Abstract
In this study we examined the effects of target membrane cholesterol depletion and cytoskeletal changes on human immunodeficiency virus type 1 (HIV-1) Env-mediated membrane fusion by dye redistribution assays. We found that treatment of peripheral blood lymphocytes (PBL) with methyl-β-cyclodextrin (MβCD) or cytochalasin reduced their susceptibility to membrane fusion with cells expressing HIV-1 Env that utilize CXCR4 or CCR5. However, treatment of human osteosarcoma (HOS) cells expressing high levels of CD4 and coreceptors with these agents did not affect their susceptibility to HIV-1 Env-mediated membrane fusion. Removal of cholesterol inhibited stromal cell-derived factor-1α- and macrophage inflammatory protein 1β-induced chemotaxis of both PBL and HOS cells expressing CD4 and coreceptors. The fusion activity as well as the chemotactic activity of PBL was recovered by adding back cholesterol to these cells. Confocal laser scanning microscopy analysis indicated that treatment of lymphocytes with MβCD reduced the colocalization of CD4 or of CXCR4 with actin presumably in microvilli. These findings indicate that, although cholesterol is not required for HIV-1 Env-mediated membrane fusion per se, its depletion from cells with relatively low coreceptor densities reduces the capacity of HIV-1 Env to engage coreceptor clusters required to trigger fusion. Furthermore, our results suggest that coreceptor clustering may occur in microvilli that are supported by actin polymerization.
Human immunodeficiency virus type 1 (HIV-1) gains entry into susceptible cells by fusion of the viral membrane with the cell plasma membrane (8, 13). This process is mediated by the interaction of the HIV-1 envelope (Env) glycoprotein with CD4 on the host cell surface and requires coreceptors, such as CXCR4 or CCR5, that determine the tropism of different HIV-1 isolates (4). The sequence of events leading to HIV-1 Env-mediated fusion is initiated by binding of the envelope gp120 to the CD4 molecule, leading to conformational changes in gp120, which results in engagement of the chemokine receptors with critical domains in gp120. The resultant conversion of HIV-1 gp41 to a fusion-active state enables the exposure of its fusion peptide domain. The triggering process leading to conformational changes in HIV-1 Env protein appears to be a highly cooperative process that is affected by receptor density as well as by Env-receptor affinity (17). Clustering of a certain number of CD4 and coreceptor molecules is presumed to be necessary for the efficient fusion of the viral and host cell membranes (35). Since both gp120-CD4 and gp120-chemokine receptor associations are reversible, the necessary number of CD4 and chemokine receptor molecules should almost simultaneously gather at the place of virus-host cell membrane fusion. In experiments using cell lines, the HIV-1 Env-mediated fusion event may be facilitated by the high expression of chemokine receptors and CD4 molecules on the cell surface. In primary cells the lower number of chemokine receptors (CD4+ T cells) or CD4 molecules (macrophages) may be a limiting factor in the fusion pore formation (16, 17). Therefore, in primary cells, existence of an active mechanism for receptor clustering may be of particular importance.
Membrane microdomains or lipid rafts are regions of host cell membrane enriched in glycosphingolipids, sphingomyelin, cholesterol, glycophosphatidylinositol-anchored proteins, and signaling proteins (25, 62). It has been hypothesized that these rafts serve as sites for recruitment of gp120-gp41-CD4-coreceptor complexes in a limited area on the cell surface. There are a number of observations that support such a model: (i) CD4 molecules associate with rafts in the plasma membrane (52); (ii) glycosphingolipids associate with CD4 (27, 67) and with CXCR4 (66) on the membrane surface, and they play a role in HIV-1 entry (29); (iii) membrane raft microdomains mediate the acquisition of cell polarity and the asymmetrical CCR5 redistribution to the leading cellular edge (24, 43); (iv) HIV-1 gp120-induced coclustering of CD4 and coreceptor into domains enriched in GM1 is prevented by the removal of cholesterol from cell plasma membranes (42, 57); and (v) depletion of cholesterol from target cells inhibits their susceptibility to HIV-1 infection (38, 42, 57). Since cholesterol plays a key role in the maintenance of membrane raft microdomains, it was argued that the inhibition of HIV-1 infection by cholesterol depletion is due to the inability of CD4 and coreceptors to form clusters in the cholesterol-depleted cells. However, alternative explanations regarding the role of cholesterol in HIV-1 entry have been offered: (i) cholesterol is required for HIV-1 Env-mediated membrane fusion (38) as is the case for alphaviruses (33), (ii) cholesterol is required for proper CXCR4 conformation and function (47), and (iii) removal of cholesterol from cells is toxic and could have myriad effects on cellular functions (34).
In previous studies effects of target cell cholesterol depletion on susceptibility to HIV-1 infection or syncytium formation (38, 42, 57) have been examined. However, in those assays effects of target membrane cholesterol depletion may have involved postfusion events. In this study we examined the direct effects of target membrane cholesterol depletion on HIV-1 Env-mediated membrane fusion by dye redistribution assays developed in our laboratory (15, 45). We found that cholesterol depletion of primary lymphocytes indeed reduces their susceptibility to HIV-1 Env-mediated membrane fusion. However, removal of cholesterol from cells expressing high levels of CD4 and coreceptors did not affect their susceptibility to HIV-1 Env-mediated membrane fusion despite the fact that the chemotaxis function of these cells for coreceptor ligands is impaired. Thus, our results suggest that cholesterol is required for HIV-1 Env-mediated fusion of cells with low coreceptor density.
It has been observed previously that HIV-1 gp120-induced copatching of CD4 and CXCR4 is blocked by cytochalasin D (30). Cytochalasins also inhibit HIV-1 Env-mediated membrane fusion with T-cell lines (22, 23). To further explore the relationship between cytoskeletal organization, receptor clustering, and HIV-1 Env-mediated fusion, we examined effects of cytochalasin treatment on HIV-1 Env-mediated fusion with primary lymphocytes and coreceptor-overexpressing cell lines. Based on the similar pattern of inhibition by cholesterol removal and cytochalasin treatment, we propose a new model for the role of CD4 and coreceptors in triggering the HIV-1 Env-mediated fusion reaction.
(Preliminary data from this work have been presented at the 44th Annual Meeting of the Biophysical Society, 12 to 16 February 2000 [abstr. 344].)
MATERIALS AND METHODS
Materials.
Antibodies and their sources were as follows: anti-CXCR4 immunoglobulin G (IgG; rabbit polyclonal antibody; Santa Cruz Biotechnology, Santa Cruz, Calif.), anti-CD4 IgG (monoclonal antibody [MAb]; Novocastra Lab, Newcastle upon Tyne, United Kingdom), and anti-Zap-70 (mouse IgG2A; BD Transduction Laboratories, Lexington, Ky.). For fluorescence-activated cell sorting analysis the following antibodies were used: phycoerythrin (PE) anti-CXCR4 IgG (MAb; PharMingen, San Diego, Calif.), PE anti-CCR5 IgG (MAb; PharMingen), and fluorescein isothiocyanate (FITC) anti-CD4 IgG (MAb; PharMingen). Recombinant HIV-1 gp120 (IIIB) was purchased from Intracell (Issaquah, Wash.). For cholesterol depletion and repletion, we used, respectively, methyl-β-cyclodextrin (MβCD) and cholesterol-MβCD complexes purchased from Sigma (St. Louis, Mo.). All other biochemicals used were of the highest purity available and were obtained from regular commercial sources.
Cell cultures.
Human CD3+ CD4+ peripheral blood T lymphocytes (PBL) were obtained from healthy donors under National Institutes of Health (NIH)-approved guidelines. Mononuclear cells were isolated by centrifugation of the blood on a Ficoll-Hypaque gradient (Sigma), and CD4+ lymphocytes were purified by using negative selection columns to yield >95% pure cell preparations (Miltenyi Biotec, Auburn, Calif.). Before being used for the chemotaxis and cell fusion experiments, PBL activated with phytohemagglutinin were further cultured with 1,000 U of recombinant human interleukin 2 (PeproTech, Rocky Hill, N.J.) per ml for 10 to 14 days. Human osteosarcoma (HOS) cells that stably express CD4 as well as CXCR4 or CCR5 were obtained from the NIH AIDS Research and Reference Reagent Program. HeLa cells expressing different levels of CD4 and coreceptor were gifts from David Kabat (Oregon Health Sciences University). HeLa cells were grown in Dulbecco modified Eagle medium plus 10% fetal bovine serum (FBS) (D10). HOS cells were grown in D10 plus 1 μg of puromycin/ml. HIV-1 envelope proteins were transiently expressed on the surface of HeLa cells with the recombinant vaccinia virus constructs vPE16 (IIIB, CXCR4 utilizing) (19a) and vCB43 (5a) (Ba-L, CCR5 utilizing) as described previously (31a).
