Abstract
A comparison of differences in incorporation and loss of radio-activity between two strains of Escherichia coli shows that: (a) three times as much irradiation is necessary to produce the same reduction in incorporation of H3-thymidine in B/r, the resistant strain, as in Bs - 1, the sensitive one; (b) radioactivity is lost from the DNA of previously labeled bacteria during the first few cell generations after X-ray exposure, and even though the initial rate of loss is similar for all strains, the sensitive one loses much more label; (c) loss of DNA is a complicated function of dose. Losses increase with dose up to 25 or 50 kr in both strains; with higher doses, losses decrease in Bs - 1 but are unchanged in B/r. Since in both strains labeled RNA is retained in irradiated cells, lysis has not occurred but the DNA is broken down into small pieces which leak from each cell. Losses from either strain do not occur at ice-bath temperature, indicating that breakdown is a function of metabolic processes. A proposed mechanism for X-ray damage and repair is advanced.
Full text
PDF

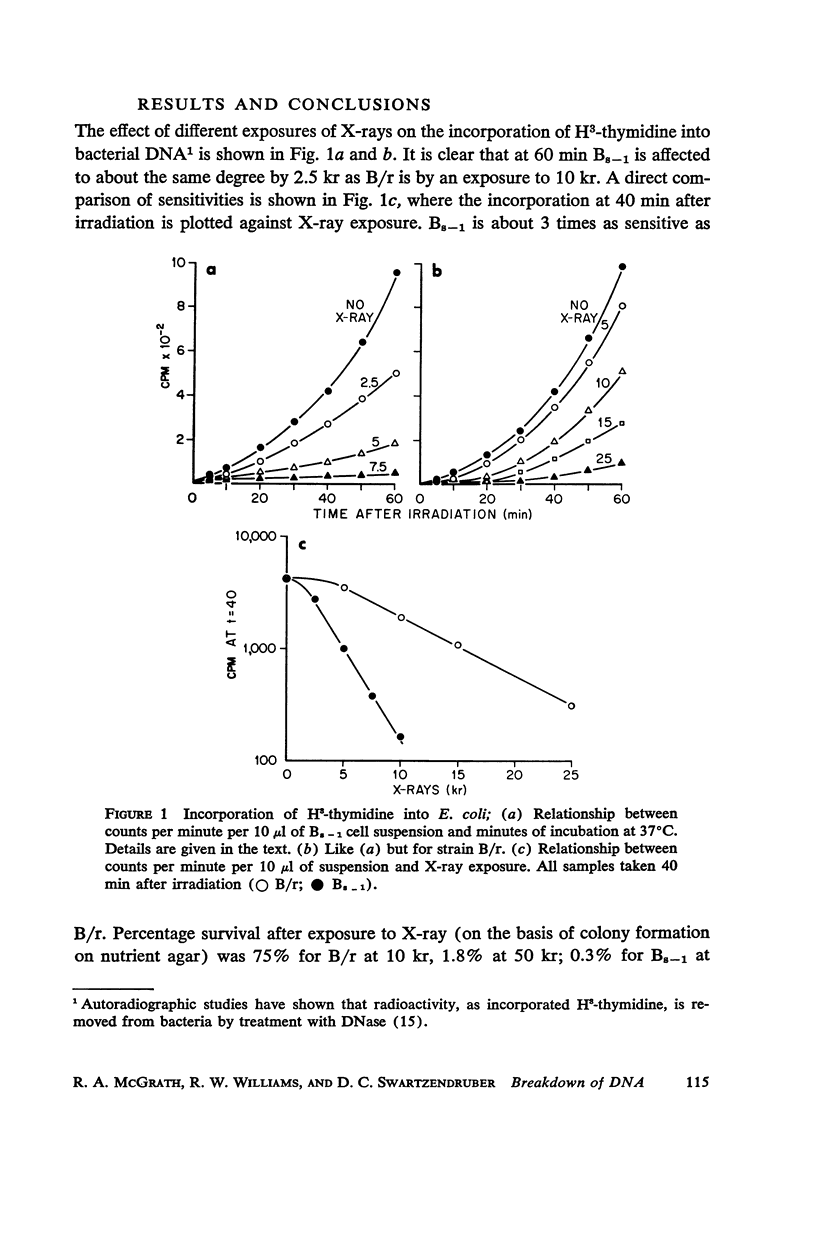
