are secreted proteins that play important roles in host resistance to viral infections. The 22 known isotypes of IFN-α share 20 to 30% amino acid homology with IFN-β. IFN-α and/or IFN-β (IFN-α/β) are secreted by most cells as an innate response to viral infection, and both bind to IFN-α/β receptors that are expressed on all cell types (47). Activation of IFN-α/β receptors modifies the transcriptional and translational environment in cells inducing an “antiviral state” (61). In contrast, IFN-γ does not share amino acid homology with IFN-α/β. IFN-γ binds to a distinct receptor, and its production is restricted to activated T cells and natural killer (NK) cells (10). While IFN-γ is a potent immunoregulatory cytokine, IFN-γ also directly inhibits the replication of hepatitis B virus, measles virus, and murine cytomegalovirus (CMV) in vivo (14, 42, 43, 48).
IFN-α/β are not effective inhibitors of HSV-1 replication in vitro. Although pretreatment with IFN-α or IFN-β reduces the pathogenesis of HSV-1 infections in mice and humans (21, 41), IFN-α and IFN-β are not effective in treating established ocular herpes infections in humans (4, 26). It has become evident that the resistance of HSV-1 to IFN-α/β is an active process that is dependent on the expression of at least two viral proteins (22, 39). The immediate-early (IE) protein ICP0 is required to prevent IFN-α/β from silencing HSV-1 IE gene expression (22, 38), and ICP34.5 is necessary to prevent the cellular double-stranded RNA-dependent protein kinase (PKR) from inhibiting protein translation in virus-infected cells (8, 31).
IFN-γ is an important component in the adaptive immune response to HSV-1. Administration of neutralizing antibodies to IFN-γ increases the severity of HSV-1 infections in mice (55). Likewise, knockout mice that lack IFN-γ or its receptor are impaired in their capacity to control HSV-1 infection as measured by higher mortality, prolonged duration of acute HSV-1 infection, and higher frequencies of induced reactivation (5, 6, 32). These phenotypes are consistent with a hypothesis that IFN-γ directly inhibits HSV-1 replication in vivo but may also be explained by a general impairment of T-cell-dependent responses in IFN-γ-knockout mice.
Recombinant IFN-γ is a weak inhibitor of HSV-1 replication in vitro (22). Although this fact appears to be incompatible with the hypothesis that IFN-γ inhibits HSV-1 replication in vivo, the in vitro test is not biologically relevant because T cells do not secrete IFN-γ into environments that are devoid of other cytokines. Secretion of IFN-α/β, proinflammatory cytokines, and chemokines probably occurs prior to, and during, the recruitment of T cells into sites of viral infection. Therefore, a more accurate test of the in vivo situation would be to treat cell cultures with IFN-γ and other cytokines of the innate immune response.
In testing this principle, we have found that IFN-γ acts in concert with the innate IFNs, IFN-α and IFN-β, to potently inhibit HSV-1 replication in vitro and in vivo. Treatment of Vero cells with either IFN-β or IFN-γ inhibits HSV-1 replication by <20-fold. However, treatment with both IFN-β and IFN-γ inhibits HSV-1 replication by ∼1,000-fold. This inhibition is neither cell type specific, nor is it virus strain specific. Moreover, pretreatment of mouse eyes with IFN-β and IFN-γ potently inhibits HSV-1 replication in vivo and prevents the development of viral pathogenesis. Because most cell types in the body secrete IFN-α/β in response to viral infection (59), the results suggest that T-cell-secreted IFN-γ may provide a second signal that suppresses HSV-1 replication in animals and thus facilitates the establishment of viral latency.
(This work was presented in part at the 2002 Experimental Biology Meeting in New Orleans and at the 27th International Herpesvirus Workshop in Cairns, Australia.)
MATERIALS AND METHODS
Cells, viruses, and IFNs.
Vero and SK-N-SH cells (American Type Culture Collection, Manassas, Va.) were propagated in Dulbecco modified Eagle medium (DMEM) containing 0.15% HCO3− supplemented with 10% fetal bovine serum, penicillin G (100 U/ml), and streptomycin (100 mg/ml), hereafter referred to as complete DMEM. Cultures of primary mouse kidney (PMK) cells were established from female ICR mice by standard dissociation methods and were propagated in complete DMEM.
Wild-type HSV-1 strains KOS (54), McKrae (25), and TU1-1 were propagated in Vero cells. TU1-1 is a clinical isolate obtained at Tulane University that is two passages removed from an oral herpes lesion. TU1-1 was confirmed as HSV-1 by immunocytochemical staining of plaques with a monoclonal antibody that is specific for HSV-1 gG, whereas no staining was observed with an HSV-2 gG-specific monoclonal antibody (Rumbaugh-Goodwin Institute, Plantation, Fla.). A recombinant strain of KOS that expresses green fluorescent protein (GFP) was kindly donated by John Balliet and Priscilla Schaffer (Harvard University, Boston). This virus, KOS-GFP, was constructed by the insertion of a CMV immediate-early promoter-GFP gene cassette into the intergenic region between the UL26 and UL27 genes of HSV-1 strain KOS (John Balliet, unpublished data).
Recombinant human (hu) universal IFN-α (A/D), hu IFN-β, hu IFN-γ, murine (mu) IFN-β, and mu IFN-γ (PBL Biomedical Laboratories, New Brunswick, N.J.) were used at concentrations of 100 U/ml, unless indicated otherwise. In all in vitro experiments, IFNs were added to cultures 12 h prior to infection, and IFN treatment was maintained continuously.
Plaque reduction and virus replication assays.
For plaque reduction assays Vero cells were seeded in 12-well plates at a density of 9 × 104 cells per well, and 6 h later 100 U/ml of IFN-α, IFN-β, or IFN-γ or both IFN-β and IFN-γ (100 U/ml of each) were added to the culture medium. Vero cells were inoculated with HSV-1 12 h later, and 1 h later the medium was replaced with complete DMEM containing 0.5% methylcellulose and the same IFNs used in the pretreatment. Plaques were counted 2 to 3 days later.
For viral replication assays, Vero, PMK, or SK-N-SH cells were seeded in 24-well plates at a density of 5 × 104 cells per well, and 6 h later cultures were treated with vehicle, 100 U/ml of IFN-β, 100 U/ml of IFN-γ, or both IFN-β and IFN-γ (100 U/ml of each). Cells were inoculated 12 h later with HSV-1 at the indicated multiplicity of infection (MOI). One hour later cells were rinsed twice with 0.5 ml of complete DMEM, and the well was treated with complete DMEM containing the same IFN(s) present during the pretreatment. The cultures were freeze-thawed 24 h after infection, and the viral titer was determined on Vero cells by a 96-well microtiter plaque assay.
PCR analysis of HSV-1-infected Vero cells.
Vero cells were plated in 24-well plates, and 6 h later the cells were treated with vehicle or both IFN-β and IFN-γ (100 U/ml of each). Cells were inoculated 12 h later with HSV-1 strain KOS at an MOI of 0.1 to 20 PFU per cell. One hour later, cells were washed twice with 0.5 ml of complete DMEM, and DNA was isolated from each culture by a phenol-chloroform extraction procedure (60). PCR was performed, as described below, on DNA samples to amplify a 243-bp fragment of the HSV-1 ribonucleotide reductase (RR) gene by using the oligonucleotide primers RR-a (5′-ATGCCAGACCTGTTTTTCAA) and RR-b (5′-GTCTTTGAACATGACGAAGG). The yield of 243-bp PCR product amplified from the DNA samples was quantified by densitometric analysis of ethidium bromide-stained agarose gels (Alpha Innotech Corp., San Leandro, Calif.).
Flow cytometry of KOS-GFP-infected Vero cells.