Preparation of detergent-resistant membrane (DRM) fractions.
Plasma membrane rafts were obtained as previously described (52). Briefly, primary lymphocytes were left untreated at 37°C or incubated with 10 μg of gp120/ml for 3 min at 37°C. Activation was stopped by ice-cold phosphate-buffered saline (PBS) washing followed by lysis at 4°C in a buffer containing 1.4 ml of 25 mM morpholineethanesulfonic acid (MES; pH 6.5), 0.15 M NaCl, 1% (vol/vol) Triton X-100, protease inhibitors (0.1 μg of phenylmethylsulfonyl fluoride/ml, 2 μg of aprotinin/ml, 2 μg of leupeptin/ml, 1 μg of pepstatin A/ml), and 1 mM sodium orthovanadate. The lysate was then homogenized and adjusted to 40% sucrose. A 5 to 30% linear sucrose gradient was formed above the homogenate. The centrifugation was carried out at 45,000 rpm for 16 to 20 h in an SW60 rotor (Beckman Instruments, Palo Alto, Calif.). A light scattering band confined to the 15 to 20% sucrose region was observed that contained lipid raft markers such as CD4 (51) but excluded most other cellular proteins. From the top of each gradient 0.35-ml fractions were collected to yield a total of 12 fractions. Proteins were quantitated by the Peterson method (53a), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and subjected to immunoblot analysis. Fractions 1 and 2 were generally lacking proteins and thus were not subjected to SDS-PAGE. Fraction 12 represents the nuclear portion.
Immunoblot analysis.
Cellular proteins were resolved by SDS-PAGE under reducing conditions and transferred to nitrocellulose membranes. Blots were incubated for 1 h in TBST (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.1% Tween 20) containing 5% powdered skim milk. After three washes with TBST, membranes were incubated for 1 h with the primary antibody (CD4 diluted 1:250, CXCR4 diluted 1:200, and Zap-70 diluted 1:5,000) in TBST and for 1 h with a horseradish peroxidase-conjugated secondary antibody (diluted 1:3,000). Immunoreactivity was detected by using an enhanced chemiluminescence detection kit (Pierce, Rockford, Ill.).
Chemotaxis assay.
The migration of PBL, HOS-CD4/CXCR4, and HOS-CD4/CCR5 cells was assessed by a 48-well microchamber technique (3a). Different concentrations of stromal cell-derived factor-1α or macrophage inflammatory protein 1β (MIP-1β) (PeproTech) were placed in the lower wells of the chamber. The HOS cells (50 μl, 106/ml) were loaded in the upper wells. The lower and upper wells were separated by a polycarbonate filter (10-μm pore size; Neuroprobe Inc., Gaithersburg, Md.) precoated with 50 μg of collagen type 1/ml for 2 h at 37°C. The chamber was incubated at 37°C for 5 h in humidified air with 5% CO2. At the end of the incubation, after removal of nonmigrating cells, the filter was fixed and stained with Diff-Quik (Richard-Allen Scientific, Kalamazoo, Mich.). By the use of three high-power fields under light microscopy, the cells migrating across the filter were counted in triplicate with all samples coded. The chemotaxis index was calculated as number of cells migrating to chemokines/number of cells migrating to medium. The significance of the difference between test and control groups was analyzed by a paired Student's t test.
HIV-1 envelope glycoprotein-mediated cell-cell fusion.
Target cells were labeled with the cytoplasmic dye 5- and 6-([(4-chloromethyl)benzoyl]-amino)tetramethylrhodamine (CMTMR) at a concentration of 1.5 μM for 1 h at 37°C. Envelope-expressing cells were labeled with calcein AM at a concentration of 1 μM for 1 h at 37°C. Calcein-labeled effector cells were cocultured with CMTMR-labeled target cells for 2 h at 37°C, and dye redistribution was monitored microscopically as described previously (29). The extent of fusion was calculated as percent fusion = 100 × number of bound cells positive for both dyes/number of bound cells positive for CMTMR.
Flow cytometry.
The cells were harvested with cell dissociation buffer from Gibco/BRL (Gaithersburg, Md.), centrifuged at 450 × g, and resuspended at 106 cells/ml in PBS with 5% FBS and 5% normal mouse serum. After incubation for 15 min at 4°C, cells were washed twice in PBS with 0.1% bovine serum albumin (BSA) and resuspended at 107 cells/ml (in 100 μl) in PBS with 5% FBS and 5% normal mouse serum. FITC-conjugated mouse IgG anti-CD4, PE-conjugated mouse IgG anti-CXCR4 (12G5), or PE-conjugated mouse IgG anti-CCR5 (2D7) from PharMingen was then added to each sample at a 1:5 dilution. Cells were incubated at 4°C for 1 h and washed twice in PBS with 0.1% BSA. Samples were fixed in PBS with 1% paraformaldehyde and resuspended in 1 ml of PBS to be read by a FACScalibur (Becton Dickinson, San Jose, Calif.) at 10,000 events per sample with respect to unlabeled cells.
Confocal laser scanning microscopy analysis.
CEM cells were left untreated or treated with 10 mM MβCD in PBS for 30 min at 37°C. After two washes with PBS, cells were labeled with MAb T4 (anti-CD4) (Coulter Corporation, Hialeah, Fla.) for 30 min at 4°C, or with anti-CXCR4 IgG MAb (PharMingen), followed by secondary antibody goat Alexa Fluor 594-conjugated anti-mouse IgG (Fab′)2 fragment (Molecular Probes, Eugene, Oreg.), for 30 min at 4°C. After repeated washes with PBS-BSA (1%), fixation was carried out at 4°C for 30 min with 3% paraformaldehyde, followed by permeabilization with 0.5% Triton X-100 for 10 min at room temperature. Cells were then incubated with Alexa Fluor 488-conjugated phalloidin (Molecular Probes) for 30 min at 37°C and seeded on a microscope slide with the Prolong reagent (Molecular Probes). Observation was carried out on a Leica TCS 4D apparatus, equipped with an argon-krypton laser and double-dichroic splitters (488-568 nm). Signals from different fluorescent probes were taken in parallel, and colocalization was detected in white (pseudocolor). Image acquisition and processing were realized by using the Scanware multicolor analysis (Leica Lasertechnik GmbH, Heidelberg, Germany) and Photoshop (Adobe Systems, Mountain View, Calif.) software. Several cells were analyzed for each labeling condition, and representative results are shown.
RESULTS
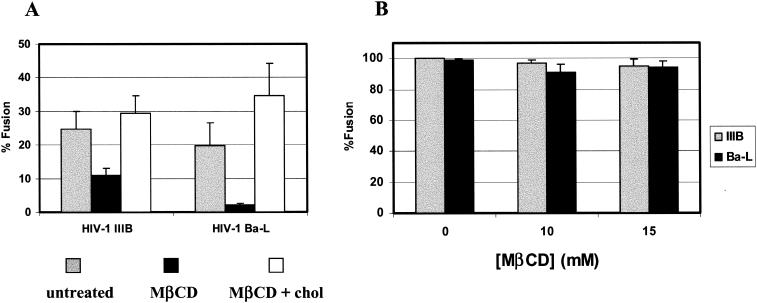
To explore the role of cholesterol in HIV envelope glycoprotein-mediated cell fusion, we used the cholesterol-solubilizing agent MβCD, which stimulates cholesterol efflux from cells. Incubation of cells with 10 mM MβCD for 30 min at 37°C reduced cholesterol levels by 40 to 50% with no significant effect on cell viability and surface expression of CD4 and coreceptors. Figure 1A shows the fusion of cells expressing either HIV-1Ba-L or HIV-1IIIB envelope glycoprotein, which utilizes CCR5 or CXCR4, respectively, with activated PBL. In the case of the R5-utilizing virus MβCD treatment resulted in about 90% inhibition of fusion, whereas the X4-utilizing virus was inhibited by about 40%. When these cells were replenished with cholesterol by incubation with preformed cholesterol-MβCD complexes, HIV-1 Env-mediated fusion was fully recovered.
FIG. 1.
HIV-1 Env-mediated fusion with PBL and HOSCD4X4R5 cells. Fusion activity was monitored as described in Materials and Methods by using HeLa cells infected with vaccinia virus vectors which express gp120-gp41 from a CXCR4-utilizing isolate (IIIB, vPE16) and a CCR5-utilizing isolate (Ba-L, vCB43). (A) Gray bars, untreated PBL; black bars, PBL treated with 10 mM MβCD for 30 min at 37°C; white bars, MβCD-treated PBL loaded with 75 μg of cholesterol/ml complexed with MβCD. (B) HIV-1IIIB (gray bars) and HIV-1Ba-L (black bars) were expressed in HeLa cells, and fusion with HOSCD4X4R5 cells treated with different amounts of cyclodextrin (at 37°C and for 30 min) was monitored as described in Materials and Methods.