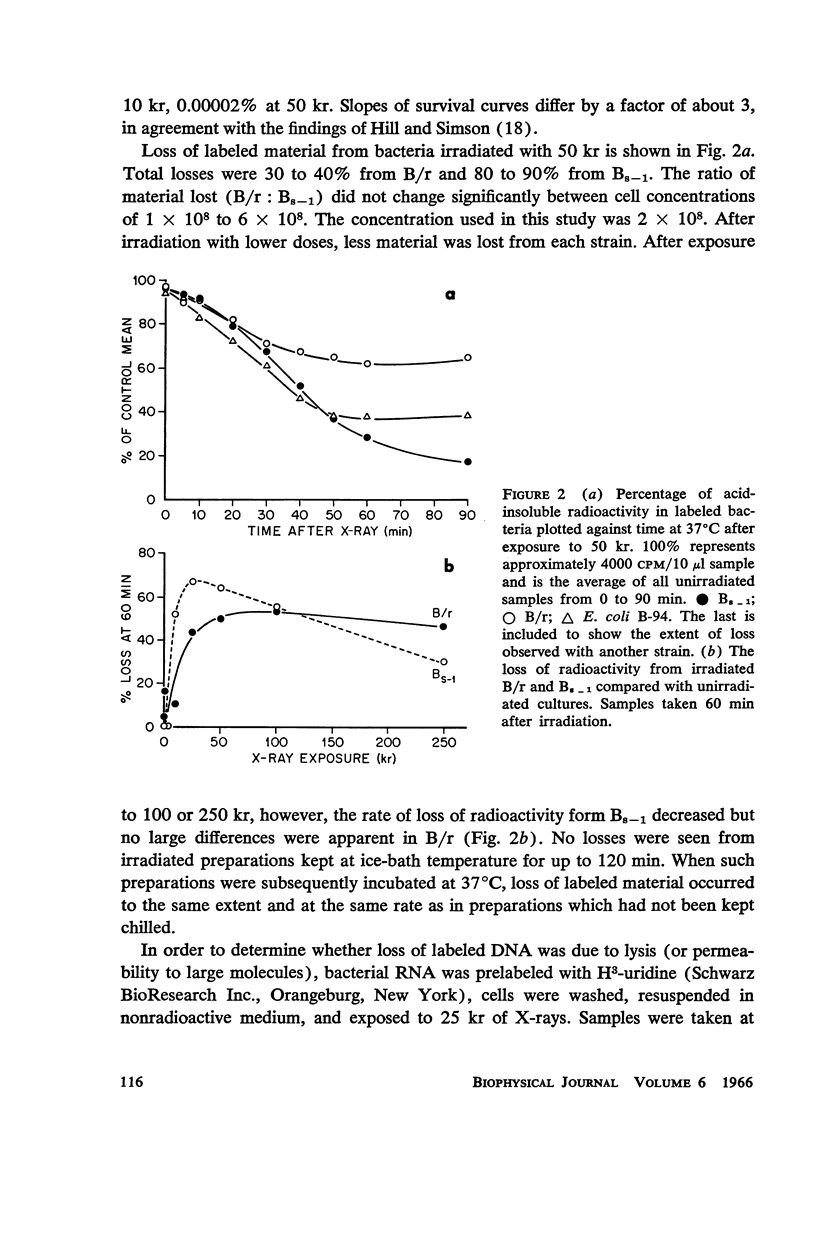
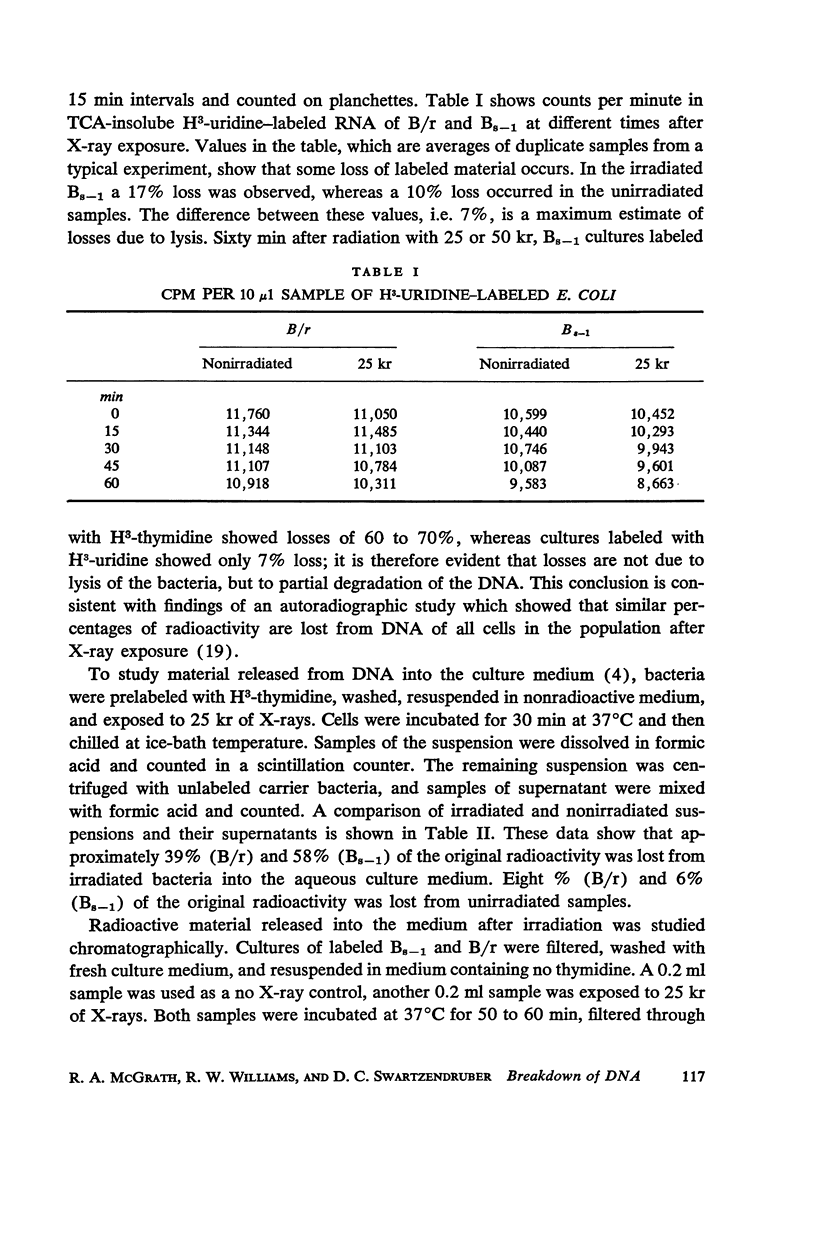



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLEN D. Modification of the release of cellular constituents by irradiated Escherichia coli. Arch Biochem Biophys. 1957 Apr;67(2):333–340. doi: 10.1016/0003-9861(57)90288-6. [DOI] [PubMed] [Google Scholar]
- Billen D., Hewitt R., Jorgensen G. X-ray-induced perturbations in the replication of the bacterial chromosome. Biochim Biophys Acta. 1965 Jul 15;103(3):440–454. doi: 10.1016/0005-2787(65)90137-1. [DOI] [PubMed] [Google Scholar]
- DAS N. K., ALFERT M. Accelerated DNA synthesis in onion root meristem during x-irradiation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:1–6. doi: 10.1073/pnas.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMERSON P. T., HOWARD-FLANDERS P. POST-IRRADIATION DEGRADATION OF DNA FOLLOWING EXPOSURE OF UV-SENSITIVE AND RESISTANT BACTERIA TO X-RAYS. Biochem Biophys Res Commun. 1965 Jan 4;18:24–29. doi: 10.1016/0006-291x(65)90876-4. [DOI] [PubMed] [Google Scholar]