Vero cells were seeded in 12-well plates at a density of 105 cells per well, and 6 h later cultures were treated with vehicle, 100 U/ml of IFN-β, 100 U/ml of IFN-γ, or both IFN-β and IFN-γ (100 U/ml of each). Cells were inoculated 12 h later with KOS-GFP at an MOI of 0.03. One hour later the cells were rinsed twice with 0.5 ml of complete DMEM, and the medium was replaced with complete DMEM containing the same IFN(s) present during the pretreatment. Half of the cultures were secondarily treated with 300 μM acyclovir. At 1, 12, 24, and 36 h postinfection, Vero cells were treated with trypsin, resuspended in phosphate-buffered saline containing 10% fetal bovine serum, and then analyzed by using a FACSCalibur and CellQuest Pro software (Becton Dickinson Biosciences, San Jose, Calif.). At each time point, n = 3 samples were analyzed per treatment group, as well as n = 3 negative controls (uninfected Vero cells) and n = 3 positive controls (Vero cells infected with 30 PFU/cell of a GFP-expressing adenovirus, Ad.CMV-GFP [19]). Of the 25,000 events evaluated per sample, 24,000 ± 550 (mean ± the standard deviation) had forward-scatter and side-scatter properties indicating that they were single, viable cells. The results are presented as the net percentage of GFP-positive cells above background fluorescence, where this background is defined as the average number of uninfected Vero cells whose fluorescence exceeded the threshold necessary to be considered GFP positive (i.e., 48 ± 5 per 24,000 cells).
Analysis of HSV-1 replication in IFN-treated mice.
Female ICR mice (3 to 5 weeks, 22 ± 2 g) were obtained from Harlan-Sprague Dawley (Indianapolis, Ind.) and handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals. At 12 h before infection, mice were anesthetized by intraperitoneal administration of xylazine (6.6 mg/kg) and ketamine (100 mg/kg), their corneas were scarified with a 26-gauge needle, tear film was blotted from the eyes, and the eyes were treated with 4 μl of DMEM alone or 4 μl of DMEM containing IFN-β (200 U), IFN-γ (200 U), or IFN-β and IFN-γ (200 U of each). These IFN treatments were repeated 8, 4, and 0 h before infection. At these subsequent time points, mice were anesthetized with 6.6 mg of xylazine and 50 mg of ketamine/kg (i.e., a half-dose). Mouse eyes were inoculated with 2 × 105 PFU of HSV-1 strain KOS in a volume of 4 μl. The ocular surface of both eyes was swabbed for seven consecutive days after infection with cotton-tipped applicators. The tips were placed in 0.4 ml of complete DMEM, and viral titers were determined by a microtiter plaque assay.
Measurement of HSV-1 DNA load in latently infected mouse trigeminal ganglia.
DNA was isolated from the combined left and right trigeminal ganglia (TG) of mice by a phenol-chloroform extraction procedure (60), and the number of HSV-1 genomes per TG was determined by competitive PCR as previously described (20). A solution containing 1× Taq buffer, 50 μM concentrations of each deoxynucleoside triphosphates, 0.25 μM concentrations of each RR primer, and 160 fg of an RR competitor template per ml (∼1,400 competitors per 50-μl reaction) was prepared. Then, 42 μl of this master mix was placed in 0.65-ml tubes and overlaid with mineral oil, and 100 ng of TG DNA (3 μl) was added to each tube. The tubes were heated to 90°C in a thermal cycler, and 2.5 U of Taq polymerase diluted in 5 μl of Taq buffer was added to each sample. PCR samples were incubated for 35 cycles of 94°C for 1 min 15 s, 57.7°C for 1 min 30 s, and 72°C for 40 s. Measurement of RR gene and competitor PCR product yields was performed as previously described (20, 23).
Statistics.
Analysis of numerical data and statistical analyses were performed with the software packages Microsoft Excel and Modstat (Modern Microcomputers, Mechanicsville, Va.). Data are presented as the mean ± the standard error of the mean (SEM). All viral titers were transformed by adding a value of 1 such that all data could be analyzed on a logarithmic scale. Statistical comparison of viral titers (or HSV-1 genome loads) in multiple treatments was performed by one-way analysis of variance (ANOVA), followed by comparison of individual treatments to vehicle-treated controls by using Duncan's multiple range test or Tukey's post hoc t test. Regression analysis was performed by the least-squares method. Two-way ANOVA was used to compare the course of viral shedding over multiple days in mice treated with vehicle or IFNs. The polynomial trendline feature of Microsoft Excel was used to describe the sigmoidal relationship between logarithm of viral genomes per TG (x, input) and the ratio of RR to competitor PCR products (y, output) amplified from viral DNA standards (e.g., x = 0.328y3 + 0.551y2 + 1.4851y + 3.4423).
RESULTS
IFN-α/β and IFN-γ synergize to inhibit HSV-1 plaque formation on Vero cell monolayers.
The efficiency of HSV-1 strain KOS plaque formation was compared in Vero cell monolayers treated with IFN-α, IFN-β, IFN-γ, or combinations of these IFNs. Plaque formation was reduced from an average of 199 plaques on vehicle-treated monolayers to 123, 42, and 53 plaques on Vero cell monolayers treated with IFN-α, IFN-β, or IFN-γ, respectively (Table 1). In contrast, HSV-1 plaque formation was reduced by >90% in Vero cells treated with both IFN-α/β and IFN-γ. An inoculum of 200 PFU formed an average of 12 plaques on cell monolayers treated with both IFN-α and IFN-γ. Likewise, only 3 of 200 PFU formed plaques on Vero cells treated with both IFN-β and IFN-γ (Table 1). Comparable levels of inhibition could not be obtained by doubling the concentration of any one IFN from 100 to 200 U/ml (Table 1). Likewise, simultaneous treatment of Vero cells with both IFN-α and IFN-β did not decrease HSV-1 plaque formation any more than IFN-β alone (Table 1).
TABLE 1.
IFN-α/β and IFN-γ synergize to inhibit HSV-1 plaque formation on Vero cells
| Treatment (U/ml)a | Mean no. of plaquesb ± SEM | Fold reductionc |
|---|---|---|
| Vehicle | 199 ± 0.7 | |
| IFN-α (100) | 123 ± 3.0∗ | 1.6 |
| IFN-β (100) | 42 ± 2.0∗ | 4.8 |
| IFN-γ (100) | 53 ± 0.5∗ | 3.8 |
| IFN-α (100) + IFN-β (100) | 43 ± 1.1∗ | 4.7 |
| IFN-α (100) + IFN-γ (100) | 12 ± 0.3∗d | 17 |
| IFN-β (100) + IFN-γ (100) | 3 ± 0.3∗ | 66 |
| IFN-α (200) | 97 ± 3.0∗ | 2.1 |
| IFN-β (200) | 32 ± 0.7∗ | 6.3 |
| IFN-γ (200) | 35 ± 1.0∗ | 5.7 |
Vero cells were treated continuously with IFN-α, IFN-β, IFN-γ, or combinations of these cytokines from 12 h before infection until the end of the experiment.
That is, the number of plaques formed in Vero cells inoculated with 200 PFU of HSV-1 strain KOS (n = 4 per group). ∗, P < 0.05, as determined by one-way ANOVA and Tukey's post hoc t test comparison of this treatment to vehicle.
The fold reduction in each group was calculated as follows: number of plaques in vehicle/number of plaques in treatment.
Boldface type indicates a >10-fold reduction in HSV-1 plaque formation.