Since the effects of MβCD on HIV-1 Env-mediated fusion may be dependent on surface levels of CD4 and/or coreceptors, we determined the surface expression of these receptors by fluorescence-activated cell sorting. The mean values of CD4 and coreceptor on PBL were compared with the mean values on engineered HeLa cells with known levels of CD4, CXCR4, and CCR5 (35, 54). Table 1 shows a slight change in the cell surface expression levels of CD4 and no significant changes in cell surface expression levels of CXCR4 and CCR5 following treatment of these cells with MβCD. In order to test the hypothesis that cholesterol is required in the membrane to facilitate the recruitment of CD4 and coreceptors into clusters sufficiently large to trigger the multiplicity of HIV-1 Env molecules needed to form a fusion pore, we performed experiments with cells that overexpress CD4 and coreceptors where such clustering may occur spontaneously. We used engineered HOS cell lines that express 5 times less CD4, but 25 times more CXCR4 or more than 300 times more CCR5, than do PBL (Table 1). Figure 1B shows that the treatment of these cells with MβCD did not alter their susceptibility to HIV-1 Env-mediated cell fusion, indicating that cholesterol may be required as an organizing principle and not for a specific interaction with the envelope glycoprotein as is the case for alphaviruses (33), or for the fusion reaction per se (5).
TABLE 1.
CD4 and coreceptor expression on different cellsa
| Cell line | Expression level on target cells (103 molecules/cell)
|
||
|---|---|---|---|
| CD4 | CXCR4 | CCR5 | |
| PBL | 70 ± 7 | 2.5 ± 1 | 0.6 ± 0.5 |
| PBL + MβCD | 50 ± 6 | 2.5 ± 1 | 0.8 ± 0.6 |
| HOSCD4X4R5 | 11 ± 3 | 64 ± 11 | 249 ± 28 |
| HeLa-RC | 10 ± 3 | 10 ± 3 | NDb |
| HeLa-JC | 150 ± 18 | 17 ± 12 | ND |
| HeLa-J10 | 196 ± 18 | ND | 6 ± 2 |
| HeLa-J53 | 345 ± 37 | ND | 190 ± 30 |
The levels of expression of CD4 and coreceptors on the different cell lines were assessed by flow cytometry. For each antibody, calibration curves between the fluorescence signal and the number of molecules were established by using cells of a known level of expression (35,54). The mean intensity and standard deviation were determined for each cell and each antibody and related to the level of expression with those calibration curves.
ND, not determined.
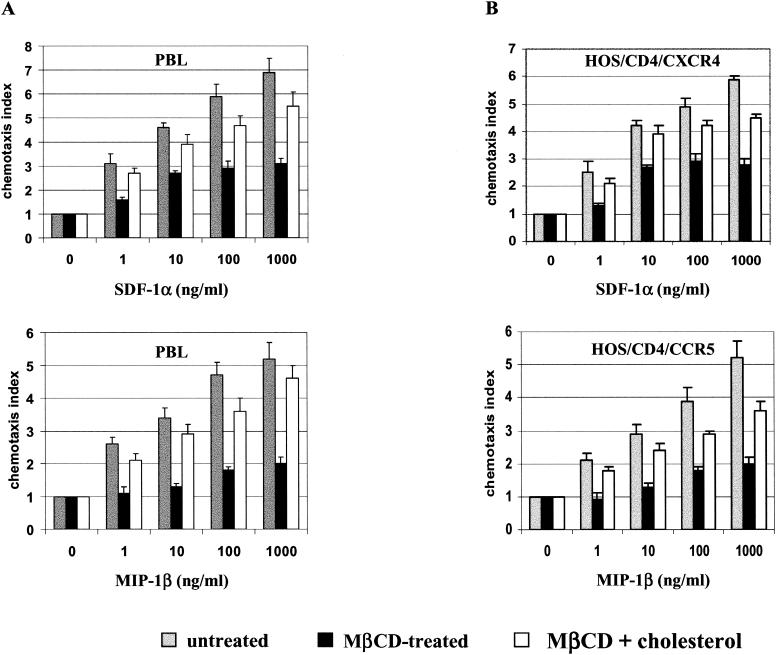
It has been shown elsewhere that the acquisition and maintenance of both spatial and functional asymmetry (polarization) between initially equivalent cell parts in response to chemokines are required for cell chemotaxis. These processes are inhibited by removal of cholesterol from the cells (24, 43). Hence, we examined whether the chemotaxis of activated PBL promoted by specific ligands to CXCR4 and CCR5 was cholesterol dependent. Figure 2A shows chemotaxis of PBL in response to SDF-1α, the ligand for CXCR4, and to MIP-1β, a ligand for CCR5. Treatment of these cells with MβCD inhibited their ability to respond to SDF-1α or MIP-1β. However, SDF-1α- and MIP-1β-induced chemotaxis was significantly restored after replenishment of the cells with cholesterol, indicating that the MβCD treatment did not result in permanent damage of cellular functions. Figure 2B shows that chemotaxis of HOS cells expressing CXCR4 or CCR5 was inhibited by depletion of plasma membrane cholesterol to levels that did not alter their susceptibility to HIV-1 Env-mediated cell fusion. These data, taken together, show that, while chemotaxis mediated by CXCR4 and CCR5 requires appropriate levels of membrane cholesterol, chemokine receptor function related to HIV-1 Env-mediated membrane fusion is less cholesterol dependent.
FIG. 2.
Migration of PBL and HOSCD4X4R5 cells in response to chemokines. Different concentrations of SDF-1α (top panels) or MIP-1β (bottom panels) were placed in the lower wells of the chemotaxis chamber; cells were placed in the upper wells, which were separated from the lower wells by a polycarbonate filter. The results are expressed as chemotaxis index representing the fold increase of migrating cells in response to chemokines over the response to control medium. Significant cell migration (P < 0.05) was detected with 10 ng of chemoattractant/ml. Gray bars, untreated cells; black bars, cells treated with 10 mM MβCD for 30 min at 37°C; white bars, MβCD-treated cells loaded with 75 μg of cholesterol/ml complexed with MβCD. (A) PBL; (B) HOSCD4X4R5.
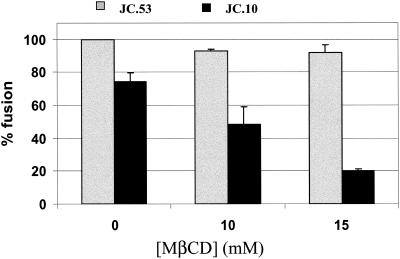
Since the effects of cholesterol depletion may be cell line dependent, we examined HIV-1 Env-mediated fusion before and after MβCD treatment of the same cell lines expressing different levels of CCR5. Figure 3 shows data obtained with HeLa cells expressing 196 × 103 and 345 × 103 molecules of CD4 and 6 × 103 and 190 × 103 molecules of CCR5 (J10 and J53 cells, respectively) (35) (Table 1). Incubation of the J53 cells with HeLa cells expressing the HIV-1Ba-L Env resulted in about 100% fusion as determined by the dye redistribution assay, and the extent of fusion was not affected by pretreatment of these cells with MβCD. On the other hand, incubation of the HeLa-HIV-1Ba-L Env cells with J10 cells, which express 32-fold-lower levels of CCR5 than do J53 cells, resulted in lower levels of fusion that were significantly inhibited by MβCD treatment. These results clearly show that HIV-1 Env-mediated cell fusion becomes sensitive to inhibition by MβCD when coreceptor levels on the target cells are low.
FIG. 3.
HIV-1 Env-mediated fusion of target cells with different surface densities of CCR5. The figure shows the fusion of HIV-1Ba-L-expressing HeLa cells with HeLa cells expressing high (J53, gray bars) and low (J10, black bars) levels of CCR5. The treatment with MβCD was performed at 37°C for 30 min. Expression levels of CD4 and CCR5 are summarized in Table 1. The fusion activity was monitored as described in Materials and Methods.