- HILL R. F., SIMSON E. A study of radiosensitive and radioresistant mutants of Escherichia coli strain B. J Gen Microbiol. 1961 Jan;24:1–14. doi: 10.1099/00221287-24-1-1. [DOI] [PubMed] [Google Scholar]
- MCGRATH R. A., LEACH W. M., CARLSON J. G. CELL STAGES REFRACTORY TO THYMIDINE INCORPORATION INDUCED BY X-RAYS. Exp Cell Res. 1965 Jan;37:39–44. doi: 10.1016/0014-4827(65)90155-2. [DOI] [PubMed] [Google Scholar]
- MCGRATH R. A., WILLIAMS R. W., SETLOW R. B. INCREASED 3H-THYMIDINE INCORPORATION INTO DNA OF IRRADIATED SLIME MOULD. Int J Radiat Biol Relat Stud Phys Chem Med. 1964;8:373–380. doi: 10.1080/09553006414550441. [DOI] [PubMed] [Google Scholar]
- MILETIC B., KUCAN Z., SASEL L. THE EFFECT OF X-IRRADIATION ON THE DEGRADATION OF DNA IN CLOSELY-RELATED STRAINS OF E. COLI. Int J Radiat Biol Relat Stud Phys Chem Med. 1963 Aug;7:141–144. doi: 10.1080/09553006314550971. [DOI] [PubMed] [Google Scholar]
- NYGAARD O. F., GUTTES S. Effects of ionizing radiation on a slime mould with synchronous mitosis. Int J Radiat Biol Relat Stud Phys Chem Med. 1962 Apr;5:33–44. doi: 10.1080/09553006214550531. [DOI] [PubMed] [Google Scholar]
- PETTIJOHN D. E., HANAWALT P. C. Deoxyribonucleic acid replication in bacteria following ultraviolet irradiation. Biochim Biophys Acta. 1963 May 28;72:127–129. [PubMed] [Google Scholar]