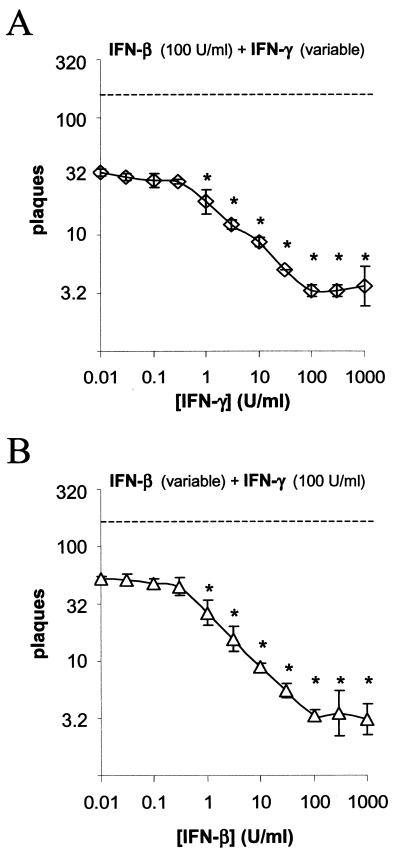
If this phenomenon was receptor dependent, then one would predict that synergy would first be observed at the minimum biologically active dose of each IFN (1 U/ml, as defined by the manufacturer). Vero cells were treated with a constant, high dose of IFN-β and 0.5-log dilutions of IFN-γ (Fig. 1A). An average of 32 plaques (out of 200 PFU) formed on Vero cells treated with IFN-β alone. When IFN-β was combined with <0.3 U/ml of IFN-γ, the number of HSV-1 plaques formed on Vero cells did not change (Fig. 1A). However, as the concentration of IFN-γ was increased from 1 to 100 U/ml, the number of plaques that formed decreased linearly from 32 to 3 plaques per culture (Fig. 1A). In a second experiment, Vero cells were treated with 0.5-log dilutions of IFN-β and a constant, high dose of IFN-γ (Fig. 1B). An average of 55 plaques (out of 200 PFU) formed on Vero cells treated with IFN-γ alone. When IFN-γ was combined with less than 0.3 U/ml of IFN-β, the number of HSV-1 plaques formed on Vero cells did not change (Fig. 1B). However, as the concentration of IFN-β was increased from 1 to 100 U/ml, the number of plaques that formed decreased linearly from 55 to 3 plaques per culture (Fig. 1B). The results suggest that simultaneous activation of IFN-α/β receptors and IFN-γ receptors is required to synergistically inhibit HSV-1 plaque formation.
FIG. 1.
IFN-β and IFN-γ synergize to inhibit HSV-1 plaque formation on Vero cells. (A) Efficiency of HSV-1 plaque formation on Vero cells treated with 100 U/ml of IFN-β and variable doses of IFN-γ (n = 3 per group). The dashed line indicates the number of plaques that formed on vehicle-treated cells. Significant reductions in plaque counts relative to cells treated with 100 U/ml of IFN-β alone are denoted by a single asterisk (P < 0.05, one-way ANOVA and Duncan's multiple-range test). (B) Efficiency of HSV-1 plaque formation on Vero cells treated with variable doses of IFN-β (n = 3 per group) and 100 U/ml of IFN-γ. The dashed line indicates the number of plaques that formed on vehicle-treated cells. Significant reductions in plaque counts relative to cells treated with 100 U/ml of IFN-γ alone are denoted by a single asterisk (P < 0.05, one-way ANOVA and Duncan's multiple-range test).
IFN-β and IFN-γ synergize to inhibit HSV-1 replication in Vero cells.
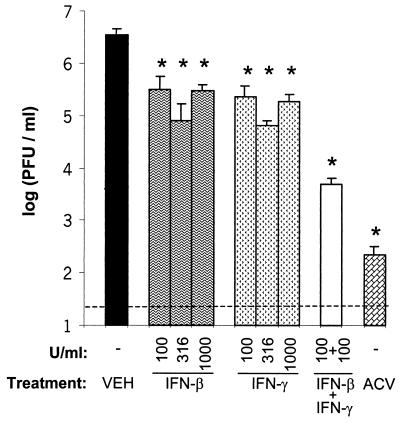
HSV-1 replication was compared in Vero cells treated with vehicle, acyclovir (ACV), IFN-β, IFN-γ, or both IFN-β and IFN-γ. In vehicle-treated cultures, HSV-1 strain KOS replicated to a titer of 3.5 × 106 PFU/ml over a 24-h period of incubation (Fig. 2). HSV-1 replicated to titers of 3.1 × 105 and 2.3 × 105 PFU/ml in cultures treated with 100 U of IFN-β or IFN-γ/ml, respectively (Fig. 2). Thus, treatment with IFN-β or IFN-γ inhibited HSV-1 replication by <20-fold. In cultures treated with both IFN-β and IFN-γ, HSV-1 replicated to titers of 4.8 × 103 PFU/ml and was inhibited by 800-fold relative to vehicle-treated cultures (Fig. 2). Comparable levels of inhibition could not be obtained by increasing the concentration of either IFN-β or IFN-γ alone to 316 or 1,000 U/ml (Fig. 2). Whereas the level of inhibition achieved by both IFN-β and IFN-γ was far greater than additive, it was considerably less than the ∼20,000-fold inhibition achieved by the HSV DNA synthesis inhibitor, ACV (Fig. 2).
FIG. 2.
IFN-β and IFN-γ synergize to inhibit HSV-1 replication in Vero cells. Virus yield in Vero cells treated with vehicle, IFN-β (100, 316, or 1,000 U/ml), IFN-γ (100, 316, or 1,000 U/ml), IFN-β and IFN-γ (100 U/ml of each), or ACV (300 μM). Virus titers were determined 24 h after infection with 0.1 PFU of HSV-1 strain KOS/cell (n = 4 per group). Treatments that significantly reduced virus titer relative to vehicle-treated cultures are indicated with an asterisk (P < 0.05, one-way ANOVA and Tukey's post hoc t test). The dashed line represents the lower limit of detection of the plaque assay used to measure viral titers.
Treatment with IFN-β and IFN-γ does not inhibit HSV-1 adsorption to Vero cells.
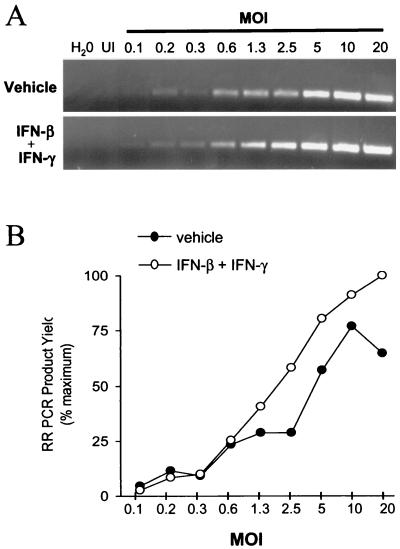
PCR was used to compare the relative efficiency with which HSV-1 adsorbed to Vero cells treated with (i) vehicle or (ii) IFN-β and IFN-γ. One hour after inoculations with MOIs of 0.1 to 20 PFU/cell, DNA was isolated from HSV-1-infected Vero cells, and PCR was used to amplify a 243-bp fragment of the HSV-1 genome (Fig. 3A). The HSV-1 RR PCR product yield increased as a function of viral MOI in Vero cells treated with vehicle (Fig. 3B; r2 = 0.89) or both IFN-β and IFN-γ (Fig. 3B; r2 = 0.98). Thus, PCR provided a valid basis for comparing the relative amount of HSV-1 DNA that entered Vero cells. The considerations that account for the inherent quantitative capacity of such noncompetitive PCR assays are addressed elsewhere (15, 16).
FIG. 3.
IFN-β and IFN-γ do not inhibit HSV-1 adsorption to Vero cells. (A) Ethidium bromide-stained RR PCR products amplified from HSV-1-infected Vero cells treated with either vehicle (upper panel) or IFN-β and IFN-γ (lower panel, 100 U/ml of each). From left to right, PCR tubes received no template (H2O), 100 ng of uninfected (UI) Vero cell DNA, or 100 ng of HSV-1-infected Vero cell DNA harvested from cells inoculated with 0.1 to 20 PFU/cell. (B) RR PCR product yield plotted as a function of viral MOI in Vero cells treated with vehicle or both IFN-β and IFN-γ. The correlation coefficient between PCR product yield and viral MOI was r = 0.81 in vehicle-treated cells and was r = 0.84 in cells treated with IFN-β and IFN-γ.
At all MOIs tested, the amount of RR PCR product amplified from Vero cells treated with IFN-β and IFN-γ was comparable to or greater than the amount of RR PCR product amplified from vehicle-treated Vero cells (Fig. 3B). Likewise, the correlation coefficient between MOI and PCR product yield did not differ between the treatments (P = 0.87, Fisher two-sided z test). Therefore, treatment with IFN-β and IFN-γ does not prevent the adsorption of HSV-1 to Vero cells.
Treatment with IFN-β and IFN-γ does not prevent HSV-1 entry into Vero cells.