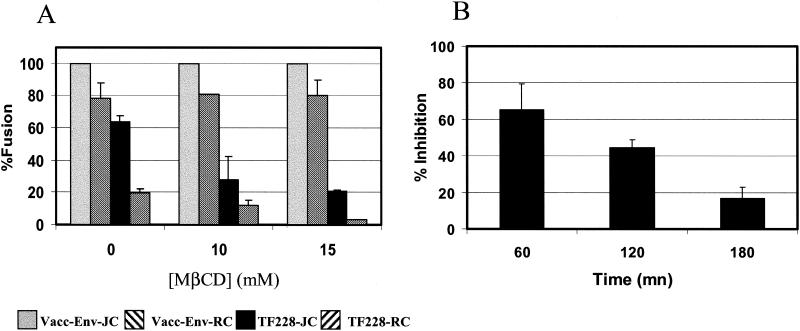
We next examined the effects of MβCD treatment on CXCR4-dependent cell fusion by using HeLa-JC and HeLa-RC cells expressing high (150 × 103) and low (10 × 103) levels of CD4, respectively (Table 1) (54). These levels are within the range of CD4 molecules found on PBL (70 × 103). However, the natural levels of CXCR4 in these HeLa cells are four to six times higher than those on the PBL that we used (Table 1). Figure 4A shows that incubation of HeLa cells expressing high levels of HIV-1IIIB Env (infected with recombinant vaccinia virus) resulted in 100 and 80% cell fusion with HeLa cells expressing high levels (JC) and low levels (RC) of CD4, respectively. However, in contrast to PBL (Fig. 1), pretreatment of target cells with MβCD did not inhibit the fusion. Presumably the CXCR4 levels in HeLa cells were high enough to render fusion of these cells with effector cells expressing high levels of HIV-1 Env insensitive to cholesterol depletion. We therefore examined whether these cells would become sensitive to the effects of cholesterol depletion if we used effector cells expressing relatively low levels of HIV-1 Env. We used TF228.16 cells that are stably transfected with HIV-1IIIB Env (31). We determined by Western analysis that these cells express about five-times-lower levels of gp120-gp41 than do cells infected with the HIV-1IIIB Env vaccinia virus recombinant vPE16 (data not shown). The TF228.16 cells produced 40 and 80% lower fusion yields with JC and RC target cells, respectively, than with cells infected with the HIV-1IIIB Env vaccinia virus recombinant (Fig. 4A). Moreover, the TF228-mediated fusion was further reduced following treatment of the target cells with MβCD. These results indicate that cholesterol depletion impairs CXCR4-dependent HIV-1 Env-mediated fusion at relatively low levels of Env. This impairment is, however, not due to a physical inability of the cell to undergo fusion and can be substantially recovered if longer times of incubation are allowed for a fusogenic interaction to take place among Env, CD4, and CXCR4 (Fig. 4B).
FIG. 4.
Effect of Env expression on HIV-1 Env-mediated fusion. (A) Fusion of HIV-1IIIB-expressing HeLa cells (gray and left diagonally striped bars) with vPE16 and of TF228 cells (black and right diagonally striped bars) with HeLa cells expressing high (JC, gray and black bars) and low (RC, striped bars) levels of CD4 (Table 1 shows levels of expression of CD4 and coreceptors). The treatment with MβCD was performed at 37°C for 30 min. The use of vaccinia virus recombinant resulted in five-times-higher Env expression than that of the TF228 cells. (B) Inhibition of the fusion of TF228 cells with JC HeLa cells as a function of the time of coincubation. JC cells were treated for 30 min at 37°C with 15 mM MβCD. Upon treatment, MβCD was washed and the JC cells were incubated in PBS until their coincubation with the TF228 cells in PBS. The TF228 cells were added to the different wells at different time points starting with the longer kinetics so that the time of incubation of the MβCD-treated cells in PBS would be the same at the end of each kinetic point. The fusion activity was monitored as described in Materials and Methods.
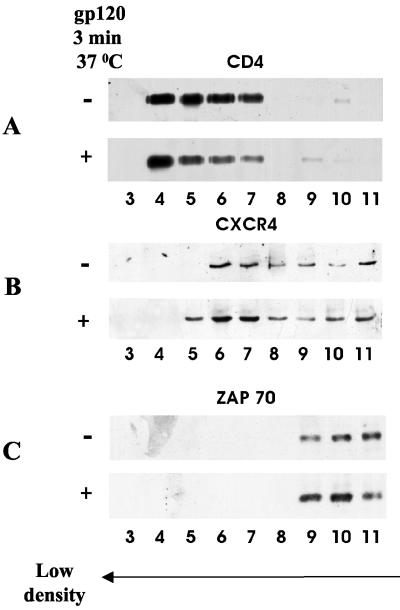
It has been hypothesized elsewhere that membrane raft microdomains mediate lateral assemblies of the receptors required for HIV-1 infection (42). The classical way to examine the association of membrane proteins with rafts is treatment of cells with Triton X-100 at 4°C followed by flotation of the cell lysate on a sucrose gradient (6). The molecules that are not solubilized by Triton X-100 under these conditions are assigned to the DRM domains (63). The presence of the molecules of interest in the soluble and DRM fractions is then examined by Western blot analysis. We performed this analysis with the same number of untreated and HIV-1 gp120-treated human primary lymphocytes. Figure 5A shows that CD4 behaves like a typical raft protein (51) in that it localized on the top of the gradient in fractions 4 to 7. As a control we used the nonraft marker in lymphocytes, Zap-70 (77), which localized in fractions 9 to 11 (Fig. 5C). By contrast CXCR4 behaved somewhere between the typical raft and nonraft proteins in that it localized in fractions 6 to 11 (Fig. 5B). Addition of gp120 to these cells had a minor effect on CD4 but resulted in a shift of CXCR4 toward fraction 5. Densitometric analysis indicates that the amount of CXCR4 in fractions 5 to 7 is enhanced threefold following treatment of the cells with gp120 (data not shown).
FIG. 5.
HIV-1 receptors in DRMs. Primary lymphocytes (5 × 108 cells) were kept at 37°C, left untreated (−) or incubated for 3 min with gp120 (10 μg/ml) (+), and then subjected to a 1% Triton X-100 sucrose gradient as described in Materials and Methods. Twelve fractions were collected and quantitated by a protein assay. Equal protein amounts (4 μg for detection of CD4 [A], 100 μg for detection of CXCR4 [B], and 8 μg for detection of Zap-70 [C]) of each fraction were resolved by SDS-PAGE and subjected to immunoblot analysis with anti-CD4, anti-CXCR4, and anti-Zap-70 antibodies as indicated. DRMs are represented by fractions 4 to 7 whereas soluble proteins appear in fractions 9 to 11.
