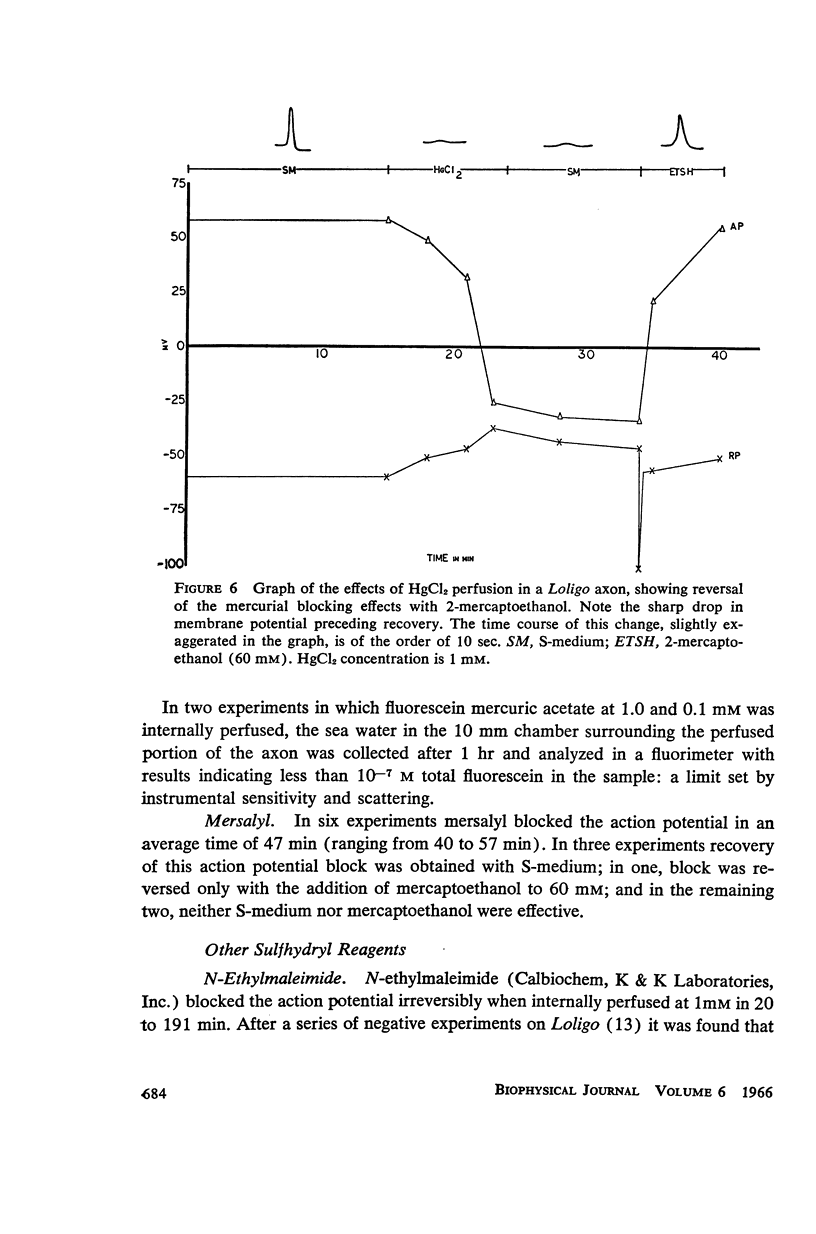
Abstract
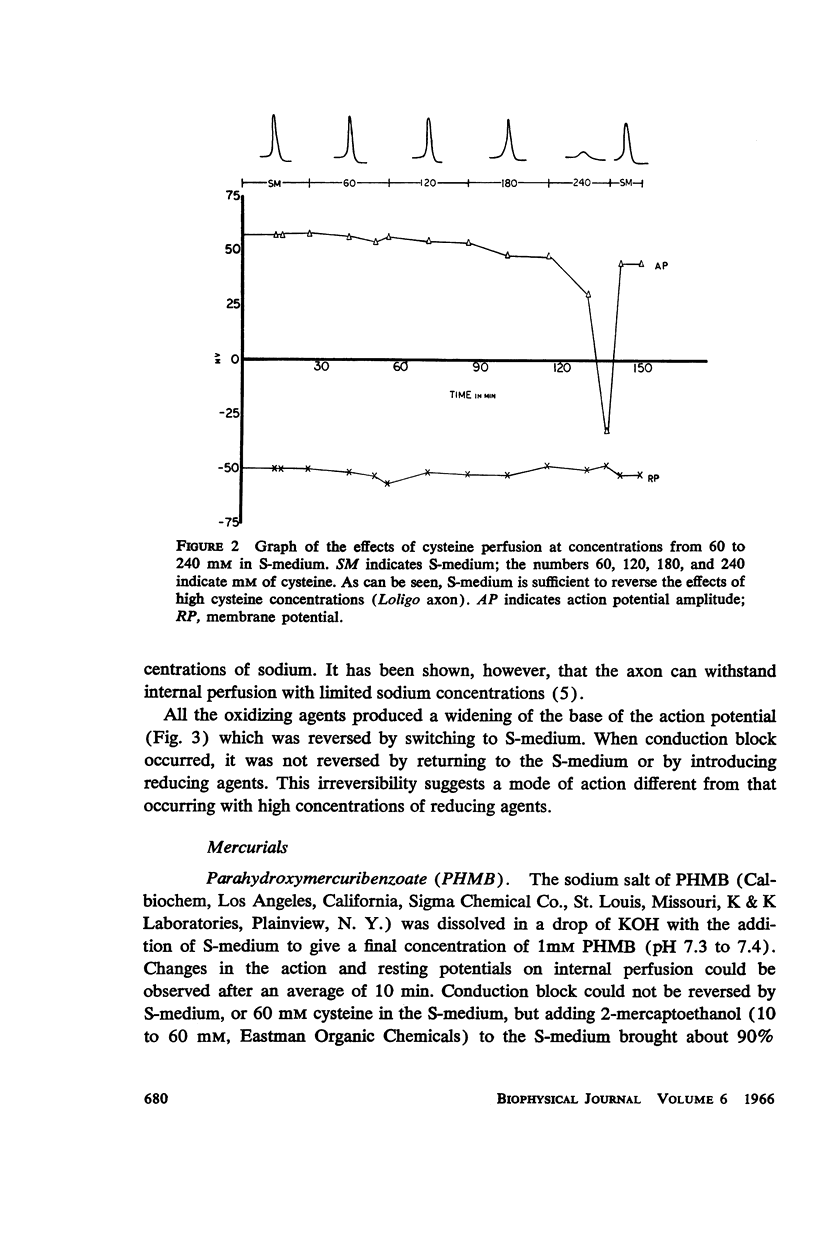
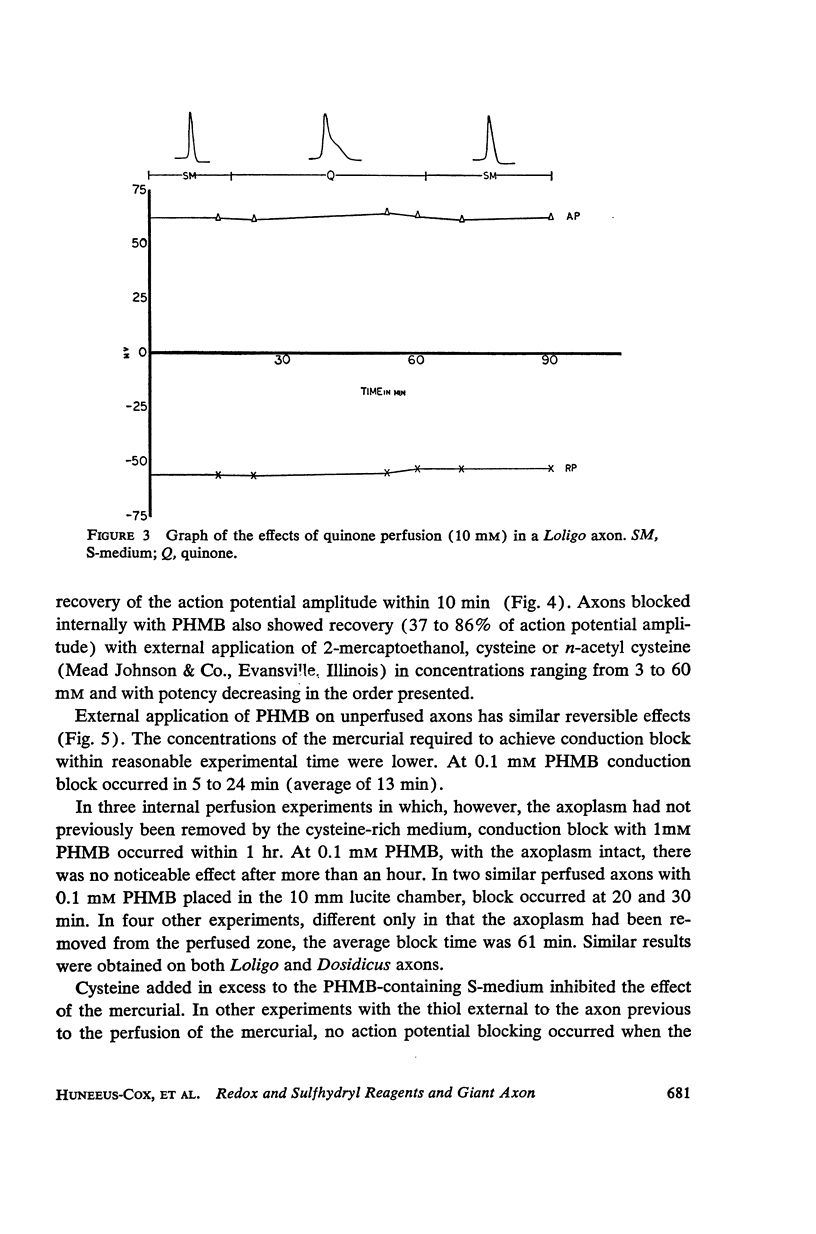
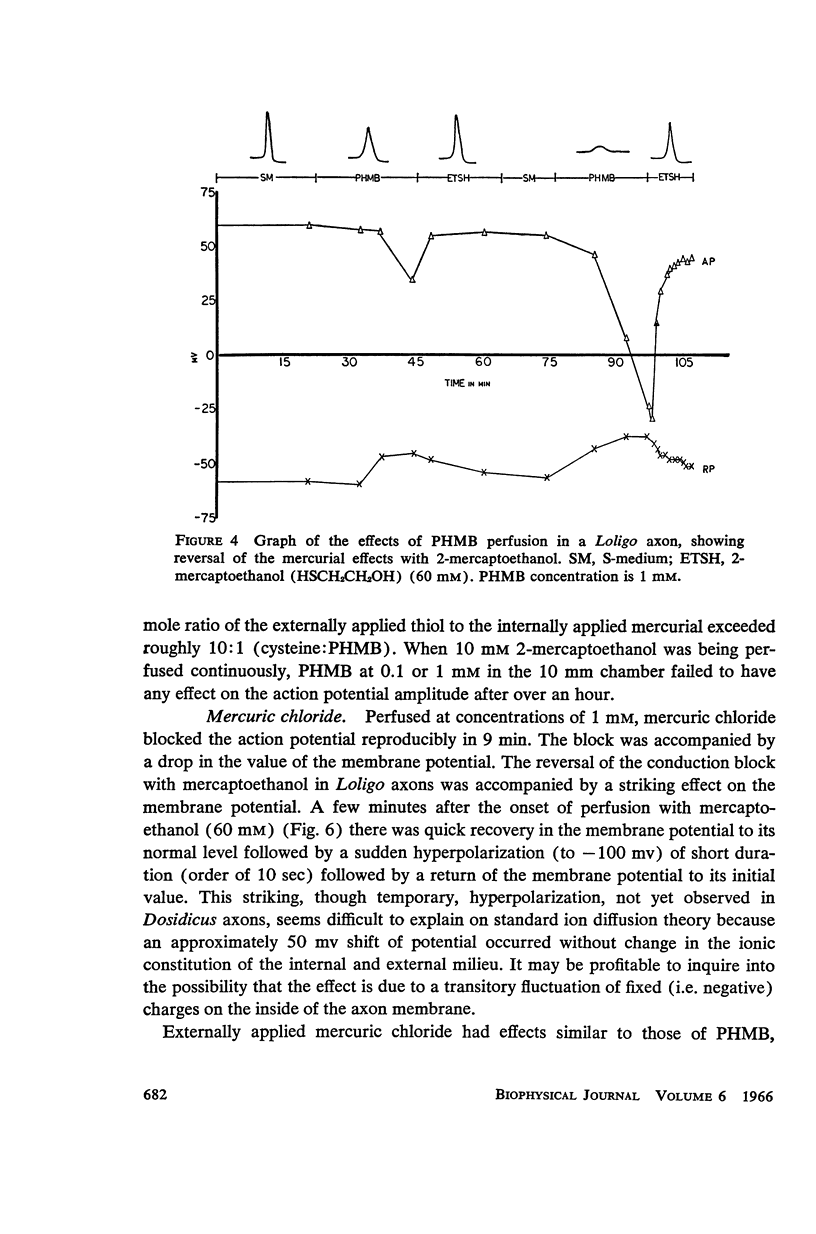
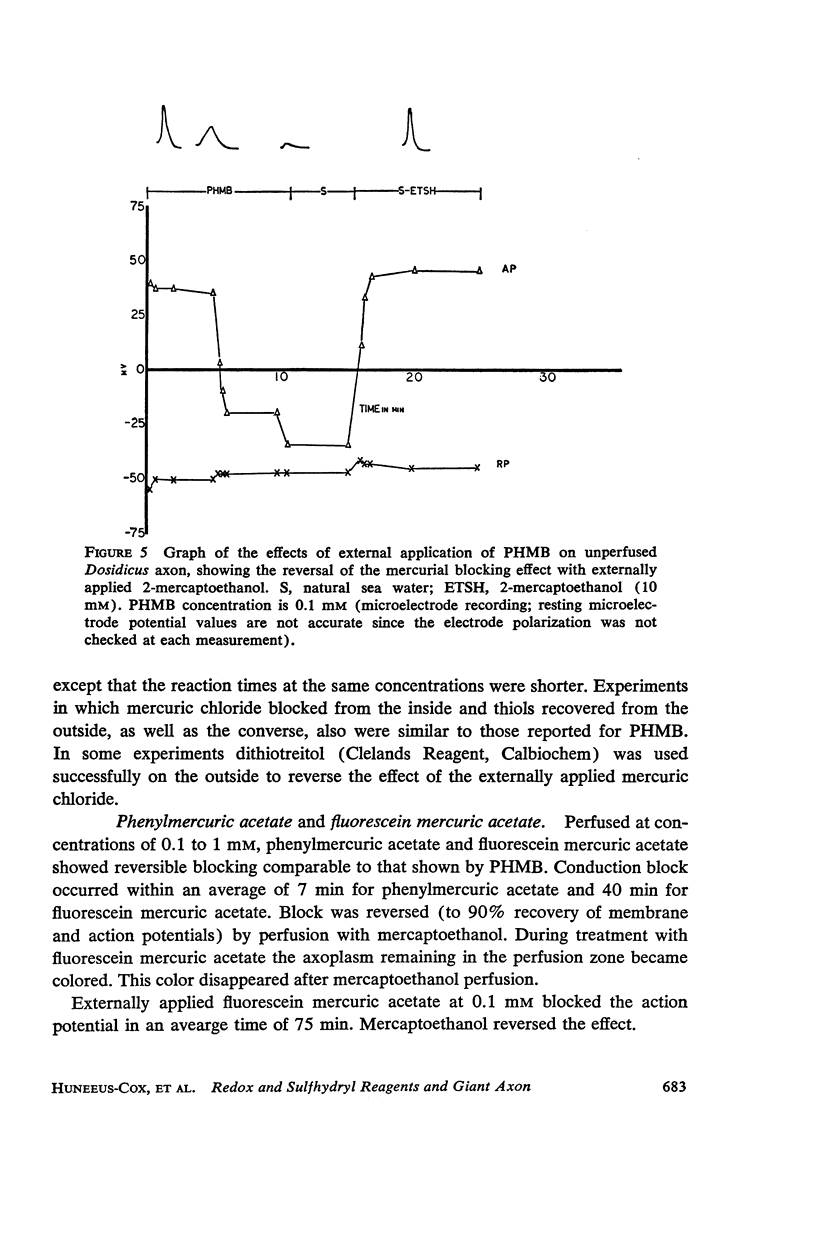
The effects of internally and externally applied sulfhydryl reagents on the bioelectric properties of the giant axon of the squid Loligo pealeii and Dosidicus gigas were studied. Cysteine-HCl (400 mM, pH 7.3) was used to remove axoplasm from the perfusion channel. Oxidizing agents (1 to 60 mM) tended to increase the duration of the action potential and had a slow, irreversible blocking effect when perfused internally; the membrane potential was little affected. Reducing agents applied internally caused a decrease in the spike duration without affecting its height or the membrane