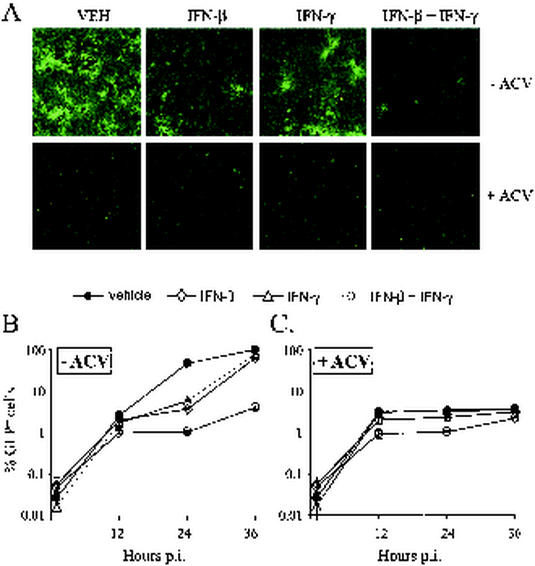
Fluorescence microscopy was used to compare the efficiency with which a recombinant virus entered Vero cells and expressed a GFP reporter in the presence of vehicle, IFN-β, IFN-γ, or both IFN-β and IFN-γ. In cells infected with 0.03 PFU/cell of KOS-GFP, treatment with either IFN-β or IFN-γ did not prevent virus from spreading to the majority of Vero cells by 36 h after infection (Fig. 4A). In contrast, treatment with both IFN-β and IFN-γ resulted in a gross decrease in the extent of GFP expression observed 36 h after infection (Fig. 4A). The presence of many single GFP+ cells and several small foci of GFP+ cells suggested that IFN-β and IFN-γ did not prevent KOS-GFP from entering Vero cells but rather inhibited a subsequent step in viral replication (Fig. 4A).
FIG. 4.
Effect of IFNs and/or ACV on the entry and spread of KOS-GFP in Vero cells. (A) Representative photomicrographs of Vero cells taken 36 h after infection with KOS-GFP (MOI = 0.03), as seen when illuminated with the 360- to 400-nm spectrum of light that excites GFP fluorescence. Magnification, ×10. Vero cells were treated with vehicle (VEH), IFN-β (100 U/ml), IFN-γ (100 U/ml), or IFN-β and IFN-γ (100 U/ml of each) and were treated with either no ACV (−ACV) or 300 μM ACV (+ACV) after infection with KOS-GFP. (B and C) Flow cytometric analysis of GFP fluorescence in Vero cells 1, 12, 24, and 36 h after infection with KOS-GFP (MOI = 0.03). IFN treatments were the same as described above, and cells were secondarily treated with either (B) no ACV (−ACV) or (C) 300 μM ACV (+ACV). The mean percentage ± the standard error (SE) of GFP-positive cells in each treatment was determined in three replicate cultures per time point. The results are presented as the mean percentage ± the SE of GFP-positive cells after subtracting out the background frequency of fluorescence observed in uninfected cultures of Vero cells (i.e., 48 ± 5 per 24,000 cells evaluated).
The kinetics of KOS-GFP spread in Vero cells treated with vehicle, IFN-β, IFN-γ, or IFN-β and IFN-γ were compared by flow cytometry. One hour after infection with KOS-GFP (MOI = 0.03), the frequency of GFP-positive cells in virus-infected cultures was indistinguishable from uninfected cultures of Vero cells. At 12, 24, and 36 h after infection, GFP expression was detectable in an average of 2.6, 46, and 98% of vehicle-treated cells, respectively (Fig. 4B). Although treatment with IFN-β or IFN-γ slowed the spread of KOS-GFP, 64% ± 3% of IFN-β-treated cells and 77% ± 3% of IFN-γ-treated cells were found to be GFP positive by 36 h after infection (Fig. 4B). In contrast, only 4.0% ± 0.3% of cells treated with both IFN-β and IFN-γ were GFP positive 36 h after infection (Fig. 4B).
In the presence of ACV, KOS-GFP was unable to replicate, and thus GFP expression was restricted to the ∼3% of cells that were initially infected (Fig. 4A). In cells treated with ACV only, flow cytometry demonstrated that GFP expression was detectable in an average of 3.1, 3.2, and 3.6% of cells by 12, 24, and 36 h after infection, respectively (Fig. 4C). When combined with ACV, treatment with IFN-β slightly delayed the expression of GFP in KOS-GFP-infected cultures (Fig. 4A and C). When combined with ACV, treatment with IFN-γ had no apparent effect on GFP expression. When combined with ACV, treatment with IFN-β and IFN-γ delayed GFP expression, and an average of 0.9, 1.0, and 2.2% of cells were found to be GFP positive by 12, 24, and 36 h after infection, respectively (Fig. 4A and C). Based on the frequency of GFP expression observed 36 h after infection, KOS-GFP entered Vero cells treated with IFN-β and IFN-γ with at least 60% of the efficiency observed in vehicle-treated cells (i.e., 2.2% ÷ 3.6%). Therefore, treatment with IFN-β and IFN-γ does not prevent HSV-1 entry into Vero cells.
Increased multiplicity of infection overcomes the inhibitory effect of IFN-β and IFN-γ.
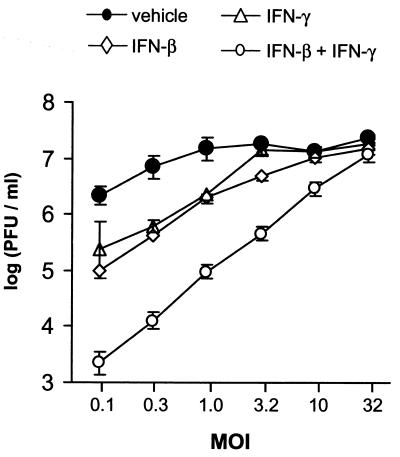
The effect of viral MOI on inhibition by IFN-β, IFN-γ, or both IFN-β and IFN-γ was compared in Vero cells inoculated with 0.1 to 32 PFU/cell. In vehicle-treated cultures inoculated with 0.1 PFU/cell, HSV-1 strain KOS replicated to titers of 2.2 × 106 PFU/ml (Fig. 5). At an MOI of 0.1, treatment with IFN-β or IFN-γ reduced HSV-1 titers by 10- to 20-fold, and treatment with IFN-β and IFN-γ reduced HSV-1 titers by 980-fold (Fig. 5). Inhibition of HSV-1 replication by IFN-β or IFN-γ was overcome by increasing the MOI to 3.2 PFU/cell. Although inhibition by both IFN-β and IFN-γ was only completely reversed when the MOI was increased to 32 PFU/cell, virus yield increased in direct proportion to MOI over the entire range tested (Fig. 5; r2 = 0.99).
FIG. 5.
Inhibition by IFN-β and IFN-γ is overcome by increasing the MOI. Virus yield was plotted as a function of MOI. Vero cells were treated with vehicle, IFN-β (100 U/ml), IFN-γ (100 U/ml), or IFN-β plus IFN-γ (100 U/ml of each). Virus titers were determined 24 h after infection with 0.1 to 32 PFU/cell of HSV-1 strain KOS (n = 4 per group). Regression analysis demonstrated that the titer of virus recovered from cells treated with IFN-β and IFN-γ was linearly dependent on MOI (r2 = 0.99, P = 10−6).
Synergistic inhibition of HSV-1 replication is not cell type or virus strain specific.
The effect of IFN-β and IFN-γ on HSV-1 replication was compared in Vero cells, SK-N-SH cells, and PMK cells inoculated with 0.1 PFU/cell. In each cell type, HSV-1 strain KOS replicated to titers of greater than 5.6 × 105 PFU/ml in vehicle-treated cells (Table 2.) Treatment with IFN-β or IFN-γ reduced HSV-1 titers in each cell type by ≤20-fold (Table 2). Treatment with both IFN-β and IFN-γ reduced HSV-1 titers in Vero, SK-N-SH, and PMK cells by 930-, 620-, and 2,600-fold, respectively (Table 2). In PMK cells, the efficiency with which IFN-β and IFN-γ inhibited HSV-1 replication was 24% of that achieved by ACV (Table 2).
TABLE 2.