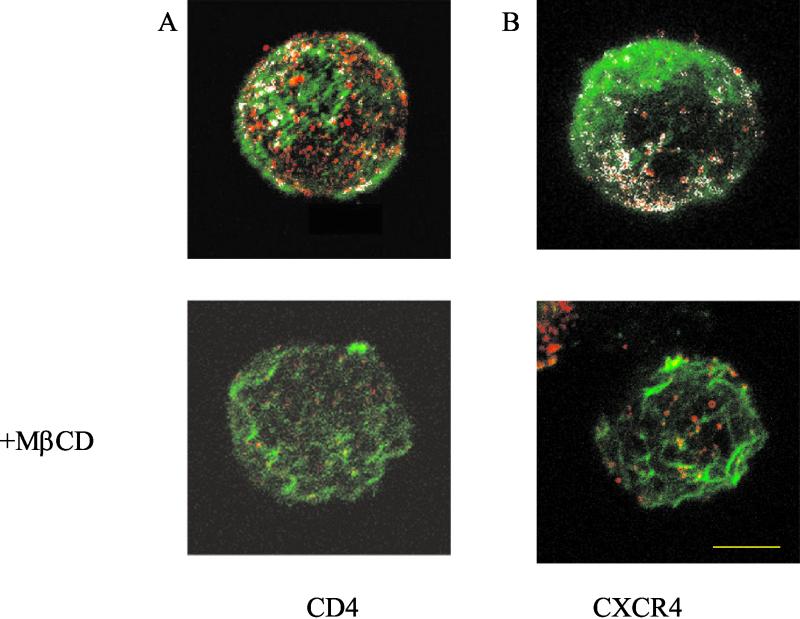
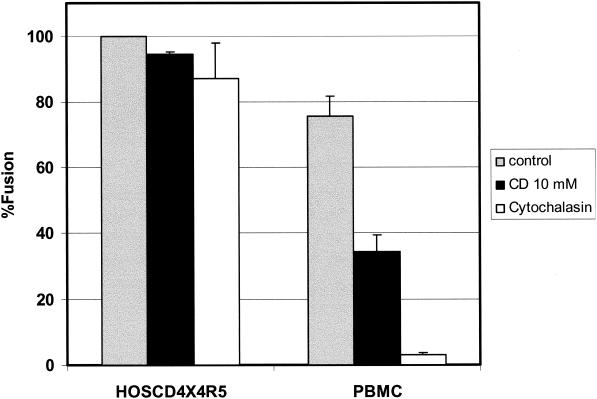
High-resolution immunogold electron microscopy demonstrated that CCR5, CXCR4, and CD4 are preferentially located on cell surface microvilli in human macrophages and T cells, as well as in genetically engineered cells (64). Moreover, each of these molecules appeared to form homogenous microclusters, which are separated by a distance of less than a viral diameter. Since plasma membrane protrusions have been identified as new cholesterol-based microdomains (11, 59), we reasoned that the depletion of cholesterol might induce changes in these protrusions. Indeed, for MDCK cells it has been shown elsewhere that cholesterol depletion reduces the number of membrane protrusions (10). F-actin polymerization plays a significant role in the formation of these structures, and cytochalasins disrupt the organization of actin filaments (20). In order to test the hypothesis that removal of cholesterol from the plasma membrane of lymphocytes leads to the disruption of microvilli, resulting in a redistribution of CD4 and coreceptors, we examined the colocalization of actin with either CD4 or CXCR4 by confocal laser scanning microscopy. Figure 6 shows that in the control cells there are a great deal of colocalizations of either CD4 (Fig. 6A, upper panel) or CXCR4 (Fig. 6B, upper panel) with actin, presumably in microvilli. These observations are consistent with the high-resolution electron microscopy images reported by Singer and coworkers (64). Treatment of the cells with MβCD causes a drastic reduction of the colocalization of CD4 or CXCR4 with actin (Fig. 6A and B, bottom panels) consistent with a disruption of microvilli. Treatment of human peripheral T lymphocytes with cytochalasins B and D has also been shown elsewhere to cause a reduction in the number of microvilli (50). Since cytochalasin B has been shown elsewhere to inhibit HIV-1 Env-mediated fusion with the lymphocyte cell line SupT1 (22, 23), we examined whether the effects of cytochalasin were similar to those of MβCD. Figure 7 shows that treatment of PBL with cytochalasin has a profound effect on fusion with HIV-1IIIB Env, while fusion with the HOS cells overexpressing CD4 and coreceptors was not effected. Cytochalasin also inhibited chemotaxis of these cells mediated by CXCR4 and CCR5 ligands (data not shown). These results, taken together, suggested that the domains affected by cholesterol depletion might be localized in microvilli.
FIG. 6.
Effect of cholesterol removal on colocalization of CD4 or CXCR4 with F actin. CEM cells were left untreated (top panels) or treated with 10 mM MβCD at 37°C for 30 min (bottom panels). The cells were stained with anti-CD4 (A) or with anti-CXCR4 (B) followed by Alexa Fluor 594-conjugated secondary (Fab)2 antibodies (red) as described in Materials and Methods. Cells were then fixed, treated with 0.5% Triton X-100, labeled with Alexa Fluor 488-conjugated phalloidin (green) at 37°C for 30 min, and examined by confocal microscopy as described in Materials and Methods. Bar, 5 μm. The white color on the images indicates CD4-actin or CXCR4-actin colocalization.
FIG. 7.
Effects of cytochalasin on HIV-1 Env-mediated fusion. Fusion activity was monitored as described in Materials and Methods between HeLa cells infected with a vaccinia virus vector which expresses gp120-gp41 from a CXCR4-utilizing isolate (IIIB, vPE16) and PBL or HOSCD4X4R5 as indicated. Gray bars, untreated cells; black bars, cells treated with 10 mM MβCD for 30 min at 37°C; white bars, cells treated with 10 μM cytochalasin B. PBMC, peripheral blood mononuclear cells.
DISCUSSION
Viral entry mediated by the HIV-1 Env protein appears to be a highly cooperative process that is affected by receptor density as well as by Env-receptor affinity (17). Kabat and coworkers (35) found a nonlinear relationship between CCR5 density and virus infection. They estimate that about six CCR5 molecules are required to produce a virus-cell fusion event leading to infection. Primary CD4+ T cells typically express fewer than 10,000 CCR5 or CXCR4 molecules and about 65,000 CD4 molecules per cell (37). We found about the same level of CD4 on PBL, but the levels of CXCR4 and CCR5 were much lower. These levels may depend on the donor and the state of cell activation. Given a surface area of activated lymphocytes of about 500 μm2 and about 1,000 CXCR4 or CCR5 molecules per cell, the average distance between coreceptors will be about 0.7 μm. Thus, random collisions between coreceptors in a fluid mosaic membrane (65) are unlikely to generate coreceptor clusters large enough to provide the cooperativity required for HIV-1 Env-mediated fusion. Therefore, lateral assemblies of glycolipids and cholesterol, called rafts (6, 63), have been invoked to recruit gp120-gp41-CD4-coreceptor complexes in a limited area on the cell surface (27, 29, 38, 42, 47, 57). Since depletion of cholesterol presumably disrupts the clustering of HIV-1 receptors in rafts, we have focused in this paper on the cholesterol dependence of HIV-1 Env-mediated fusion. Treatment of PBL with MβCD inhibited fusion mediated by envelope glycoproteins from HIV-1 isolates that utilize CXCR4 and CCR5, as well as chemotaxis triggered by the chemokines SDF-1α and MIP-1β, respectively (Fig. 1A and 2A). The fusion activity as well as the chemotactic activity was recovered by adding back cholesterol to the host cells. However, the link between induction of chemotaxis and susceptibility to HIV-1 Env-mediated fusion was severed when we used engineered cell lines expressing high levels of HIV-1 receptors (55). Whereas cholesterol removal inhibited SDF-1α- and MIP-1β-induced chemotaxis of HOS cells expressing relatively high levels of CXCR4 and CCR5, fusion with X4- and R5-utilizing HIV-1 Env-expressing cells was not affected (Fig. 1B and 2B). Moreover, HeLa-CD4 cells expressing high levels of CCR5 did not alter their susceptibility to HIV-1 Env-mediated fusion following cholesterol removal, whereas fusion with the same cells expressing low levels of CCR5 was inhibited following the same treatment (Fig. 3). These results indicate that the effects of MβCD treatment are not related to membrane fusion per se (38) or to toxic effects (34) but rather to the impaired capacity of gp120 to engage the HIV-1 receptor clusters required to trigger the fusion event when the receptors are at lower levels. Moreover, they are consistent with the notion that, while adequate levels of cholesterol are required for chemokine receptor disposition and function (42, 43, 47), these are not absolutely required for HIV-1 Env-mediated fusion.
Although there appears to be strong evidence for the importance of lipid rafts for viral budding (3, 39, 46, 49), their role in viral entry is not that clear. The best-studied example of the dependence of viral fusion on target membrane cholesterol and sphingomyelin is that of Semliki Forest virus (SFV) (33). Kielian and coworkers showed that the fusion peptide of SFV, but not that of influenza virus hemagglutinin, associates with detergent-resistant membrane domains (1). However, studies with cholesterol analogs indicate that this association reflects specific SFV Env-lipid interactions rather than a clear requirement for rafts in SFV fusion. Enveloped viruses that appear to require rafts for entry include ecotropic murine leukemia virus (40), human T-cell leukemia virus type 1 (48), and Ebola and Marburg viruses (3). In the case of ecotropic murine leukemia virus the cholesterol dependence of fusion appears to be confined to the receptor-bearing membrane (41), whereas in the case of HIV-1 the Env-bearing membranes also require cholesterol for efficient fusion (49). In a recent elegant series of studies Helenius and coworkers (53) showed that simian virus 40, a nonenveloped virus, utilizes caveolae (a subset of lipid rafts [63]) for infectious entry into host cells. They found that, after binding to caveolae, virus particles induced transient breakdown of actin stress fibers. These events depended on the presence of cholesterol in the target membrane and on the activation of tyrosine kinases that phosphorylate proteins in caveolae.
One of the problems with the raft model for HIV-1 entry is the controversy regarding the localization of CXCR4 in the raft domains. DRM flotation studies show little CXCR4 in the raft fraction in the absence of gp120 (34, 42, 57). However, following treatment of HEK293-CD4 cells at 4°C with gp120, Manes and coworkers (42) showed substantive recruitment of CXCR4 into the raft fraction. By contrast, Kabat and coworkers (34) show no such recruitment of CXCR4 into rafts following treatment of H9 cells with gp120 for 1 h at 37°C. Our data (Fig. 5) show that CXCR4 in PBL behaved somewhere between the typical raft and nonraft proteins in that it localized between the DRM and soluble protein fractions. However, treatment of PBL with gp120 within a time frame and at a temperature (3 min at 37°C) that are relevant to the HIV-1 Env-mediated fusion reaction (58) resulted in a definite shift of CXCR4 toward the DRM fraction. It should be kept in mind that lipid rafts are dynamic entities whose associations with proteins are unstable and rapidly fluctuating in physiological conditions (32, 63).