potential, although at high concentrations there was reversible deterioration of the action potential. Both external and internal perfusion of mercaptide-forming reagents caused deterioration in the action and membrane potentials with conduction block occurring in 5 to 45 min. 2-mercaptoethanol reversed the effects. Thiol alkylating reagents, iodoacetate and iodoacetamide, were without effect. N-ethylmaleimide did, however, block. Tests with chelating agents for nonheme iron in the membrane brought about no change in the electrical parameters. The implications of the present findings with regard to the macromolecular mechanism of excitation are discussed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. Ferredoxin and photosynthesis. Science. 1965 Sep 24;149(3691):1460–1470. doi: 10.1126/science.149.3691.1460. [DOI] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the protoplasm of a giant nerve fibre with artificial solutions. Nature. 1961 Jun 3;190:885–887. doi: 10.1038/190885a0. [DOI] [PubMed] [Google Scholar]
- Cecil R., Thomas M. A. Nature of the unreactive sulphydryl groups in human haemoglobin. Nature. 1965 Jun 26;206(991):1317–1320. doi: 10.1038/2061317a0. [DOI] [PubMed] [Google Scholar]
- DEFFNER G. G., HAFTER R. E. Chemical investigations of the giant nerve fibers of the squid. II. Detection and identification of cysteic acid amide (beta-sulfoalaninamide) in squid nerve axoplasm. Biochim Biophys Acta. 1959 Oct;35:334–340. doi: 10.1016/0006-3002(59)90382-8. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOTZ I. M. Non-covalent bonds in protein structure. Brookhaven Symp Biol. 1960 Nov;13:25–48. [PubMed] [Google Scholar]