Effect of IFN-β and IFN-γ on HSV-1 replication in three different cell types
| Treatmenta | Mean HSV-1 log titer ± SE (fold inhibition)b
|
||
|---|---|---|---|
| Vero cells | SK-N-SH cells | PMK cells | |
| Vehicle | 6.31 ± 0.9 | 6.19 ± 0.3 | 5.75 ± 0.1 |
| IFN-β | 5.01 ± 0.3∗c (20) | 5.47 ± 0.1 (5) | 5.16 ± 0.2∗ (4) |
| IFN-γ | 5.37 ± 0.5∗ (14) | 5.99 ± 0.1 (2) | 5.19 ± 0.1∗ (4) |
| IFN-β + IFN-γ | 3.34 ± 0.2∗ (930) | 3.36 ± 0.2∗ (620) | 2.33 ± 0.1∗ (2,600) |
| ACV | 1.96 ± 0.2∗ (22,000) | 2.65 ± 0.2∗ (5,400) | 1.69 ± 0.2∗ (11,000) |
Vero and SK-N-SH cells were treated with vehicle, 100 U/ml of hu IFN-β, 100 U/ml of hu IFN-γ, hu IFN-β and hu IFN-γ (100 U/ml of each), or with ACV (300 μM). PMK cells received identical treatments with murine IFNs. Viral titers were determined 24 h after infection with 0.1 PFU/cell of HSV-1 strain KOS.
HSV-1 titers (log [PFU/ml]) recovered from Vero, SK-N-SH, or PMK cells (n = 4 per group). The fold inhibition was calculated within each cell type as the titer in vehicle/titer in treatment.
∗, P < 0.05, as determined by one-way ANOVA and Tukey's post hoc t test comparison of this treatment to vehicle-treated cultures of the same cell type.
The effect of IFN-β and IFN-γ on the replication of HSV-1 strain KOS in Vero cells was compared to two other HSV-1 strains, McKrae and TU1-1. All three HSV-1 strains replicated to titers of greater than 2.5 × 105 PFU/ml in vehicle-treated cells inoculated with 0.1 PFU/cell (Table 3). Treatment with IFN-β or IFN-γ reduced titers of KOS, McKrae, and TU1-1 by ≤20-fold. In contrast, treatment with both IFN-β and IFN-γ reduced the titers of KOS, McKrae, and TU1-1 by 1,000-, 1,400-, and 1,800-fold, respectively (Table 3). The efficiency with which IFN-β and IFN-γ inhibited the replication of McKrae and TU1-1 was 25% of that achieved by ACV (Table 3).
TABLE 3.
Effect of IFN-β and IFN-γ on the replication of three different HSV-1 strains
| Treatmenta | Mean HSV-1 log titer ± SE (fold inhibition)b
|
||
|---|---|---|---|
| KOS | McKrae | TU1-1 | |
| Vehicle | 6.66 ± 0.1 | 5.81 ± 0.1 | 5.40 ± 0.1 |
| IFN-β | 5.38 ± 0.2∗c (19) | 4.52 ± 0.1∗ (20) | 4.30 ± 0.2∗ (14) |
| IFN-γ | 5.94 ± 0.2∗ (7) | 4.87 ± 0.1∗ (9) | 4.32 ± 0.1∗ (12) |
| IFN-β + IFN-γ | 3.70 ± 0.1∗ (1,000) | 2.78 ± 0.3∗ (1,400) | 2.15 ± 0.1∗ (1,800) |
| ACV | 2.64 ± 0.1∗ (11,000) | 2.07 ± 0.1∗ (5,500) | 1.58 ± 0.1∗ (7,000) |
Vero cells were treated with vehicle, 100 U/ml of hu IFN-β, 100 U/ml of hu IFN-γ, hu IFN-β and hu IFN-γ (100 U/ml of each), or ACV (300 μM). Viral titers were determined 24 h after infection with 0.1 PFU/ml of HSV-1 strains KOS, McKrae, or TU1-1.
HSV-1 titers (log [PFU/ml]) recovered from Vero, SK-N-SH, or PMK cells (n = 4 per group). The fold inhibition was calculated within each cell type as the titer in vehicle/titer in treatment.
∗, P < 0.05, as determined by one-way ANOVA and Tukey's post hoc t test comparison of this treatment to vehicle-treated cultures infected with the same HSV-1 strain.
Pretreatment with IFN-β and IFN-γ inhibits HSV-1 replication in mouse eyes.
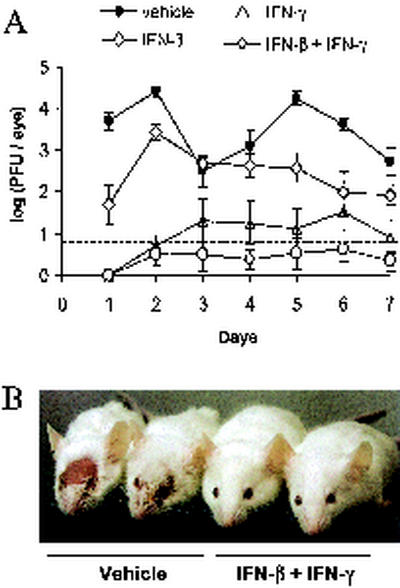
Viral replication was compared in mouse eyes that were pretreated with vehicle, IFN-β, IFN-γ, or both IFN-β and IFN-γ at 12, 8, 4, and 0 h prior to HSV-1 infection. In vehicle-treated mice, an average of 5,000 PFU/eye was detected 24 h after infection, and average titers ranged from 300 to 25,000 PFU/eye over the next 6 days (Fig. 6A). Treatment with IFN-β caused an ∼100-fold reduction in ocular HSV-1 shedding 24 h after infection, but HSV-1 titers increased to 2,700 PFU/eye by 48 h after infection (Fig. 6A). Treatment with IFN-γ reduced ocular HSV-1 shedding to undetectable levels 24 h after infection, and five of eight mice shed 10 to 40 PFU/eye between 2 and 7 days after infection. (Fig. 6A). Treatment with both IFN-β and IFN-γ reduced ocular HSV-1 shedding to undetectable levels 24 h after infection, and only two of eight mice shed detectable levels of virus between 2 and 7 days after infection. (Fig. 6A). HSV-1 infection killed one of eight vehicle-treated mice and none of the IFN-treated mice. Vehicle-treated mice developed extensive periocular skin lesions by 8 days after infection (Fig. 6B). IFN-β-treated mice developed periocular skin lesions, but the severity of the lesions was reduced (not shown). Only two of eight IFN-γ treated mice developed visible skin lesions. None of the eight mice treated with IFN-β and IFN-γ developed periocular skin lesions by 8 days after infection, or at any other subsequent time during the 40-day course of the experiment (Fig. 6B). These results are representative, as similar results were obtained in two independent experiments.
FIG. 6.
Pretreatment with IFN-β and IFN-γ inhibits HSV-1 replication in vivo. (A) Virus titers recovered from mouse eyes treated with vehicle, IFN-β, IFN-γ, or both IFN-β and IFN-γ (n = 8 mice per group). Mouse eyes were scarified and treated 0, 4, 8, and 12 h later with vehicle, 200 U of IFN-β, 200 U of IFN-γ, or IFN-β and IFN-γ (200 U of each). After the final treatment, mouse eyes were inoculated with 2 × 105 PFU of HSV-1 strain KOS. The titer of virus recovered from mouse eyes was measured by plaque assay, and the dashed line indicates the lower limit of detection of this assay. Two-way ANOVA demonstrated that each IFN treatment significantly reduced the course of ocular HSV-1 shedding in mice relative to vehicle-treated mice (P < 0.001). (B) Photograph taken 8 days after ocular HSV-1 infection of mice treated with vehicle or both IFN-β and IFN-γ.
Pretreatment with IFN-β and IFN-γ inhibits the establishment of latent HSV-1 infection.