The other method of choice to identify molecules in rafts is antibody patching and immunofluorescence microscopy (28). A number of groups, using fluorescence microscopy, have found CD4 and coreceptors to be randomly distributed on the surface in an unstimulated state (2, 30, 42, 57, 71). However, at high surface densities of CD4 and coreceptor, interactions between these molecules can be detected by coimmunoprecipitation experiments (75). Following addition of gp120, colocalization of these molecules has been detected by fluorescence microscopy (2, 30, 42, 57, 71), and their associations have been observed by coimmunoprecipitation experiments (36). By contrast, Kozak et al. (34) did not observe detectable redistribution of CD4 and CXCR4 between their separate domains following treatment of cells with gp120 for 1 h at 37°C. The antibody patching approach with light microscopy appears to be a rather blunt instrument to study in situ associations between membrane molecules. High-resolution immunogold electron microscopy, on the other hand, has revealed the localization of CCR5, CXCR4, and CD4 molecules in homogeneous microclusters that are separated by a distance of less than a viral diameter (64). These molecules appear to be preferentially located on cell surface microvilli. It is important to appreciate the difference between colocalization at the resolution of light microscopy (∼0.2 μm) and that of electron microscopy where mixed microclusters of CD4 and chemokine receptor were not observed. At the resolution of light microscopy these molecules would be seen as being coclustered. However, the light microscopic observation appropriately places CD4 or CXCR4 with actin at the microvilli (Fig. 6). Treatment of cells with MβCD disrupts this pattern.
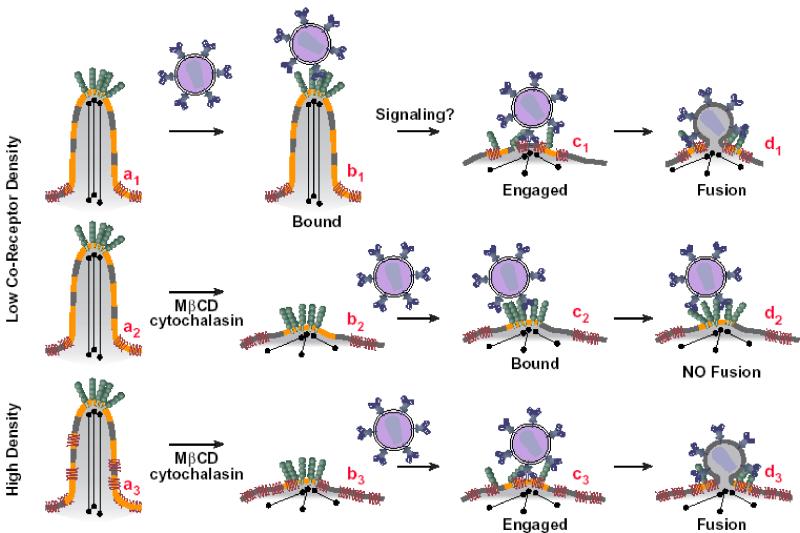
In T lymphocytes the association of CD4 with microvilli appears to be mediated by p56Lck, a CD4-associated tyrosine kinase (21). Treatment with cytochalasin D disrupted this association, suggesting the involvement of cytoskeletal elements in CD4 anchoring to microvilli. Moreover, the CD4-p56Lck complex appears to be enriched within raft domains as opposed to conditions in which CD4 does not interact with p56Lck. However, similar localization of CD4 and coreceptors has been seen on various p56Lck-negative cell types (64), indicating that other components may mediate association of these molecules with microvilli. To further explore the link between the involvement of target cell lipid domains and cytoskeletal elements in HIV-1 Env-mediated fusion, we performed inhibition experiments with cytochalasin B. Figure 7 shows a similar pattern of treatment of cells with MβCD or with cytochalasin in that inhibition was seen when PBL, but not cells overexpressing coreceptors, were used as targets. Based on these observations, we propose a new model diagrammed in Fig. 8 for the role of CD4 and coreceptors in triggering the HIV-1 Env-mediated fusion reaction.
FIG. 8.
Model for the effect of the cell surface disposition of CD4 and coreceptor on the triggering of HIV-1 Env-mediated fusion. CD4 is located on the tip of a microvillus whereas CXCR4 or CCR5 is located more toward the base (64). Interaction of gp120 on HIV-1 or on HIV-1 Env-expressing cells with CD4 has two consequences: (i) triggering of conformational changes in Env that expose binding sites for coreceptor (70, 74) and (ii) signaling events that lead to rearrangements of actin in the microvilli (26). As a result the triggered Env is able to engage coreceptor, leading to gp41 six-helix bundle formation and fusion (23). Treatment of cells with MβCD or inhibitors of F-actin polymerization disrupts the microvilli. As a consequence CD4-triggered Env becomes less capable of engaging coreceptors on cells expressing low levels of coreceptor. However, the effect of these treatments can be overcome in cells expressing levels of coreceptor high enough to engage CD4-triggered Env.
We had hypothesized that the CD4- and coreceptor-induced triggering events leading to HIV-simian immunodeficiency virus Env-mediated fusion are stochastic, meaning that the events will happen in time and space according to a certain probability distribution (58). According to the model, HIV-1 Envs are initially attached to CD4 on the tips of target cell microvilli (64) (Fig. 8a1 to a3).
Since many of the CD4 molecules form cell surface microclusters, it is likely that the initial interactions between the HIV Env trimers (7, 72) and multiple CD4 molecules will be cooperative (Fig. 8b1). These multiple interactions will then result in the “cocking” of HIV Env so that binding sites on gp120 become available for coreceptor engagement (18, 19, 23, 44, 60, 70, 74). The activated Envs then need to take hold of a cluster of coreceptors (35) to trigger the fusion reaction. The probability of coreceptor engagement is enhanced by localization of both CD4 and the coreceptors on microvilli; CCR5 and CXCR4 molecules are often found within a viral diameter of many of the CD4 microclusters (64). The changes in the cytoskeletal organization induced by interactions between HIV-1 Env and CD4 may lead to a transient intermixing between CD4 and coreceptor, enabling the triggered Env to engage coreceptors (Fig. 8b1→c1). This process then leads to gp41 six-helix bundle formation and fusion (23) (Fig. 8d1). Pretreatment of cells with MβCD (10) or inhibitors of F-actin polymerization (50) prevents the raft formation and cytoskeletal reorganization, possibly disrupting the microvilli (Fig. 8a2 and a3→b2 and b3). As a consequence CD4-triggered Env becomes less capable of engaging coreceptors on cells expressing low levels of coreceptor (Fig. 8c2 and d2). However, the effect of these treatments can be overcome by providing levels of coreceptor in target membranes high enough to engage CD4-triggered Envs (Fig. 8c3 and d3). Based on our model, HIV-1 Env-mediated fusion might eventually occur with cholesterol-depleted cells if sufficient time is provided for the random interactions that bring Env-CD4 complexes in juxtaposition with coreceptor. This prediction has been borne out by the experiment presented in Fig. 4B showing reduced inhibition of HIV-1 Env-mediated fusion with cholesterol-depleted targets after longer incubation times. In experiments with HIV-1 virions we would not expect such a recovery because at longer times fusion-incompetent virus may be directed toward the nonproductive endocytic pathway (61).
We have observed that fusion mediated by CD4-independent HIV-1 Envs that engage coreceptors directly is also cholesterol dependent (S. Ablan, A. Puri, and R. Blumenthal, unpublished observations). Absent CD4 on the target membrane, glycosphingolipids may serve as initial attachment sites for HIV-1 Env (29), and cholesterol may support a membrane domain (presumably on microvilli) where glycosphingolipids and coreceptors can be juxtaposed to form a fusion-competent complex. The observation that CXCR4 and CCR5 associate with GM3-enriched domains on lymphocytes (24, 66) is consistent with this notion.
Cell surface microvilli appear to play a crucial role in cell adhesion and fusion processes (73). In the case of HIV-1 Env-mediated fusion we have observed by image-enhanced Nomarski differential interference contrast optics that cells made contact by using microspikes to “touch” and adhere to the neighboring cells prior to fusion (14). Pseudopod extension and condensation of F actin at cell-cell adhesion sites during HIV-1 Env-mediated fusion indicate the involvement of the cytoskeleton in the adhesion and/or subsequent fusion event (69). The notion that plasma membrane protrusions, rich in actin, are new cholesterol-based microdomains (11, 59) may provide a link between the observed cholesterol and F-actin dependence of HIV-1 Env-mediated fusion. For lymphocytes it has been shown previously that CD4 cross-linking activates tyrosine kinases that then induce simultaneous association of CD4 and p56Lck with cytoskeleton (26).