- POLLARD E. C., ACHEY P. M. RADIATION ACTION ON DNA IN BACTERIA: EFFECT OF OXYGEN. Science. 1964 Oct 2;146(3640):71–73. doi: 10.1126/science.146.3640.71. [DOI] [PubMed] [Google Scholar]
- STUY J. H. Studies on the radiation inactivation of microorganisms. VI. X-ray induced breakdown of deoxyribonucleic acid in Haemophilus influenzae and in other bacteria. J Bacteriol. 1960 May;79:707–715. doi: 10.1128/jb.79.5.707-715.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer C. R., McGrath R. A. The effects of x-irradiation on the DNA of Escherichia coli. An autoradiographic study. Exp Cell Res. 1965 Sep;39(2):604–606. doi: 10.1016/0014-4827(65)90062-5. [DOI] [PubMed] [Google Scholar]
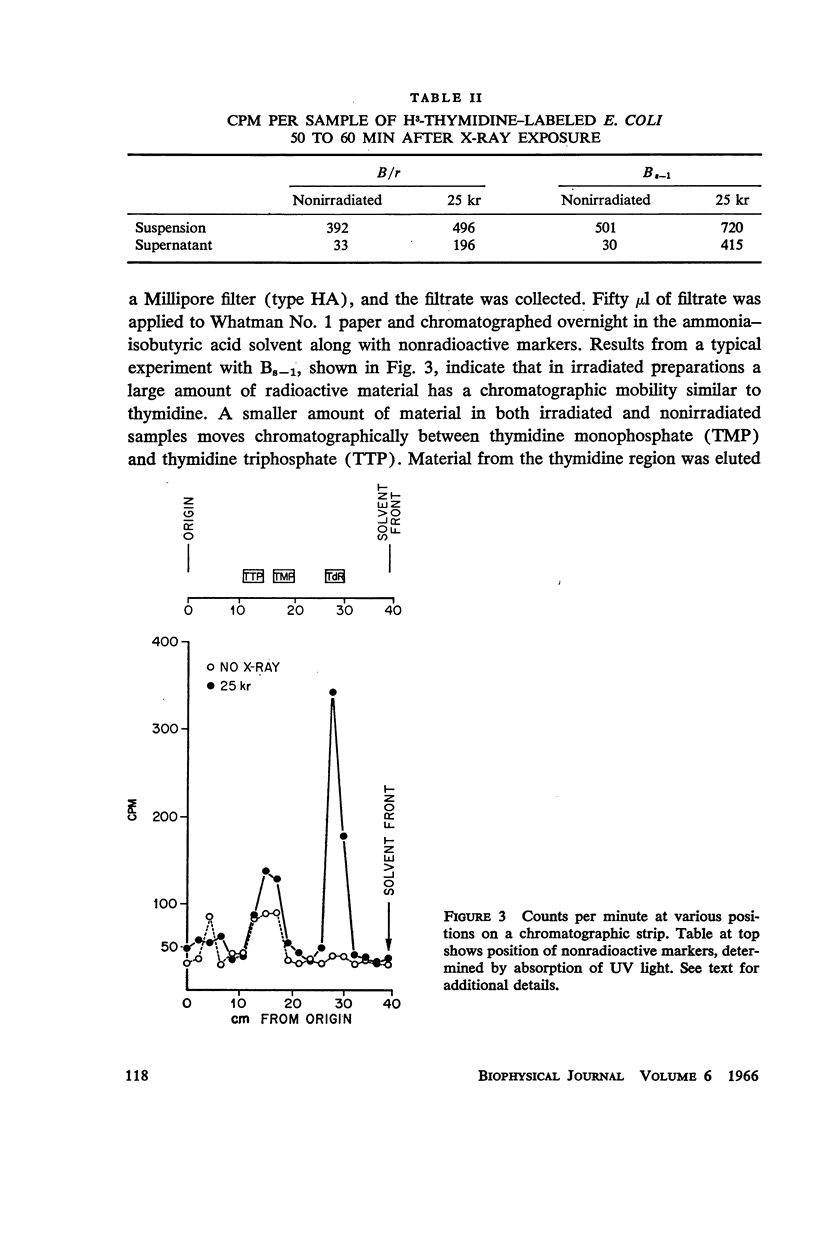
- VAN TUBERGEN R. P., SETLOW R. B. Quantitative radioautographic studies on exponentially growing cultures of Escherichia coli. The distribution of parental DNA, RNA, protein, and cell wall among progeny cells. Biophys J. 1961 Sep;1:589–625. doi: 10.1016/s0006-3495(61)86911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]