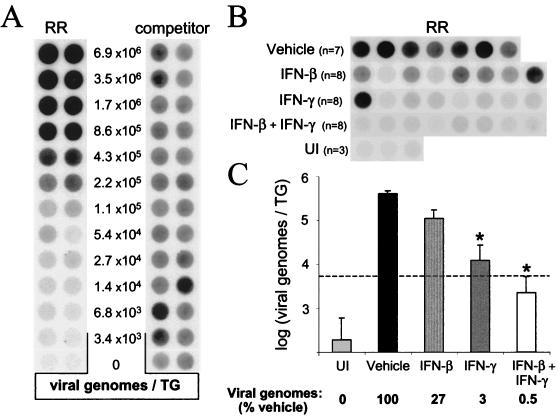
The efficiency with which HSV-1 established latent infections in the TG of mice treated with vehicle, IFN-β, IFN-γ, or both IFN-β and IFN-γ was compared 40 days after infection. HSV-1 RR and competitor PCR products were coamplified from viral DNA standards in order to define the relationship between RR PCR product yield (normalized to the competitor) and the number of HSV-1 genomes per TG (Fig. 7A). In parallel reactions, RR and competitor PCR products were coamplified from TG DNA samples isolated from HSV-1-infected mice and uninfected controls (Fig. 7B; competitor product yields not shown). Based on the yield of RR PCR products (normalized to the competitor), the average number of latent HSV-1 genomes in vehicle-treated mice was 4.3 × 105 viral genomes per TG (Fig. 7C).
FIG. 7.
Pretreatment with IFN-β and IFN-γ reduces the establishment of latent HSV-1 in the TG. (A) RR and competitor PCR products amplified from viral DNA standards. These DNA standards consisted of (i) twofold dilutions of HSV-1 genomes, (ii) a constant amount of competitor (∼1,400 copies), and (iii) 100 ng of uninfected TG DNA. Duplicate blots of PCR products were hybridized to RR-specific or competitor-specific probes, and the ratio of RR to competitor PCR product was used to define the “normalized RR PCR product yield.” The relationship between the number of viral genomes per TG (x, input) and the normalized RR PCR product yield (y, output) was sigmoidal and was described by the polynomial equation: x = 0.328y3 + 0.551y2 + 1.4851y + 3.4423 (r2 = 0.99). The linear range of the standard curve was between 6.8 × 103 and 1.7 × 106 viral genomes/TG. Only ∼1/230th of the DNA from each TG was included in the PCR (i.e., 100 ng); thus, the lower limit of accurate quantitation was 30 HSV-1 genomes per PCR. (B) Dot blot of HSV-1 RR PCR products amplified from TG of 3 uninfected (UI) mice and 31 HSV-1 latently infected mice (sacrificed 40 days after inoculation) that were treated prior to HSV-1 infection with vehicle (n = 7), IFN-β (n = 8), IFN-γ (n = 8), or both IFN-β and IFN-γ (n = 8). (C) Effect of IFN-β, IFN-γ, or IFN-β plus IFN-γ on HSV-1 genome load in latently infected TG. The dashed line indicates the lower limit of the linear range of the assay. Significant differences in viral genome load relative to vehicle-treated mice are indicated by an asterisk (P < 0.05, one-way ANOVA and Tukey's post hoc t test).
Pretreatment of mouse eyes with IFN-β or IFN-γ reduced the number of latent HSV-1 genomes per TG by 4- and 33-fold, respectively (Fig. 7C). An average of 2.2 × 103 viral genomes per TG were detected in mice treated with both IFN-β and IFN-γ (Fig. 7C). This latter value may be somewhat of an overestimate because the amount of amplified RR PCR product was slightly below the linear range of the competitive PCR assay (dashed line in Fig. 7C). Therefore, pretreatment with IFN-β and IFN-γ reduced the number of latent HSV-1 genomes per TG by ∼200-fold relative to the number of HSV-1 genomes detected in TG of vehicle-treated mice.
DISCUSSION
Although HSV-1 is highly resistant to the antiviral activities of IFN-α/β or IFN-γ, it is possible that the combined activities of IFN-α/β and IFN-γ could synergize to inhibit HSV-1 replication. Several reports from the 1980s contain limited evidence that is consistent with this hypothesis (9, 40, 64). Balish et al. (2) provide definitive evidence that IFN-α and IFN-γ synergize to inhibit HSV-1 strain KOS replication in human corneal fibroblast cells, but the generality of the effect was not further investigated (2). Therefore, the combined effect of IFN-α/β and IFN-γ on HSV-1 replication has never been rigorously evaluated.
The results of the present study establish that the combined activities of IFN-α/β and IFN-γ inhibit HSV-1 replication in vitro with a potency that approaches that of ACV. The results of dose-response analyses with recombinant IFN proteins indicate that (i) the effect is far greater than additive and (ii) the synergy appears to be dependent on the simultaneous activation of IFN-α/β receptors and IFN-γ receptors. Therefore, we propose that coactivation of IFN-α/β and IFN-γ receptors renders cells highly resistant to HSV-1 replication. The fact that this synergy is observed in multiple cell types and against three different HSV-1 strains suggests that this is a generally applicable principal.
The results of the present study establish for the first time that the combined activities of IFN-α/β and IFN-γ potently inhibit HSV-1 replication in vivo. The potency with which IFN-β and IFN-γ inhibited HSV-1 replication in vivo appeared to be considerably greater than is typically achieved when animals are treated with ACV or other antiviral agents in vivo (12, 18, 30, 36, 58). In mice pretreated with IFN-β and IFN-γ, HSV-1 replication was not detected in the eyes of six of the eight mice, and the average level of virus replication in the remaining two mice was ∼500-fold less than observed in vehicle-treated controls. Consequently, treatment with IFN-β and IFN-γ prevented HSV-1-associated disease in mice and reduced the number of latent HSV-1 genomes per TG by ∼200-fold relative to vehicle-treated mice. While IFN-β reduced the severity of disease, HSV-1 still replicated to high titers in mice treated with IFN-β alone. Treatment with IFN-γ alone, however, inhibited HSV-1 replication with nearly the same potency as that of IFN-β and IFN-γ together. We hypothesize that the high level of inhibition achieved by exogenous IFN-γ alone is the result of synergy with endogenous IFN-α/β that is locally produced in response to HSV-1 infection. The validity of this hypothesis remains to be tested.
Mechanism(s) by which IFN-β and IFN-γ may synergize to inhibit HSV-1 replication.
The results of the present study do not establish the specific mechanism(s) by which IFN-β and IFN-γ synergize to inhibit HSV-1 replication. In the absence of such knowledge, it is theoretically possible that IFN-β and IFN-γ interfere with HSV-1 replication at one, or several, of the following steps: (i) adsorption, (ii) entry, (iii) viral gene expression, (iv) viral DNA replication, (v) virus assembly, or (vi) virus maturation and egress. The results of the present study argue strongly against the first two possibilities. PCR analysis of HSV-1 DNA levels associated with Vero cells 1 h after infection demonstrate that IFN-β and IFN-γ do not prevent HSV-1 from binding to Vero cells. Likewise, fluorescence microscopy and flow cytometry indicate that IFN-β and IFN-γ have a <2-fold effect on the efficiency with which KOS-GFP enters Vero cells. Thus, it is highly unlikely that inhibition of viral adsorption and/or entry account for the mechanism(s) by which IFN-β and IFN-γ achieve an ∼1,000-fold inhibition of HSV-1 replication in Vero cells. It remains to be formally proven that treatment with IFN-β and IFN-γ does not inhibit HSV-1 entry into PMK, SK-N-SH, or mouse eye cells.
If IFN-β and IFN-γ inhibited an essential cellular function, viral replication would have been efficiently inhibited at all MOIs. However, the degree of inhibition achieved by IFN-β and IFN-γ was highly dependent on the multiplicity of HSV-1 infection (r2 = 0.99). Thus, we hypothesize that IFN-β and IFN-γ synergize to inhibit the expression of at least one virus-encoded function that is necessary for HSV-1 replication. For example, if IFN-α/β and IFN-γ synergistically block the expression of ICP0 or ICP34.5, HSV-1 would be highly sensitive to inhibition by IFN-α/β (22, 39). If this hypothesis is correct, then increasing the copy number of either ICP0 and/or the ICP34.5 genes should be sufficient to overcome inhibition by IFN-α/β and IFN-γ. Investigations are currently in progress to test these and other hypotheses regarding the mechanism(s) by which IFN-α/β and IFN-γ synergistically block HSV-1 replication.