The model may provide a rationale for the observed relationship between HIV-1 gp120-induced signaling and HIV-1 entry-fusion (68). It has been reported elsewhere that the tyrosine kinase inhibitor herbimycin A inhibits syncytium formation among Jurkat cells infected with HIV-1 (9). Also, another tyrosine kinase inhibitor, genistein, was reported previously to inhibit syncytium formation between HIV-1IIIB Env-expressing Jurkat cells and the U937 cell line (76). Although the precise mechanisms by which tyrosine kinase inhibitors reduce HIV-1 Env-mediated fusion are unclear, the fact that genistein inhibits cytoskeletal changes induced by CD4 cross-linking (26) may point to the role of tyrosine kinases in HIV-1 Env-mediated fusion. Other signaling events induced by binding of HIV-1 Env to its receptors include tyrosine phosphorylation of mitogen-activated protein kinase and extracellular signal-regulated kinase (56) and of the protein tyrosine kinase Pyk-2 (12). According to our model, tyrosine kinase signaling may be needed for actin cytoskeleton rearrangements and subsequent CD4-chemokine receptor commingling. The precise molecular rearrangements involved following the interactions of HIV-1 Env with target cells need to be further explored.
Acknowledgments
We are grateful to L. Samelson, D. Kabat, Z. Jonak, and the NIH AIDS Research and Reference Reagent Program for providing cell lines and reagents. We thank Santos Mañes for helpful suggestions regarding the cholesterol reloading experiments; Wanghua Gong for assistance with the chemotaxis experiments; and Xiaodong Xiao, Dimiter Dimitrov, and the members of the Blumenthal labortory for helpful suggestions.
REFERENCES
- 1.Ahn, A., D. L. Gibbons, and M. Kielian. 2002. The fusion peptide of Semliki Forest virus associates with sterol-rich membrane domains. J. Virol. 76:3267-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfano, M., H. Schmidtmayerova, C. A. Amella, T. Pushkarsky, and M. Bukrinsky. 1999. The B-oligomer of pertussis toxin deactivates CC chemokine receptor 5 and blocks entry of M-tropic HIV-1 strains. J. Exp. Med. 190:597-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Ben-Baruch, A., L. Xu, P. R. Young, K. Bengali, J. J. Oppenheim, and J. M. Wang. 1995. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors. C-C CKR1, a receptor for macrophage inflammatory protein-1 alpha/Rantes, is also a functional receptor for MCP3. J. Biol. Chem. 270:22123-22128. [DOI] [PubMed] [Google Scholar]
- 4.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal, R. 1987. Membrane fusion. Curr. Top. Membr. Transp. 29:203-254. [Google Scholar]
- 5a.Broder, C. C., and E. A. Berger. 1995. Fusogenic selectivity of the envelope glycoprotein is a major determinant of human immunodeficiency virus type 1 tropism for CD4+ T-cell lines vs. primary macrophages. Proc. Natl. Acad. Sci. USA 92:9004-9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 7.Center, R. J., P. Schuck, R. D. Leapman, L. O. Arthur, P. L. Earl, B. Moss, and J. Lebowitz. 2001. Oligomeric structure of virion-associated and soluble forms of the simian immunodeficiency virus envelope protein in the prefusion activated conformation. Proc. Natl. Acad. Sci. USA 98:14877-14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, D. I., Y. Tani, H. Tian, E. Boone, L. E. Samelson, and H. C. Lane. 1992. Participation of tyrosine phosphorylation in the cytopathic effect of human immunodeficiency virus-1. Science 256:542-545. [DOI] [PubMed] [Google Scholar]
- 10.Colarusso, P., and K. R. Spring. 2002. Reticulated lipid probe fluorescence reveals MDCK cell apical membrane topography. Biophys. J. 82:752-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbeil, D., K. Roper, C. A. Fargeas, A. Joester, and W. B. Huttner. 2001. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic 2:82-91. [DOI] [PubMed] [Google Scholar]
- 12.Davis, C. B., I. Dikic, D. Unutmaz, C. M. Hill, J. Arthos, M. A. Siani, D. A. Thompson, J. Schlessinger, and D. R. Littman. 1997. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J. Exp. Med. 186:1793-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitrov, D. S. 2000. Cell biology of virus entry. Cell 101:697-702. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrov, D. S., and R. Blumenthal. 1994. Photoinactivation and kinetics of membrane fusion mediated by the human immunodeficiency type 1 envelope glycoprotein. J. Virol. 68:1956-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimitrov, D. S., H. Golding, and R. Blumenthal. 1991. Initial steps in HIV-1 envelope glycoprotein mediated cell fusion monitored by a new assay based on redistribution of fluorescence markers. AIDS Res. Hum. Retrovir. 7:799-805. [DOI] [PubMed] [Google Scholar]
- 16.Dimitrov, D. S., D. Norwood, T. S. Stantchev, Y. Feng, X. Xiao, and C. C. Broder. 1999. A mechanism of resistance to HIV-1 entry: inefficient interactions of CXCR4 with CD4 and gp120 in macrophages. Virology 259:1-6. [DOI] [PubMed] [Google Scholar]
- 17.Doms, R. W. 2000. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology 276:229-237. [DOI] [PubMed] [Google Scholar]
- 18.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151:F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doranz, B. J., S. S. Baik, and R. W. Doms. 1999. Use of a gp120 binding assay to dissect the requirements and kinetics of human immunodeficiency virus fusion events. J. Virol. 73:10346-10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forscher, P., and S. J. Smith. 1988. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 107:1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foti, M., M. A. Phelouzat, A. Holm, B. J. Rasmusson, and J. L. Carpentier. 2002. p56Lck anchors CD4 to distinct microdomains on microvilli. Proc. Natl. Acad. Sci. USA 99:2008-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey, S., M. Marsh, S. Gunther, A. Pelchen-Matthews, P. Stephens, S. Ortlepp, and T. Stegmann. 1995. Temperature dependence of cell-cell fusion induced by the envelope glycoprotein of human immunodeficiency virus type 1. J. Virol. 69:1462-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo, S. A., A. Puri, and R. Blumenthal. 2001. HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process. Biochemistry 40:12231-12236. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Mouton, C., J. L. Abad, E. Mira, R. A. Lacalle, E. Gallardo, S. Jimenez-Baranda, I. Illa, A. Bernad, S. Manes, and A. Martinez. 2001. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. USA 98:9642-9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hakomori, S. 1998. New insights in glycosphingolipid function: “glycosignaling domain,” a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology 8:xi-xix. [DOI] [PubMed] [Google Scholar]
- 26.Ha-Lee, Y. M., Y. Lee, Y. K. Kim, and J. Sohn. 2000. Cross-linking of CD4 induces cytoskeletal association of CD4 and p56lck. Exp. Mol. Med. 32:18-22. [DOI] [PubMed] [Google Scholar]
- 27.Hammache, D., N. Yahi, M. Maresca, G. Pieroni, and J. Fantini. 1999. Human erythrocyte glycosphingolipids as alternative cofactors for human immunodeficiency virus type 1 (HIV-1) entry: evidence for CD4-induced interactions between HIV-1 gp120 and reconstituted membrane microdomains of glycosphingolipids (Gb3 and GM3). J. Virol. 73:5244-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141:929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hug, P., H. M. Lin, T. Korte, X. Xiao, D. S. Dimitrov, J. M. Wang, A. Puri, and R. Blumenthal. 2000. Glycosphingolipids promote entry of a broad range of human immunodeficiency virus type 1 isolates into cell lines expressing CD4, CXCR4, and/or CCR5. J. Virol. 74:6377-6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyengar, S., J. E. Hildreth, and D. H. Schwartz. 1998. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J. Virol. 72:5251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonak, Z. L., R. K. Clark, D. Matour, S. Trulli, R. Craig, E. Henri, E. V. Lee, R. Greig, and C. Debouck. 1993. A human lymphoid recombinant cell line with functional human immunodeficiency virus type 1 envelope. AIDS Res. Hum. Retrovir. 9:23-32. [DOI] [PubMed] [Google Scholar]
- 31a.Jones, P. L., T. Korte, and R. Blumenthal. 1998. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J. Biol. Chem. 273:404-409. [DOI] [PubMed] [Google Scholar]