- PAULING L. A molecular theory of general anesthesia. Science. 1961 Jul 7;134(3471):15–21. doi: 10.1126/science.134.3471.15. [DOI] [PubMed] [Google Scholar]
- ROJAS E., LUXORO M. MICRO-INJECTION OF TRYPSIN INTO AXONS OF SQUID. Nature. 1963 Jul 6;199:78–79. doi: 10.1038/199078b0. [DOI] [PubMed] [Google Scholar]
- ROJAS E. MEMBRANE POTENTIALS, RESISTANCE, AND ION PERMEABILITY IN SQUID GIANT AXONS INJECTED OR PERFUSED WITH PROTEASES. Proc Natl Acad Sci U S A. 1965 Feb;53:306–311. doi: 10.1073/pnas.53.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI H., MURAI T., SASAKI T. Plateau formation and sulphydryl groups in the plasma membrane. Nature. 1958 Dec 13;182(4650):1675–1677. doi: 10.1038/1821675a0. [DOI] [PubMed] [Google Scholar]
- TASAKI I., TAKENAKA T. EFFECTS OF VARIOUS POTASSIUM SALTS AND PROTEASES UPON EXCITABILITY OF INTRACELLULARLY PERFUSED SQUID GIANT AXONS. Proc Natl Acad Sci U S A. 1964 Sep;52:804–810. doi: 10.1073/pnas.52.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASAKII, WATANABE A., TAKENAKA T. Resting and action potential of intracellularly perfused squid giant axon. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1177–1184. doi: 10.1073/pnas.48.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I., Singer I., Takenaka T. Effects of internal and external ionic environment on excitability of squid giant axon. A macromolecular approach. J Gen Physiol. 1965 Jul;48(6):1095–1123. doi: 10.1085/jgp.48.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]