Implications. (i) Host immunity to HSV-1.
Three pieces of evidence are consistent with a hypothesis that T cells inhibit HSV-1 replication in the peripheral nervous system through noncytolytic mechanisms. First, despite their best known role as cytotoxic T lymphocytes, CD8+ T cells actually contribute to the survival of HSV-1 infected neurons in mice (53). Second, during acute HSV-1 infection, ganglionic neurons surrounded by CD8+ T cells remain healthy in appearance (35). Finally, CD8+ T cells are sufficient to block HSV-1 reactivation from TG neurons ex vivo, and this activity can be blocked with neutralizing antibodies to IFN-γ (33, 34).
The results of the present study are consistent with a hypothesis that secretion of IFN-γ suppresses HSV-1 replication in vivo. If IFN-α and/or IFN-β are produced as an innate response to viral infection (61), then it follows that IFN-α/β are ubiquitous in virus-infected tissues. Extrapolating from the in vitro results, one can imagine that the ∼10-fold inhibition achieved by IFN-α/β would reduce the rate of HSV-1 replication but would not stop the spread of virus in vivo. Secretion of IFN-γ from T cells and/or NK cells, however, may provide a second signal that synergizes with IFN-α/β and renders cells far less permissive to HSV-1 replication. Thus, the ∼1,000-fold inhibition achieved by the combined activities of IFN-α/β and IFN-γ may effectively stop HSV-1 infection from spreading in vivo.
Under this hypothesis, cells that are capable of secreting IFN-γ should be essential components of the host immune response to HSV-1. Depletion of either CD4+, CD8+, or γδ+ T-cell subsets grossly impairs the host immune response to HSV-1 (37, 49, 53). Likewise, depletion of NK cells significantly increases the pathogenesis of HSV-1 infections (1, 45). Therefore, the primary cell types that are capable of secreting IFN-γ in vivo are all important for host immunity to HSV-1.
(ii) Establishment of latent infections.
The fact that IFN-β and IFN-γ synergize to inhibit the replication of HSV-1 has important implications for the coevolution of herpesviruses and their vertebrate hosts. Most viral infections have two possible outcomes: (i) the virus kills the host or (ii) the host clears the virus. In vertebrate animals, it is the clonal proliferation and dissemination of virus-specific T cells that allows the host to clear the virus. The results of the present study raise the intriguing possibility that HSV, and perhaps other herpesviruses, have evolved to respond to T-cell-secreted cytokines as cues that trigger the efficient suppression of viral replication at appropriate times. By coupling repression of virus replication to the infiltration of T cells into infected tissues, a third possible outcome of viral infection may be created: the establishment of latent infections that persist in an equilibrium with the host's immune system. In light of this hypothesis, it is intriguing that secretion of IFN-γ from T cells also inhibits the replication of hepatitis B virus, an unrelated persistent virus (13, 14, 46).
(iii) Clinical relevance.
Although IFNs are not considered clinically effective in treating HSV-1 infections, only IFN-α and IFN-β have been tested in humans (24). In the present study, IFN-γ was a far more potent inhibitor of HSV-1 replication in vivo than IFN-β. Recombinant IFN-γ has never been clinically evaluated for its potential in treating human HSV-1 infections, nor has recombinant IFN-γ been rigorously evaluated in animal models of HSV-1 infection.
If IFN-α/β are ubiquitous in HSV-infected tissues, then treatment with IFN-α or IFN-β can only increase the levels of a preexisting cytokine. However, if secretion of IFN-γ from T cells is truly rate limiting (particularly in the immunodeficient), then there is a clear theoretical rationale for determining if exogenous IFN-γ can be used to limit the spread of HSV-1 infection in vivo. Further investigation will be required to determine whether IFN-γ has any potential application in limiting the replication and pathogenesis of HSV-1 in humans.
Acknowledgments
This work was supported by a grant from the W. M. Keck Foundation of Los Angeles and the Louisiana Board of Regents Support Foundation (LEQSF-2001-2004-RD-A-33).
We are indebted to John Balliet and Priscilla Schaffer (Harvard University, Boston, Mass.) for kindly donating the recombinant virus KOS-GFP. We also thank Bryan Gebhardt, Daniel Carr, and Robert Hendricks for critical review of the manuscript.
REFERENCES
- 1.Adler, H., J. L. Beland, N. C. Del-Pan, L. Kobzik, R. A. Sobel, and I. J. Rimm. 1999. In the absence of T cells, natural killer cells protect from mortality due to HSV-1 encephalitis. J. Neuroimmunol. 93:208-213. [DOI] [PubMed] [Google Scholar]
- 2.Balish, M. J., M. E. Abrams, A. M. Pumfery, and C. R. Brandt. 1992. Enhanced inhibition of herpes simplex virus type 1 growth in human corneal fibroblasts by combinations of interferon-alpha and -gamma. J. Infect. Dis. 166:1401-1403. [DOI] [PubMed] [Google Scholar]
- 3.Buddingh, G. J., D. I. Schrum, J. C. Lanier, and D. J. Guidry. 1953. Studies of the natural history of herpes simplex virus infections. Pediatrics 11:593-605. [PubMed] [Google Scholar]
- 4.Cantell, K. 1995. Development of antiviral therapy with alpha interferons: promises, false hopes, and accomplishments. Ann. Med. 27:23-28. [DOI] [PubMed] [Google Scholar]
- 5.Cantin, E., B. Tanamachi, and H. Openshaw. 1999. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J. Virol. 73:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin, E., B. Tanamachi, H. Openshaw, J. Mann, and K. Clarke. 1999. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J. Virol. 73:5196-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantin, E. M., D. R. Hinton, J. Chen, and H. Openshaw. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassady, K. A., M. Gross, and B. Roizman. 1998. The second-site mutation in the herpes simplex virus recombinants lacking the γ134.5 genes precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2α. J. Virol. 72:7005-7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czarniecki, C. W., C. W. Fennie, D. B. Powers, and D. A. Estell. 1984. Synergistic antiviral and antiproliferative activities of Escherichia coli-derived human alpha, beta, and gamma interferons. J. Virol. 49:490-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrar, M. A., and R. D. Schreiber. 1993. The molecular cell biology of interferon-gamma and its receptor. Annu. Rev. Immunol. 11:571-611. [DOI] [PubMed] [Google Scholar]
- 11.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Field, H. J., S. E. Bell, G. B. Elion, A. A. Nash, and P. Wildy. 1979. Effect of acycloguanosine treatment of acute and latent herpes simplex infections in mice. Antimicrob. Agents Chemother. 15:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti, L. G., K. Ando, M. V. Hobbs, T. Ishikawa, L. Runkel, R. D. Schreiber, and F. V. Chisari. 1994. Cytotoxic T lymphocytes inhibit hepatitis B virus gene expression by a noncytolytic mechanism in transgenic mice. Proc. Natl. Acad. Sci. USA 91:3764-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 15.Halford, W. P. 1999. The essential prerequisites for quantitative RT-PCR. Nat. Biotechnol. 17:835. [DOI] [PubMed] [Google Scholar]
- 16.Halford, W. P., V. C. Falco, B. M. Gebhardt, and D. J. J. Carr. 1999. The inherent quantitative capacity of the reverse transcription-polymerase chain reaction. Anal. Biochem. 266:181-191. [DOI] [PubMed] [Google Scholar]
- 17.Halford, W. P., B. M. Gebhardt, and D. J. Carr. 1996. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 157:3542-3549. [PubMed] [Google Scholar]
- 18.Halford, W. P., B. M. Gebhardt, and D. J. Carr. 1997. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology 238:53-63. [DOI] [PubMed] [Google Scholar]