- 32.Kenworthy, A. K., N. Petranova, and M. Edidin. 2000. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol. Biol. Cell 11:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kielian, M., P. K. Chatterjee, D. L. Gibbons, and Y. E. Lu. 2000. Specific roles for lipids in virus fusion and exit. Examples from the alphaviruses. Subcell. Biochem. 34:409-455. [DOI] [PubMed] [Google Scholar]
- 34.Kozak, S. L., J. M. Heard, and D. Kabat. 2002. Segregation of CD4 and CXCR4 into distinct lipid microdomains in T lymphocytes suggests a mechanism for membrane destabilization by human immunodeficiency virus. J. Virol. 76:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhmann, S. E., E. J. Platt, S. L. Kozak, and D. Kabat. 2000. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J. Virol. 74:7005-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapham, C. K., J. Ouyang, B. Chandrasekhar, N. Y. Nguyen, D. S. Dimitrov, and H. Golding. 1996. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science 274:602-605. [DOI] [PubMed] [Google Scholar]
- 37.Lee, B., M. Sharron, L. J. Montaner, D. Weissman, and R. W. Doms. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 96:5215-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 39.Lindwasser, O. W., and M. D. Resh. 2001. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges: raft-like membrane microdomains. J. Virol. 75:7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu, X., and J. Silver. 2000. Ecotropic murine leukemia virus receptor is physically associated with caveolin and membrane rafts. Virology 276:251-258. [DOI] [PubMed] [Google Scholar]
- 41.Lu, X., Y. Xiong, and J. Silver. 2002. Asymmetric requirement for cholesterol in receptor-bearing but not envelope-bearing membranes for fusion mediated by ecotropic murine leukemia virus. J. Virol. 76:6701-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manes, S., G. Del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, P. Sánchez-Palomino, R. Delgado, J. Alcamí, E. Mira, and C. Martinez-A. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manes, S., E. Mira, C. Gomez-Mouton, R. A. Lacalle, P. Keller, J. P. Labrador, and C. Martinez-A. 1999. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J. 18:6211-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz-Barroso, I., S. Durell, K. Sakaguchi, E. Appella, and R. Blumenthal. 1998. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 140:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen, D. H., and D. Taub. 2002. CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168:4121-4126. [DOI] [PubMed] [Google Scholar]
- 48.Niyogi, K., and J. E. Hildreth. 2001. Characterization of new syncytium-inhibiting monoclonal antibodies implicates lipid rafts in human T-cell leukemia virus type 1 syncytium formation. J. Virol. 75:7351-7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otteskog, P., L. Wanger, and K. G. Sundqvist. 1983. Cytochalasins distinguish by their action resting human T lymphocytes from activated T-cell blasts. Exp. Cell Res. 144:443-454. [DOI] [PubMed] [Google Scholar]
- 51.Parolini, I., M. Sargiacomo, M. P. Lisanti, and C. Peschle. 1996. Signal transduction and glycophosphatidylinositol-linked proteins (lyn, lck, CD4, CD45, G proteins, and CD55) selectively localize in Triton-insoluble plasma membrane domains of human leukemic cell lines and normal granulocytes. Blood 87:3783-3794. [PubMed] [Google Scholar]
- 52.Parolini, I., S. Topa, M. Sorice, A. Pace, P. Ceddia, E. Montesoro, A. Pavan, M. P. Lisanti, C. Peschle, and M. Sargiacomo. 1999. Phorbol ester-induced disruption of the CD4-Lck complex occurs within a detergent-resistant microdomain of the plasma membrane. Involvement of the translocation of activated protein kinase C isoforms. J. Biol. Chem. 274:14176-14187. [DOI] [PubMed] [Google Scholar]
- 53.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535-539. [DOI] [PubMed] [Google Scholar]
- 53a.Peterson, G. L. 1983. Determination of total protein. Methods Enzymol. 91:95-119. [DOI] [PubMed] [Google Scholar]
- 54.Platt, E. J., S. L. Kozak, and D. Kabat. 2000. Critical role of enhanced CD4 affinity in laboratory adaptation of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:871-882. [DOI] [PubMed] [Google Scholar]
- 55.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popik, W., J. E. Hesselgesser, and P. M. Pitha. 1998. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J. Virol. 72:6406-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Popik, W., T. M. Alce, and W. C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raviv, Y., M. Viard, J. Bess, Jr., and R. Blumenthal. 2002. Quantitative measurement of fusion of HIV-1 and SIV with cultured cells using photosensitized labeling. Virology 293:243-251. [DOI] [PubMed] [Google Scholar]
- 59.Roper, K., D. Corbeil, and W. B. Huttner. 2000. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat. Cell Biol. 2:582-592. [DOI] [PubMed] [Google Scholar]
- 60.Salzwedel, K., E. D. Smith, B. Dey, and E. A. Berger. 2000. Sequential CD4-coreceptor interactions in human immunodeficiency virus type 1 Env function: soluble CD4 activates Env for coreceptor-dependent fusion and reveals blocking activities of antibodies against cryptic conserved epitopes on gp120. J. Virol. 74:326-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 63.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 64.Singer, I. I., S. Scott, D. W. Kawka, J. Chin, B. L. Daugherty, J. A. DeMartino, J. DiSalvo, S. L. Gould, J. E. Lineberger, L. Malkowitz, M. D. Miller, L. Mitnaul, S. J. Siciliano, M. J. Staruch, H. R. Williams, H. J. Zweerink, and M. S. Springer. 2001. CCR5, CXCR4, and CD4 are clustered and closely apposed on microvilli of human macrophages and T cells. J. Virol. 75:3779-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singer, S. J., and G. L. Nicolson. 1972. The fluid mosaic model of the structure of cell membranes. Science 175:720-731. [DOI] [PubMed] [Google Scholar]
- 66.Sorice, M., T. Garofalo, R. Misasi, A. Longo, V. Mattei, P. Sale, V. Dolo, R. Gradini, and A. Pavan. 2001. Evidence for cell surface association between CXCR4 and ganglioside GM3 after gp120 binding in SupT1 lymphoblastoid cells. FEBS Lett. 506:55-60. [DOI] [PubMed] [Google Scholar]
- 67.Sorice, M., I. Parolini, T. Sansolini, T. Garofalo, V. Dolo, M. Sargiacomo, T. Tai, C. Peschle, M. R. Torrisi, and A. Pavan. 1997. Evidence for the existence of ganglioside-enriched plasma membrane domains in human peripheral lymphocytes. J. Lipid Res. 38:969-980. [PubMed] [Google Scholar]
- 68.Stantchev, T. S., and C. C. Broder. 2001. Human immunodeficiency virus type-1 and chemokines: beyond competition for common cellular receptors. Cytokine Growth Factor Rev. 12:219-243. [DOI] [PubMed] [Google Scholar]
- 69.Sylwester, A., D. Wessels, S. A. Anderson, R. Q. Warren, D. C. Shutt, R. C. Kennedy, and D. R. Soll. 1993. HIV-induced syncytia of a T cell line form single giant pseudopods and are motile. J. Cell Sci. 106:941-953. [DOI] [PubMed] [Google Scholar]
- 70.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 71.Ugolini, S., M. Moulard, I. Mondor, N. Barois, D. Demandolx, J. Hoxie, A. Brelot, M. Alizon, J. Davoust, and Q. J. Sattentau. 1997. HIV-1 gp120 induces an association between CD4 and the chemokine receptor CXCR4. J. Immunol. 159:3000-3008. [PubMed] [Google Scholar]
- 72.Weiss, C. D., J. A. Levy, and J. M. White. 1990. Oligomeric organization of gp120 on infectious human immunodeficiency virus type 1 particles. J. Virol. 64:5674-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson, N. F., and W. J. Snell. 1998. Microvilli and cell-cell fusion during fertilization. Trends Cell Biol. 8:93-96. [DOI] [PubMed] [Google Scholar]
- 74.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 75.Xiao, X., L. Wu, T. S. Stantchev, Y. R. Feng, S. Ugolini, H. Chen, Z. Shen, J. L. Riley, C. C. Broder, Q. J. Sattentau, and D. S. Dimitrov. 1999. Constitutive cell surface association between CD4 and CCR5. Proc. Natl. Acad. Sci. USA 96:7496-7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoshida, H., Y. Koga, Y. Moroi, G. Kimura, and K. Nomoto. 1992. The effect of p56lck, a lymphocyte specific protein tyrosine kinase, on the syncytium formation induced by human immunodeficiency virus envelope glycoprotein. Int. Immunol. 4:233-242. [DOI] [PubMed] [Google Scholar]
- 77.Zhang, W., R. P. Trible, and L. E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9:239-246. [DOI] [PubMed] [Google Scholar]