- 19.Halford, W. P., C. D. Kemp, J. A. Isler, D. J. Davido, and P. A. Schaffer. 2001. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 75:6143-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halford, W. P., and P. A. Schaffer. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J. Virol. 74:5957-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harle, P., E. Lauret, P. M. Pitha, E. De Maeyer, and D. J. Carr. 2001. Expression of human and macaque type I IFN transgenes interferes with HSV-1 replication at the transcriptional and translational levels: IFN-β is more potent than IFN-α2. Virology 290:237-248. [DOI] [PubMed] [Google Scholar]
- 22.Harle, P., B. Sainz, Jr., D. J. Carr, and W. P. Halford. 2002. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology 293:295-304. [DOI] [PubMed] [Google Scholar]
- 23.Hill, J. M., W. P. Halford, R. Wen, L. S. Engel, L. C. Green, and B. M. Gebhardt. 1996. Quantitative analysis of polymerase chain reaction products by dot blot. Anal. Biochem. 235:44-48. [DOI] [PubMed] [Google Scholar]
- 24.Ho, M. 1990. Interferon as an agent against herpes simplex virus. J. Investig. Dermatol. 95(Suppl. 6):158S-160S. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman, H. E., A. B. Nesburn, and E. D. Maloney. 1962. IDU therapy of herpes simplex. Arch. Ophthalmol. 67:583-591. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman, H. E., J. Sugar, and E. D. Varnell. 1973. Effect of exogenous interferon on herpetic keratitis in rabbits and monkeys. Investig. Ophthalmol. 12:378-380. [PubMed] [Google Scholar]
- 27.Kinghorn, G. R. 1994. Epidemiology of genital herpes. J. Int. Med. Res. 22(Suppl. 1):14A-23A. [PubMed] [Google Scholar]
- 28.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koelle, D. M., and A. Wald. 2000. Herpes simplex virus: the importance of asymptomatic shedding. J. Antimicrob. Chemother. 45(Suppl. T3):1-8. [DOI] [PubMed] [Google Scholar]
- 30.LeBlanc, R. A., L. Pesnicak, M. Godleski, and S. E. Straus. 1999. The comparative effects of famciclovir and valacyclovir on herpes simplex virus type 1 infection, latency, and reactivation in mice. J. Infect. Dis. 180:594-599. [DOI] [PubMed] [Google Scholar]
- 31.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lekstrom-Himes, J. A., R. A. LeBlanc, L. Pesnicak, M. Godleski, and S. E. Straus. 2000. Gamma interferon impedes the establishment of herpes simplex virus type 1 latent infection but has no impact on its maintenance or reactivation in mice. J. Virol. 74:6680-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, T., K. M. Khanna, B. N. Carriere, and R. L. Hendricks. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178-11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, T., Q. Tang, and R. L. Hendricks. 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 70:264-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loutsch, J. M., B. Sainz, Jr., M. E. Marquart, X. Zheng, P. Kesavan, S. Higaki, J. M. Hill, and R. Tal-Singer. 2001. Effect of famciclovir on herpes simplex virus type 1 corneal disease and establishment of latency in rabbits. Antimicrob. Agents Chemother. 45:2044-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercadal, C. M., D. M. Bouley, D. DeStephano, and B. T. Rouse. 1993. Herpetic stromal keratitis in the reconstituted scid mouse model. J. Virol. 67:3404-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 76:1995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neumann-Haefelin, D., R. Sundmacher, H. Frey, and W. Merk. 1985. Recombinant HuIFN-γ prevents herpes simplex keratitis in African green monkeys: demonstration of synergism with recombinant HuIFN-α2. Med. Microbiol. Immunol. 174:81-86. [DOI] [PubMed] [Google Scholar]
- 41.Noisakran, S., I. L. Campbell, and D. J. Carr. 1999. Ectopic expression of DNA encoding IFN-α1 in the cornea protects mice from herpes simplex virus type 1-induced encephalitis. J. Immunol. 162:4184-4190. [PubMed] [Google Scholar]
- 42.Orange, J. S., B. Wang, C. Terhorst, and C. A. Biron. 1995. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 182:1045-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson, C. E., D. M. Lawrence, L. A. Echols, and G. F. Rall. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 76:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posavad, C. M., D. M. Koelle, and L. Corey. 1998. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat. Med. 4:381-382. [DOI] [PubMed] [Google Scholar]
- 45.Rager-Zisman, B., P. C. Quan, M. Rosner, J. R. Moller, and B. R. Bloom. 1987. Role of NK cells in protection of mice against herpes simplex virus-1 infection. J. Immunol. 138:884-888. [PubMed] [Google Scholar]
- 46.Robek, M. D., S. F. Wieland, and F. V. Chisari. 2002. Inhibition of hepatitis B virus replication by interferon requires proteasome activity. J. Virol. 76:3570-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuel, C. E. 1998. Reoviruses and the interferon system. Curr. Top. Microbiol. Immunol. 233:125-145. [DOI] [PubMed] [Google Scholar]
- 48.Schultz, U., and F. V. Chisari. 1999. Recombinant duck interferon gamma inhibits duck hepatitis B virus replication in primary hepatocytes. J. Virol. 73:3162-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sciammas, R., P. Kodukula, Q. Tang, R. L. Hendricks, and J. A. Bluestone. 1997. T-cell receptor-gamma/delta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J. Exp. Med. 185:1969-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott, D. A., W. A. Coulter, and P. J. Lamey. 1997. Oral shedding of herpes simplex virus type 1: a review. J. Oral Pathol. Med. 26:441-447. [DOI] [PubMed] [Google Scholar]
- 51.Shimeld, C., J. L. Whiteland, S. M. Nicholls, D. L. Easty, and T. J. Hill. 1996. Immune cell infiltration in corneas of mice with recurrent herpes simplex virus disease. J. Gen. Virol. 77(Pt. 5):977-985. [DOI] [PubMed] [Google Scholar]
- 52.Shimeld, C., J. L. Whiteland, S. M. Nicholls, E. Grinfeld, D. L. Easty, H. Gao, and T. J. Hill. 1995. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J. Neuroimmunol. 61:7-16. [DOI] [PubMed] [Google Scholar]
- 53.Simmons, A., and D. C. Tscharke. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 175:1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith, K. O. 1964. Relationship between the envelope and the infectivity of herpes simplex virus. Proc. Soc. Exp. Biol. Med. 115:814-816. [DOI] [PubMed] [Google Scholar]
- 55.Smith, P. M., R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1994. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-γ). Virology 202:76-88. [DOI] [PubMed] [Google Scholar]
- 56.Stanberry, L. R., S. A. Floyd-Reising, B. L. Connelly, S. J. Alter, M. J. Gilchrist, C. Rubio, and M. G. Myers. 1994. Herpes simplex viremia: report of eight pediatric cases and review of the literature. Clin. Infect. Dis. 18:401-407. [DOI] [PubMed] [Google Scholar]
- 57.Stewart, J. A., S. E. Reef, P. E. Pellett, L. Corey, and R. J. Whitley. 1995. Herpesvirus infections in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 21(Suppl. 1):S114-S120. [DOI] [PubMed] [Google Scholar]
- 58.Thackray, A. M., and H. J. Field. 1998. Famciclovir and valaciclovir differ in the prevention of herpes simplex virus type 1 latency in mice: a quantitative study. Antimicrob. Agents Chemother. 42:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomson, A. 1998. The cytokine handbook, 3rd ed. Academic Press, San Diego, Calif.
- 60.Treco, D. A. 1990. Preparation and analysis of DNA, p.2.0.3-2.2.3. In F. M. Ausubel (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 61.Vilcek, J., and J. Sen. 1996. Interferons and other cytokines, p. 375-400. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Raven Publishers, Philadelphia, Pa.
- 62.Wald, A., L. Corey, R. Cone, A. Hobson, G. Davis, and J. Zeh. 1997. Frequent genital herpes simplex virus 2 shedding in immunocompetent women: effect of acyclovir treatment. J. Clin. Investig. 99:1092-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wald, A., J. Zeh, S. Selke, T. Warren, A. J. Ryncarz, R. Ashley, J. N. Krieger, and L. Corey. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844-850. [DOI] [PubMed] [Google Scholar]
- 64.Zerial, A., A. G. Hovanessian, S. Stefanos, K. Huygen, G. H. Werner, and E. Falcoff. 1982. Synergistic activities of type I (alpha, beta) and type II (gamma) murine interferons. Antiviral Res. 2:227-239. [DOI] [PubMed] [Google Scholar]